Abstract
This study aimed to evaluate the effect of forest roadside on plant diversity and composition in the Hyrcanian temperate forest of northern Iran. To collect vegetation data, 116 rectangular sampling plots were established at two microhabitats (i.e., cut and fill slopes) of forest roads. For both microhabitats, the highest life form belonged to hemicryptophytes. Among the 85 identified species, 58 were native, 12 invasive, 9 potentially invasive, 5 pioneer, and 1 exotic. On the roadside microhabitats, the ground cover of the Rosaceae family, with 11 species, was the highest among plant families. The highest frequency of Grime’s life history strategies was the competitive strategy. Shannon–Wiener diversity and species richness indices of cut slopes were significantly higher than those of fill slopes. Partial canonical correspondence analysis indicated that species composition on roadsides was primarily influenced by microhabitats, elevation, gradient, canopy cover, story layer, occurrence of bare soil, and clay content. Our study contributes to understanding how roadside microhabitats affect environmental conditions, which might be helpful to forest road managers when implementing best management practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest roads are essential in providing connectivity and enabling mobility to access and manage forest ecosystems. They provide quick and easy access to forest resources, enabling forest management and intervention in the case of calamity/disturbance, and facilitating the use of other services provided by forests, such as ecotourism (Papierowska et al. 2020). While being useful in forest management, forest roads also cause environmental disturbances with negative consequences (Deljouei et al. 2018; Rahbarisisakht et al. 2021). Additionally, they contribute to environmental degradation, landscape fragmentation, and biodiversity loss by affecting the ecosystems in which they are developed (Nayak et al. 2020).
Constructing forest roads creates unique microhabitats, such as the cut and fill slopes and ditches that disrupt the ecosystem’s connectivity (Son et al. 2020). About half of all disturbances caused by road construction in mountainous areas are due to the cut and fill slopes (Grace 1999). Furthermore, these microhabitats are characterized by the highest erosion and sediment production among road components, low soil nutrient content, and increased soil compaction (Solgi et al. 2021). Roadside microhabitat conditions are limiting factors for some native species due to vegetation loss and soil disturbance, which alter the light and moisture regimes (Avon et al. 2010). Accordingly, plants with better adaptations to new environmental conditions can establish themselves in roadside microhabitats (Follak et al. 2018), and during their establishment and growth, they can create conditions that favor non-native and invasive species (Barbosa et al. 2010). Invasive species are considered to be a major factor causing native biodiversity loss and changes in the environmental, social, and economic conditions, as well as ecosystem services (Kotowska et al. 2021). Moreover, roads play an important role in spreading exotic plants, by the space they create and the associated transport of propagules by vehicles (Sharma and Raghubanshi 2009).
To develop feasible strategies for restoring plant communities along roads, it is essential to understand the structure and composition of the vegetation established on road microhabitats (i.e., cut and fill slopes). Therefore, it is important to evaluate the plant communities by inventories and the factors that influence their establishment, with a special focus on reducing the adverse impacts of forest roads by providing ecological restoration solutions that address site-specific concerns (Martín-Sanz et al. 2015). Vegetation establishment, structure, and floristic composition are influenced by various environmental factors, including topography, soil properties, and nutrient conditions (Feng et al. 2016; Tilk et al. 2017).
Several studies have already reported the effect of different factors on vegetation composition, such as the road material type, e.g., paved or unpaved roads (e.g., Carias et al. 2021; Phillips et al. 2021), traffic level (Sharma and Raghubanshi 2009), vehicle exhaust gases (Viskari et al. 2000), and applicable technical revegetation methods (Paschke et al. 2000). However, only a few studies have investigated the distribution, patterns, and establishment of vegetation along different road microhabitats such as the cut and fill slopes (i.e., Feng et al. 2016; Lázaro-Lobo and Ervin 2019). Roadside studies are needed to evaluate the decline in species diversity and richness (Deljouei et al. 2017). Although many studies were conducted on the impact of roadside on vegetation composition in temperate deciduous mixed forests (see, e.g., Parendes and Jones 2000; Flory and Clay 2009), few have been carried out in the Hyrcanian mixed forest of northern Iran (Hosseini et al. 2011; Lotfalian et al. 2012; Deljouei et al. 2017, 2018). Hyrcanian forest is a unique Arcto-Tertiary forest, where several tree species survived the last ice age, including the Caucasian wingnut (Pterocarya fraxinifolia (Lam.) Spach), Persian silk tree (Albizia julibrissin Durazz), Persian ironwood (Parrotia persica (DC.) C.A.Mey.), and Caspian locust (Gleditsia capsica Desf.) (Akhani et al. 2010). Since the early 1970s, about 60% of Iranian forests have been managed, and in recent years, forest roads have had an ecological impact on species diversity and composition. In contrast to other temperate forests discussed in the literature, Hyrcanian forest has not been managed for a long time. Based on the importance of forest roads as the core infrastructure for forest management, the objectives of this study were the following: (i) to assess the floristic composition and identify indicator species on cut and fill slopes of forest roads; (ii) to evaluate the differences in vegetation diversity between roadside microhabitats (cut and fill slopes); and (iii) to identify the key environmental factors affecting vegetation composition along roadside microhabitats. In addition, the paper discusses the implication of findings on forest road management.
Materials and methods
Study site
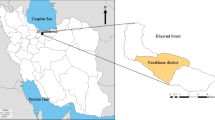
Hyrcanian forest is a temperate deciduous forest located in northern Iran, where it covers an area of ~ 1.9 million hectares (Heshmatol Vaezin et al. 2022). The study site is located near Nowshahr city, in the Mazandaran province, at the educational and experimental forest of the University of Tehran. The mean annual precipitation is 1300 mm, and the mean annual temperature is 17 °C in the study area (Haghshenas et al. 2015). Lithologically, the substrate is mostly calcareous parent material. The study was focused on the road network from the Namkhane district (Fig. 1). The study site covers 1083 ha, ranging from 350 to 1350 m a.s.l.; in the area, slope varies from 0 to 80%. As a part of the forest management in Namkhane, single and group selective cutting regimes are implemented to maintain mixed uneven aged high forests. The most important forest plant associations are oriental beech (Fagetum orientalis), oak-hornbeam (Querceto-Carpinetum betulii), and oak-oriental beech (Carpino-Fagetum orientalis). The most important species in the area are box holly (Ruscus Hyrcanus Woronow), yellow archangel (Lamium galeobdelon (L.) Crantz), ostrich fern (Matteuccai struthiopteris (L.) Tod.), and dog’s mercury (Mercurialis perennis L.) mixed with the oriental beech (Fagetum orientalis) community. Wild service tree (Sorbus torminalis (L.) Crantz), Primula (Primula heterochroma Stapf), China Redkotzvetkovaya (Lathyrus laxiflorus (Desf.) Kuntze), wood dock (Rumex sanguineus L.), purple tails (Teucrium hyrcanicum), hawthorn (Crataegus microphylla K. Koch), cow parsley (Anthriscus sylvestris (L.) Hoffm.), Cretan brake (Pteris cretica L.), medlar (Mespilus germanica L.), Alexandrian laurel (Danae racemose (L.) Moench), Persian Holly (Ilex spinigera (Loes.) Loes.), and field elm (Ulmus minor Mill.) are mixed with the Querceto-Carpinetum betulii community; bishop’s hat (Epimedium pinnatum Fisch. ex DC.), common chickweed (Stellaria media (L.) Vill.), Caucasian alder (Alnus subcordata C.A.Mey.), glandular crane's-bill (Geranium platypetalum Fisch. & C.A.Mey.), lesser calamint (Calamintha officinalis (L.) Kuntze), remote sedge (Carex remota L.), horse mint (Mentha longifolia (L.) Huds.), and symphyandra odontosepala (Campanula odontosepala Boiss.) are mixed with the Carpineto-Fagetum orientalis community.
Sampling design
Field data were collected in late July of 2020, when most species were expected to be present in the vegetation composition. Sampling plots were established at the verge of forest roads categorized as all season main forest roads. These roads have gravel surfacing, 5.5 m in width and a longitudinal slope of 3 to 8%. Overall, road network length is 15.8 km in the study site, and the road density is 14.6 m per hectare. These roads were constructed in 1988 from river run gravel, which has a carbonate origin, and limestone, which was used for stabilization in some areas. Approximately 10 km of the main road network was selected for the study.
Systematic sampling was used to collect vegetation data in the study area. The first sampling plot was located at the beginning of the road, followed by plots positioned and marked at 150-m intervals along each side of the road. Geographical coordinates of the stations corresponding to each plot on the road centerline were recorded by a Garmin GPSMAP 64 s GPS unit (Garmin Ltd., Olathe, USA). We sampled 116 plots on cut and fill slopes (58 sampling plots on each microhabitat) with an area of 20 m2 (10 × 2 m; Fig. 2) and recorded the frequency of herbaceous species in each plot. The abundance–dominance coefficients for the species have been quantified using the Londo decimal scale (Londo 1976). All the plants were identified according to the Flora Iranica (Rechinger 1963–1998) and Flora of Iran (Assadi et al. 1989) guidebooks.
Plant species classification
In each sampling plot, the proportions of different life forms (Raunkiaer 1934) were inventoried. Raunkiaer’s (1934) classification scheme for life forms is based on how the buds develop during unfavorable seasons. This classification system consists of five main groups for plant species: phanerophytes (Ph), chamaephytes (Ch), hemicryptophytes (He), cryptophytes (Cr), and therophytes (Th). Life span was classified as annual or biennial (AB), herbaceous perennial (P), and tree or shrub (W). The chorology of the species in the study site was assessed, according to Zohary (1973), which are Euro-Siberian (ES), Pluriregional (PL), Mediterranean (M), Irano-Turanian (IT), Cosmopolitan (COSM), Subcosmopolitan (SCOSM), Hyrcanian (HYR), and Euxino-Hyrcanian (Euxino-HYR). Nativity status was determined based on five classes, namely native (N), invasive (I), pioneer (P), exotic (E), and potentially invasive (PI) species. Grime’s CSR strategy model categorizes species based on their responses to stress and disturbance, which are two major groups of external environmental factors. Grime’s strategy model (Grime 2002) has outlined three primary strategies, including competitive (C), stress-tolerant (S), and ruderal (R), also, four secondary strategies, namely competitive-ruderal (CR), competitive-stress-tolerant (CS), stress-tolerant-ruderal (SR), and competitive-stress-tolerant-ruderal (CSR).
Plant species diversity
The heterogeneity of species within a community was described with various indices. Each plot was evaluated for herbaceous species diversity using the Shannon–Wiener index (H), species richness (number of herbaceous species; S), and Pielou’s index (E), as described in Eqs. (1) to (4) (Pielou 1966):
where pi is the proportion/share of individuals of species i, ni is the number of individuals of a given species (s), and N is the number of individuals of all species found within a plot. All indices were estimated using the PAST software (ver. 4.8).
Environmental data
Soil samples were collected on dry days at four corners and the center of the sampling plots at 10 cm depth. A sieve with a 2-mm aperture was used to filter soil samples. Using Bouyoucos hydrometers (Makineci et al. 2007), the sand, silt, and clay shares in soil samples were determined. The electrical conductivity (EC, in µs cm−1) was measured using an EC meter and a 1:1 solution of soil–distilled water (Gee and Baude 1986). In order to determine pH, a pH meter and a 1:1 soil–water extract were used (Rayment and Lyons 2011). Available calcium (Ca) and magnesium (Mg) in ppm were estimated with an atomic absorption spectrophotometer, along with cation exchange capacity (CEC), which was measured with a flame photometer (Bower et al. 1952). The soil analysis was carried out in the laboratory at the Faculty of Natural Resources, University of Tehran.
Environmental factors such as the gradient (steepness), litter depth, the percentage of bare soil (as a proxy for soil disturbance), topography factors (aspect, and elevation), soil drainage classes (excessively, well, moderately well, poorly, very poorly drained), story layers (i.e., canopy layer), and canopy cover (%) were recorded (Møller et al. 2019).
Statistical analysis
All the analysis was conducted using R software version 3.6.1 (R Core Team 2021). The indicator species were identified by the use of indicator species analysis (ISA) provided by the indicspecies package (Cáceres and Legendre 2009). The mean values of the Shannon–Wiener index, species richness, and Pielou’s index were compared at roadside microhabitats using an independent sample t-test. Partial canonical correspondence analysis (pCCA) was used to partition the variance in plant community caused by environmental variables (roadside microhabitat, slope, gradient, bare soil, canopy, litter depth, tree layers, elevation, aspect, and drainage). Spatial factors (longitude and latitude) were also included as covariates in the pCCA to analyze the variance in the plant community composition caused by the geographic location of the plots. Variance inflation factors (VIF) were used to remove highly correlated variables (VIF > 10) from the analysis, to avoid collinearity issues (Borcard et al. 2011). In the initial model, VIF ranged from 1.2 to 16.1. However, in the final model, all VIFs were less than two due to the exclusion of highly correlated variables (sand and longitude). As such, the longitude was removed from the analysis. In the final model, all VIFs were less than two. The significance of the variables was tested with automatic forward selection and Monte Carlo permutation tests (1,000 unrestricted permutations). A pCCA test was performed using the vegan R package (Oksanen et al. 2019).
Results
Floristic composition of plant species
We recorded 85 plant species, including 67 herbaceous species, 12 trees, and 6 shrubs belonging to 49 families (Table A1). Splitting these values by microhabitats, 76 plant species were identified on the cut slope, of which 17 species belonging to 14 families were uniquely recorded in this microhabitat; 68 species were identified on the fill slope, of which 9 species belonging to 9 families were exclusively recorded in this microhabitat (Table A1). Approximately 69% of species were common in both microhabitats. The family with the highest number of species was Rosaceae (11 species, 9 of which occurred in both microhabitats; Fig. 3). The majority of families (67.3%) were represented by only a single species. The sampling plots had the highest number of species belonging to the Carex genus, with four species.
In the study plots, the hemicryptophyte life form dominated (37.6%), followed by phanerophytes (24.7%), cryptophytes (22.4%), therophytes (12.9%), and chamaephytes (2.4%) (Table A1). The share of life forms for cut and fill slopes is shown in Fig. 4. For both microhabitats, the highest and the lowest share of life forms belonged to hemicryptophytes and chamaephytes, respectively (Fig. 4).
Approximately 69.4% of the species were perennial herbs, 21.2% were trees or shrubs, and 9.4% were biennial (Table A1). For the cut slope, these values were 71%, 21%, and 8%, respectively (Fig. 5a). The values were similar to those of the fill slope, where 68% of the species were perennial herbs, 22% trees or shrubs, and 10% biennial (Fig. 5a). Based on chorology, species were identified as Euro-Siberian (17.6%), Euro-Siberian, Irano-Turanian (15.3%), Euro-Siberian, Irano-Turanian, Mediterranean (14.1%), and Pluriregional (12.9%). Euro-Siberian species had the highest frequency, while Euro-Siberian, Mediterranean, Irano-Turanian, and Pluriregional had the lowest frequency on the cut and fill slopes (Fig. 5b). The share of Euro-Siberian species was 18.4% and 16.2% for cut and fill slopes, respectively, whereas the share of Euro-Siberian, Mediterranean, Irano-Turanian, and Pluriregional was only 1.5% for fill slope (Fig. 5b).
Among the identified species (Table A1), 58 were native, 12 invasive, 9 potentially invasive, 5 pioneer, and 1 exotic, which belongs to the Poaceae family (Microstegium vimineum (Trin.) A. Camus.). Native species had the highest frequency among all species (68% at the cut slope and 63% at the fill slope), followed by invasive (12% at the cut slope and 15% at the fill slope), potentially invasive (12% at the cut slope and 13% at the fill slope), pioneer (7% for both microhabitats), and exotic (approximately 2%) species (Fig. 6a). Almost all species were assigned to 19 secondary or transient Grime’s strategies (C/CR, C/CS, C/SC, C/CSR, CR, CS, CR/CSR, CS/CSR, R/CR, R/SR, R/CSR, S/SC, S/SR, S/CSR, SC, SC/CSR, SR, SR/CSR, and CSR; Table A1). Ten out of the 85 species exhibited a C, two S, and one R strategy (Table A1). On main Grime’s strategies, the highest species share was found for the C strategy, with 13.2% and 11.8% for the cut and fill slopes, respectively, and the lowest share was that of the R strategy, with 1.3% and 1.5% for the cut and fill slopes, respectively (Fig. 6b). S was not observed at the fill slope, whereas its share at the cut slope was 2.6% (Fig. 6b). CR had the highest share of secondary Grime’s strategies, with 13.2% for the cut slope and 14.7% for the fill slope (Fig. 6b). The lowest share of secondary Grime’s strategies was S/SC, S/SR, CS/CSR, SC/CSR, and R/SR with 1.3% at the cut slope; C/CS was not found in the case of cut slope (Fig. 6b). In addition, for the fill slope, the lowest share of secondary Grime’s strategies was that of S/SR, CS/CSR, SC/CSR, and C/CS with 1.5%, whereas SR and R/SR strategies were not observed at the fill slope (Fig. 6b).
Indicator species
Thirteen species were identified as an indicator for the two microhabitats (Table 1), of which nine species were for the cut slope (69%) and four species for the fill slope (31%). The life forms of indicator species were hemicryptophytes (six species, 46%), phanerophytes (four species, 31%), cryptophytes, and therophytes (three species, 23%). No indicator species belonged to the chamaephytes. Therophytes with one species were found on the fill slopes. Eight indicators species (61%) were native species, three were potentially invasive species (23%), one was a pioneer (8%), and one (8%) was an invasive species. All the species were found in both microhabitats except for Setaria glauca ((L.) (P. Beauv.), which is an invasive species that was found only on the fill slope. Overall, the main indicator species were perennial herbs (8/13, 61%), followed by trees or shrubs (4/13, 31%), and annual or biennial (1/13, 8%) species.
Diversity indices
According to the Shannon–Wiener diversity index, a higher diversity was observed on the cut slope as compared to the fill slope (t = 2.64, p < 0.05; Fig. 7). The results indicated that the Shannon–Wiener diversity index ranged from 0.52 to 1.14 at the cut slope and from 0.41 to 1.00 at the fill slope (Fig. 7a). The average values (± standard deviation) of the Shannon–Wiener diversity index were 0.81 (± 0.02) for the cut and 0.75 (± 0.02) for the fill slope, respectively (Fig. 7a). Species richness was also significantly higher at the cut as opposed to the fill slope microhabitat (t = 2.24, p < 0.05; Fig. 7b). Furthermore, in terms of species richness, the mean values of the cut and fill slopes were calculated at 15.98 (± 0.40) and 14.78 (± 0.36), respectively (Fig. 7b). Species richness varied from 10 to 23 and from 6 to 20 at the cut and fill slope microhabitats, respectively (Fig. 7b). In addition, Pielou’s evenness index values of the two microhabitats were quite similar (t = 1.63, p > 0.05; Fig. 7c). In terms of Pielou’s evenness index, the corresponding mean values for the cut and fill slopes were calculated as 0.84 (± 0.02) and 0.80 (± 0.02), respectively (Fig. 7c). In addition, the results showed that the minimum and maximum values for the two microhabitats were 0.63 and 1.27 (cut slope) and 0.56 and 1.22 (fill slope), respectively (Fig. 7c).
Environmental effects on species composition
The pCCA indicated that species composition on roadsides was primarily influenced by roadside microhabitats (cut or fill slope), elevation, gradient, canopy cover, story layer, bare soil, and clay content (p < 0.05; Table 2). However, the aspect, litter depth, soil drainage, silt content, calcium, magnesium, EC, and pH did not significantly contribute to developing the pCCA models (p > 0.05; Table 2). The first two axes of the pCCA models were statistically significant (p < 0.003) and described 55.7% of the variation in the plant community caused by environmental variables (Fig. 8).
Discussion
Species compositional differences by cut and fill slope microhabitats
Compared with the findings of studies on road ecology of temperate regions, the results of this study indicate that forest roadside microhabitats (cut and fill slopes) had a significant impact on vegetation diversity and composition (e.g., Deljouei et al. 2017, 2018; Vanneste et al. 2020). In this study, we focused on the impact of two microhabitats created by road construction on plant diversity and composition, by considering roadside microhabitat (cut and fill slopes) effects on the plant communities. Roadsides are contrasting microhabitats compared to surrounding forest ecosystems, being characterized by unique light, moisture, and temperature regimes (Tiţă et al. 2019). Corridor opening causes canopy gaps, and as a result, herbaceous, fast-growing, and light-demanding species become established around the road (Avon et al. 2010; Deljouei et al. 2018), influencing plant growth and competition patterns, particularly between shade-tolerant and shade-intolerant species (Horn 1971). As a result, roadside disturbances reduce the abundance of trees and shrubs.
A total of 67 out of 85 plants were identified as herbaceous species, findings which agree with those of Deljouei et al. (2017) reported for the Namkhane district, Kheyrud forest. Esmailzadeh et al. (2012) found 58 herbaceous species in the east part of the Hyrcanian forest (Gorgan forest). Factors such as ecological disturbances, topography, light intensity, and humidity significantly affect the species pool and vegetation composition of the Hyrcanian forest of northern Iran (Shakeri et al. 2021). In the collected set of species, Rosaceae was the most representative family, followed by Poaceae, Cyperaceae, and Labiatae. These results are not surprising since Rosaceae is a family containing a large number of species with a wide ecological range and strong adaptability (Yu et al. 2002). Moreover, many species in this family can grow well at an altitude of 400–4200 m in a low-humidity environment and can safely pass over winter in snow and temperatures of − 34 °C (Yang et al. 2021). Tolerance and hyperaccumulation are characteristics of the Poaceae (Kensa et al. 2018), and Cyperaceae is the third largest monocotyledons family that occurs in a large variety of altitudes, from 0 to 5000 m a.s.l. (Mline and Mline 1975). Moreover, most of the species in this family tend to tolerate extreme temperatures as well as poor soils (Mline and Mline 1975). In this regard, there is no doubt that the ecological potential of the dominant herbaceous species along roadsides could be explored further to understand ecological indications of tolerance.
Plant life forms are a reflection of the environmental conditions in which they grow and provide clues about their habitat (Siadati et al. 2010). The highest proportion of life forms in this study was allocated to hemicryptophytes (40%), which is in agreement with the findings of Deljouei et al. (2017). Esmailzadeh et al. (2011) reported that hemicryptophytes, phanerophytes, and cryptophytes are the dominating life forms in the central part of the Hyrcanian forest. According to Ehrendorfer (1965), perennial herbs hemicryptophytes and annual therophytes can be adapted to different microhabitats in pioneer communities. Houssard et al. (1980) reported that phanerophytes are rare in the early stages of succession, whereas therophytes are abundant. It was stated that phanerophytes and chamaephytes were absent along the roadside, although these species are common in the forest interior (Avon et al. 2010). In a hornbeam-beech forest, chamaephytes did not exist farther than 5 m from the roadside, and at distances beyond this, there were changes from phanerophytes to hemicryptophyte species (Deljouei et al. 2017). In temperate regions with moist to the humid climate, hemicryptophytes are dominant and most frequent in broadleaf and mid-altitudinal forests (Raju et al. 2014) as a consequence of adaptation to regional ecological conditions (Talebi et al. 2014). Furthermore, the frequency of hemicryptophytes increases with forest age (Hermy et al. 1999). The abundance of hemicryptophytes in this study supports the claim of others that the Hyrcanian forest may be the world’s last remaining natural deciduous temperate forest (Fallahchai et al. 2018).
Based on the results, Euro-Siberian elements were more common than other floristic elements in the roadside microhabitats. Since the study area is located in the Hyrcanian forest, there is a floristic link between the study site and the Euro-Siberian phytogeographical area (Zohary 1973). In addition, Irano-Turanian elements were present because phytogeographically Irano-Turanian floristic elements are found in large parts of Iran (Akhani et al. 2013). These results agree with those of other studies carried out in other parts of the Hyrcanian forest (Atashgahi et al. 2009; Asadi et al. 2011). Several species of alpine flora occur on the plateau of Iran and belong to the Irano-Turanian region, while only a few species are found in the northern hemisphere or in the Euro-Siberian region. In terms of geomorphology and climate, the Alborz Mountains represent one of the most heterogeneous mountain systems in the southwest of Asia. This is due to the northern slopes, which are exposed to humid air masses that originate in the southern Caspian Sea, regulating the vegetation of Euro-Siberian phytochoria within the Hyrcanian ecoregion (Akhani et al. 2010). Nonetheless, on southern slopes and above forest line, various endemic species dominate in the semi-arid Irano-Turanian phytochorion (Noroozi et al. 2008). Irano-Turanian elements are found at higher altitudes (Ghahreman et al. 2006) because the upper mountain areas of Alborz are characterized by a large number of Irano-Turanian elements (Noroozi et al. 2008). This occurrence may be explained by two reasons. Firstly, there is a phytogeographical floristic link between our study sites (Hyrcanian district) and other areas in the Euro-Siberian region. Secondly, human activities are responsible for establishing weeds (Ghahreman et al. 2006).
Native species had the highest frequency in our study site. The presence of native species in a habitat is a criterion of habitat quality and sustainability (Sukopp et al. 1979). Spreading the topsoil enhances the recovery of biotic communities and ecosystem functions, while promoting the establishment of native plants (Lázaro-Lobo and Ervin 2019). Regardless, invasive and exotic plants can establish and spread through roads as the roads act as suitable corridors and microhabitats (e.g., Godefroid and Koedam 2004; Flory and Clay 2006); meanwhile, native species are adapting themselves to disturbed environments (e.g., Ervin 2009; Ahrens et al. 2014) causing changes in species diversity, composition, and vegetation abundance (Lázaro-Lobo and Ervin 2019). It is more likely to expect roadsides to influence native species positively and it is well known that constructing forest roads causes changes in light conditions and increases disturbance. Generally, abiotic conditions allow exotic species to establish easily and tend to increase vegetation diversity (e.g., Bernhardt-Römermann et al. 2006). Roadside microhabitats are ideal microhabitats for invasive and exotic species and can facilitate the transport of plant propagules over long distances (Follak et al. 2018). We identified the presence of only one exotic species (Microstegium vimineum), which seemed to be restricted to higher light conditions. The establishment of M. vimineum in forest understories alters forest succession dynamics, suppresses native species, and intensifies prescribed fires (Wagner and Fraterrigo 2015). The following processes are potentially driving the arrival and establishment of exotic species in our study area: (1) an initial mechanical disturbance caused by constructing and subsequently utilizing forest roads; (2) creating suitable seedbeds with mineral soil, high level of light, moisture, and nutrients which are the requirement of some exotic species; and (3) the presence of multiple dispersal vectors, including human and natural sources (Deljouei et al. 2017).
Species with competitor–ruderal behavior are the most frequent species that contribute moderately to grassland biomass, since they complement the performance of ecosystems occupied primarily by dominant species and exploit relatively unfavorable microhabitats (Grime 2006). Competition and disturbance influenced the evolution of this functional type. As defined by Grime (2002), competitor–ruderals are found in habitats where moderate disturbance prevents competitors from dominating the vegetation. As the traditional management of lands includes cattle and sheep grazing in the study site, plant strategies have changed from S, SC, S/SC, and SC/CSR to CR, C/CR, R/CR, and C/SR. Ruderalism varies from place to place, depending on the level of disturbance. Additionally, Pierce et al. (2007) found that disturbance intensity enhanced species diversity as well as functional diversity, particularly among primitives and species with low co-dominance, whereas species with high stress tolerance were suppressed. A variety of species representing the S and SR strategies can also succeed in managed sites (Huhta and Rautio 1998). Accordingly, the presence of CR strategists in the study site confirms that disturbances significantly influence niche segregation; fast-growing competitive–ruderals consume local nutrients to establish their genes before ephemeral nutrient patches are exhausted (Pierce et al. 2007; Hüseyinova et al. 2013). The results of this study were compared with those of others (e.g., Hüseyinova et al. 2013; Grime et al. 2014), finding differences that may be explained by features of the microhabitats in the study area, including altitude, climate, location, soil structure, light conditions, nutrient content and intensity of disturbance. For instance, alpine plants are exposed to high stress levels due to the high altitudes, low temperatures, strong winds, dryness, and UV radiation (Grime et al. 2014). A species strategy within the CSR space is likely to change in such circumstances. As a result, species often exhibit different strategies in different habitats; therefore, it is uncertain about assuming that they will follow the same strategy irrespective of the habitat.
Indicator species
Analysis of indicator species revealed that 13 species exhibited a significant affinity for either the cut or the fill slope microhabitat. Son et al. (2020) identified 48 indicator species in various roadways and microhabitats and reported 50% of the species as native and 46% as invasive, which agrees with our results. Native species are directly impacted by high temperatures, soil disturbance, and drought, whereas habitat disturbance has an indirect impact (Salinitro et al. 2018). Invasive species can be dispersed along the road due to environmental disturbances (Avon et al. 2010). Invasive species presence in road seed banks is due to their high seed dispersion by wind, vehicles, humans, and animals (Taylor et al. 2012). Timber harvesting (Venanzi et al. 2020), road construction, and maintenance processes cause soil disturbance, which creates an environment favorable for the establishment and growth of invasive non-native plants (Follak et al. 2018). Invasive species can adapt to new habitats and environmental changes by having characteristics such as potent scattering ability, fast growth, short reproduction time, and a high tolerance to various habitat conditions (Poland et al. 2021). Consequently, it is necessary to manage invasive species in new regions in order to avoid their spreading and dispersing (Son et al. 2020). The majority of species are light and moisture demanding, and a large number of their seeds are found in soil seed banks of Hyrcanian forest; in proper conditions, they will establish in disturbed habitats (Esmailzadeh et al. 2011). With regard to the types of indicator species identified, we found that perennial herb species were most established on the roadside microhabitats, while annual/biennial plant species, as well as trees and shrubs, were less abundant. In contrast, Son et al. (2020) reported that annual/biennial species predominate in microhabitats along roadsides. Despite the ability of perennial herb species to tolerate harsh roadways, the major limiting factors of growing perennial herbs and trees in newly created habitats are an inadequate soil and nutrient supply, lack of deep soil, or symbiotic soil conditions (Paschke et al. 2000). Almost all herbaceous perennial plants that grow in disturbed sites require appropriate soils to establish and survive (Paschke et al. 2000). However, as soil conditions improve, annuals and biennials, as well as shortlived perennial herbs, decline significantly due to competition with perennial herbs and shrubs (Foster and Tilman 2000). Based on this pattern, in this study, the annual/biennial indicator species were not dominant along the roadside microhabitats. Thus, the study sites will need to be monitored for longer periods to assess whether succession toward dominance of those species is in progress (Lupardus et al. 2019).
Diversity differences between microhabitats
Shortly after road construction, different species can establish in the roadside environmental conditions and increase the species diversity and richness on the roadside (Martín-Sanz et al. 2015). Plant diversity and richness were significantly influenced by microhabitats (cut and fill slopes), whereas the evenness index did not show any significant differences. Furthermore, the diversity and richness at the cut slope were higher than the fill slope, which is consistent with other results reported for the Hyrcanian forest (Hosseini et al. 2011; Lotfalian et al. 2012), and temperate forests of South Korea (Chu et al. 2019). On cut slopes constructed by excavation, vegetation establishment has been reported to follow a primary succession process (Jiménez et al. 2013). The parent materials, construction techniques, and construction period may differ despite the similarity in geology (Rentch et al. 2005). Cut slopes have more thin soil and soil that comes from lower horizons, meaning that there is a smaller volume of soil for plants to absorb water and nutrients. Fill slopes consist of steep rock fills and moderate to flat fills composed of unconsolidated materials. The soil can be compacted in order to increase soil stability; therefore, the potential of root growth and development will be limited (Miller et al. 2002). After the road construction, sometimes trees are left on the fill slope to enhance the construction’s stability (Ji et al. 2013). Hence, fill slopes tend to be less favorable to plant species, leading to a reduction in species richness and diversity.
Environmental factors affecting species composition
pCCA showed that vegetation communities are significantly influenced by elevation and microhabitats (cut and fill slopes) as primary factors, followed by bare soil, canopy cover, clay content, slope gradient, and story layer (i.e., dominant understory vs. overstory layer). Previous studies showed that elevation is a key factor in landscape and plant diversity as it controls or influences environmental factors (Sang 2009). Although elevation does not directly drive biological processes, it correlates with several factors that influence organismic performance (Körner 2007), especially climatic factors such as the length of the growth period or the average ambient temperature during this period (Rumpf et al. 2018). The second most important factor was the microhabitat (i.e., cut or fill slope). This finding agrees with Rentch et al. (2005), who showed that the type of road habitat has strongly impacted the plant community. The construction of roads in mountainous regions is generally accompanied by great physical disturbances caused by cut-and-fill operations that change the landscape significantly (Paiaro et al. 2011). The third most influential factor was the presence of bare soil, and this finding is in line with Christiansen and Lyon (1975), who showed that the most vulnerable sites for vegetation establishment on the cut and fill slopes are those where the bare soil occurs. Increasing the amount of bare ground on the road verge or compaction may facilitate the establishment of ruderal plants (Truscott et al. 2005). The next important environmental factor was the canopy cover percentage. Canopy cover plays an important role in establishing species that require light (Sefidi et al. 2022). Deljouei et al. (2017) showed that the highest species richness was found to be at the road verge. The fifth important variable was the clay content. Skrindo and Pedersen (2004) showed that clay content affects the roadside vegetation composition in Norway. The next parameter affecting plant composition was the slope gradient, a result that is similar to other studies (Bochet et al. 2010). Bochet et al. (2010) studied the effect of slope gradient on vegetation cover and plant species composition near Valencia city (east of Spain). Their study indicated that the main factor influencing the vegetation variables on road slopes was the slope gradient, as well the absence of vegetation on roads with slopes greater than 45°. In contrast, vegetation cover on gentler slopes ranged from 44 to 78%. The last significant environmental factor on species composition was the story layer, which was also reported in past studies (Barbier et al. 2008) and correlates well with the importance of canopy cover (Fig. 8).
Management implications
Developing new forest roads is important in many mountainous regions to access resources and services and to connect and develop regional economies. In order to reduce the effects of forest roads on the environment, mitigation is more useful than stopping building or abandoning roads. This study revealed that roads that have been in use for a long period have some impacts on natural ecosystems. The cut slopes had a higher species diversity and richness than the fill slopes. We suggest that in order to ensure sustainable management of forest ecosystems, monitoring of vegetation should be included in local (i.e., study’s area) forestry plans during and after road construction. As part of road construction and maintenance activities, it is particularly important to avoid introducing and spreading exotic invasive species.
Knowledge of ecology, morphology, phenology, reproductive biology, physiology, and phytochemistry of vegetation species is essential for effective management. The impact of forest road construction on forest structure can be minimized by careful planning of road construction activities, and forest management based on ecological principles. Planning or managing areas around roads should consider microclimatological shifts along roadsides, assess their spatiotemporal stability, and determine how they relate to ecological processes. As a result of this approach, roads could be designed so as not to adversely affect plant diversity in forests and could be managed and constructed in a way that promotes forest conservation. The cumulative impact of road design, construction, maintenance, and use on forests needs to be predicted, planned, monitored, and assessed more carefully. Currently, environmental assessment methods are deficient for gaining rapid assessment objectives. Nonetheless, we suggest monitoring vegetation based on qualitative methods (seasonally), quantitative methods (using phytosociological methods), and mapping using ground-based techniques (via map overlays or GPS), as well as remotely sensed images (aerial photographs, high-resolution multi-spectral digital data). Using modern technology, practitioners can gain immediate access to such tools for monitoring and managing data. In recent years, the environmental assessment of road impacts on vegetation communities has been improved through the use of new tools (Transportation Research Board and National Research Council 2005).
Conclusion
In this study, we investigated the impact of forest roadside microhabitats on vegetation diversity and composition. Sixty-seven herbaceous species, 12 trees, and 6 shrubs belonging to 49 families were observed within the road verge. On the roadside microhabitats, the ground cover of the Rosaceae family with 11 species was the highest among plant families. Hemicryptophytes and Euro-Siberian elements had the largest proportion compared with other life forms and chorotypes, respectively. Persistent native and perennial herbs and to a lesser extent colonizing annual plants led to significant differences in vegetation diversity and composition on forest roadside microhabitats. Roads contributed to 19 of Grime’s secondary or transient strategies, indicative of the high functional vegetation diversity in the study sites. Diversity and richness indices at the cut slope were higher than at the fill slope. Identifying the response of different species along roadside microhabitats can be useful for understanding how the road corridor affects local biodiversity. It is likely that more natural habitat is being converted to roadside microhabitats as a result of road construction over time, which has considerable implications in terms of forest road impact. We expect that these results will serve as additional information for local land managers and decision makers in their attempt to manage plant species and maintain the integrity of biological communities. A greater focus should be placed on the impacts of anthropogenic activities on the diversity and composition of plants in this study area.
Our findings indicate that roadside microhabitats (cut and fill slopes) from the Hyrcanian temperate forest affect vegetation diversity and composition after constructing roads. In this regard, dispersal and settlement of various species are possible on cut and fill slopes due to the newly created microhabitats. In order to achieve better results, local or regional conditions should be considered in the management plans.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ahrens CW, Meyer TH, Auer CA (2014) Distribution models for Panicum virgatum (Poaceae) reveal an expanded range in present and future climate regimes in the northeastern United States. Am J Bot 101:1886–1894
Akhani H, Djamali M, Ghorbanalizadeh A, Ramezani E (2010) Plant biodiversity of hyrcanian relict forests, N Iran: an overview of the flora, vegetation, palaeoecology and conservation. Pakistan J Bot 42:231–258
Akhani H, Mahdavi P, Noroozi J, Zarrinpour V (2013) Vegetation patterns of the Irano-Turanian steppe along a 3,000 m altitudinal gradient in the Alborz Mountains of Northern Iran. Folia Geobot 48:229–255
Asadi H, Hosseini SM, Esmailzadeh O, Ahmadi A (2011) Flora, life form and chronological study of box tree (Buxus hyrcanus Pojark.) sites in Khybus protected forest. Mazandaran J Plant Biol 3:27–40
Assadi M, Ramak Maassoumi AA, Khatamsaz M (1989) Flora of Iran. Ministry of Agriculture, Iran
Atashgahi Z, Ejtehadi H, Zare H (2009) Study of floristics, life form and chorology of plants in the east of Dodangeh forests, Mazandaran province. Iran J Biol 22:193–203
Avon C, Bergès L, Dumas Y, Dupouey JL (2010) Does the effect of forest roads extend a few meters or more into the adjacent forest? A study on understory plant diversity in managed oak stands. For Ecol Manag 259:1546–1555
Barbier S, Gosselin F, Balandier P (2008) Influence of tree species on understory vegetation diversity and mechanisms involved—a critical review for temperate and boreal forests. For Ecol Manag 254:1–15
Barbosa NP, Fernandes GW, Carneiro MA, Júnior LA (2010) Distribution of non-native invasive species and soil properties in proximity to paved roads and unpaved roads in a quartzitic mountainous grassland of southeastern Brazil (rupestrian fields). Biol Invasions 12:3745–3755
Bernhardt-Römermann M, Kirchner M, Kudernatsch T, Jakobi G, Fischer A (2006) Changed vegetation composition in coniferous forests near to motorways in southern Germany: the effects of traffic-born pollution. Environ Pollut 143:572–581
Bochet E, García Fayos P, Tormo J (2010) How can we control erosion of roadslopes in semiarid Mediterranean areas? Soil improvement and native plant establishment. Land Degrad Dev 21:110–121
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, USA
Bower CA, Reitemeier RF, Fireman M (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73:251–261
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Carias M, Hascall E, Pennington K, Summers-Evans J (2021) Paving the way for habitat disturbance: different road types have distinct ecological impacts on annual plant communities and gall-forming insects in the Mojave Desert. CEC Res 5:1–12
Christiansen PA, Lyon DL (1975) A research report on roadside vegetation management. No. HR-159
Chu Y, Jin SN, Son D, Park S, Cho H, Lee H (2019) Introduction of alien plants on the fill and cut slopes of the road construction in South Korea. Ecol Resil Infrastruct 6:191–199
Deljouei A, Abdi E, Marcantonio M, Majnounian B, Amici V, Sohrabi H (2017) The impact of forest roads on understory plant diversity in temperate hornbeam-beech forests of Northern Iran. Environ Monit Assess 189:1–15
Deljouei A, Sadeghi SMM, Abdi E, Bernhardt-Römermann M, Pascoe EL, Marcantonio M (2018) The impact of road disturbance on vegetation and soil properties in a beech stand, Hyrcanian forest. Eur J for Res 137:759–770
Ehrendorfer F (1965) Uber stammesgeschichtliche differenzierungsmuster bei den dipsacaceae. Ber Dtsch Bot Ges 77:83–94
Ervin GN (2009) Distribution, habitat characteristics, and new county-level records of Baccharis halimifolia L. on a portion of its present US range boundary. SE Nat 8:293–304
Esmailzadeh O, Hosseini SM, Tabari M (2011) Relationship between soil seed bank and above-ground vegetation of a mixed-deciduous temperate forest in northern Iran. J Agr Sci Tech 13:411–424
Esmailzadeh O, Hosseini SM, Asadi H, Ghadiripour P, Ahmadi A (2012) Plant biodiversity in relation to physiographical factors in Afratakhteh yew (Taxus baccata L.) habitat. NE Iran J Plant Biol 4:1–12
Fallahchai MM, Haghverdi K, Mojaddam MS (2018) Ecological effects of forest roads on plant species diversity in Caspian forests of Iran. Acta Ecol Sin 38:255–261
Feng C, Ai Y, Chen Z, Liu H, Wang K, Xiao J, Chen J, Huang Z (2016) Spatial variation of soil properties and plant colonisation on cut slopes: a case study in the semitropical hilly areas of China. Plant Ecol Divers 9:81–88
Flory SL, Clay K (2006) Invasive shrub distribution varies with distance to roads and stand age in eastern deciduous forests in Indiana, USA. Plant Ecol 184:131–141
Flory SL, Clay K (2009) Effects of roads and forest successional age on experimental plant invasions. Biol Conserv 142:2531–2537
Follak S, Eberius M, Essl F, Fürdös A, Sedlacek N, Trognitz F (2018) Invasive alien plants along roadsides in Europe. EPPO Bull 48:256–265
Foster BL, Tilman D (2000) Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol 146:1–10
Gee GW, Baude JM (1986) Methods of soil analysis part 1, physical and mineralogical methods. A. Klute, Ed., Am. Soc. Agron Madison, Wisconsin 9:383–411
Ghahreman A, Naqinezhad A, Hamzehee B, Attar F, Assadi M (2006) The flora threatened black alder forests in the Caspian lowlands, Northern Iran. Rostaniha 7:5–30
Godefroid S, Koedam N (2004) The impact of forest paths upon adjacent vegetation: effects of the path surfacing material on the species composition on soil compaction. Biol Conserv 119:405–419
Grace JM III (1999) Erosion control techniques on forest road cutslopes and fillslopes in North Alabama. Transp Res Rec 1652:227–234
Grime JP (2002) Plant strategies, vegetation processes and ecosystem properties. John Wiley and Sons
Grime JP (2006) Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J Veg Sci 17:255–260
Grime JP, Hodgson JG, Hunt R (2014) Comparative plant ecology: a functional approach to common British species. Springer, USA
Haghshenas M, Marvi Mohadjer MR, Attarod P, Pourtahmasi K, Feldhaus J, Sadeghi SMM (2015) Climate effect on tree-ring widths of Fagus orientalis in the Caspian forests, northern Iran. For Sci Tech 12(4):176–182
Hermy M, Honnay O, Firbank L, Grashof-Bokdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conserv 91:9–22
Heshmatol Vaezin SM, Moftakhar Juybari M, Sadeghi SMM, Banaś J, Marcu MV (2022) The seasonal fluctuation of timber prices in Hyrcanian temperate forests, northern Iran. Forests 13(5):761
Horn HS (1971) The adaptive geometry of trees. Princeton University Press, Princeton
Hosseini SA, Jalilvand H, Pourmajidian MR, Parsakhoo A (2011) Effects of forest road clearings on understory diversity beneath Alnus subcordata L. stands in Iran. Maejo Int J Sci Technol 5:241–251
Houssard C, Escarré J, Bomane F (1980) Development of species diversity in some Mediterranean plant communities. Vegetation 43:59–72
Huhta AP, Rautio P (1998) Evaluating the impacts of mowing: a case study comparing managed and abandoned meadow patches. Ann Bot Fenn 35:85–99
Hüseyinova R, Kılınç M, Kutbay HG, Kılıç DD, Bilgin A (2013) The comparison of Grime’s strategies of plant taxa in Hacı Osman Forest and Bafra Fish Lakes in the Central Black Sea region of Turkey. Turk J Bot 37:725–734
Ji BY, Hwang JS, Jeong DH (2013) The construction method of the forest road for wood production. Nat Inst Forest Sci 526:44–45
Jiménez MD, Ruiz-Capillas P, Mola I, Pérez-Corona E, Casado MA, Balaguer L (2013) Soil development at the roadside: a case study of a novel ecosystem. Land Degrad Dev 24:564–574
Kensa MV, Sangeetha M, Lekshmi JL (2018) Road side flora of Pazhavoor, Tirunelveli district, Tamil Nadu, South India. GSC Biol Pharm Sci 4:053–063
Körner C (2007) The use of altitude in ecological research. Trends Ecol Evol 22(11):569–574
Kotowska D, Pärt T, Żmihorski M (2021) Evaluating Google Street View for tracking invasive alien plants along roads. Ecol Indic 121:107020
Lázaro-Lobo A, Ervin GN (2019) A global examination on the differential impacts of roadsides on native vs. exotic and weedy plant species. Global Ecol Conserv 17:e00555. https://doi.org/10.1016/j.gecco.2019.e00555
Londo G (1976) The decimal scale for releves of permanent quadrats. Vegetation 33:61–64
Lotfalian M, RiahiFar N, Fallah A, Hodjati SM (2012) Effects of roads on understory plant communities in a broadleaved forest in Hyrcanian zone. J for Sci 58:446–455
Lupardus RC, McIntosh ACS, Janz A, Farr D (2019) Succession after reclamation: identifying and assessing ecological indicators of forest recovery on reclaimed oil and natural gas well pads. Ecol Indic 106:105515
Makineci E, Demir M, Yilmaz E (2007) Long-term harvesting effects on skid road in a fir (Abies bornmulleriana Mattf.) plantation forest. Build Environ 42:1538–1543
Martín-Sanz RC, Fernández-Santos B, Martínez-Ruiz C (2015) Early dynamics of natural revegetation on roadcuts of the Salamanca province (CW Spain). Ecol Eng 75:223–231
Miller RL, Sencindiver JC, Skousen J (2002) Evaluation of highways and other transportation rights-of-way in West Virginia as habitats for invasive species: soil report. West Virginia Department of Transportation, Division of Highways, Charleston, WV, in press
Mline LJ, Mline MJG (1975) Living plants of the world. Random House, New York City
Møller AB, Iversen BV, Beucher A, Greve MH (2019) Prediction of soil drainage classes in Denmark by means of decision tree classification. Geoderma 352:314–329
Nayak R, Karanth KK, Dutta T, Defries R, Karanth KU, Vaidyanathan S (2020) Bits and pieces: forest fragmentation by linear intrusions in India. Land Use Policy 99:104619
Noroozi J, Akhani H, Breckle SW (2008) Biodiversity and phytogeography of the alpine flora of Iran. Biodivers Conserv 17:493–521
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Package ‘vegan’. Community ecology package, version 2.5–6.
Paiaro V, Cabido M, Pucheta E (2011) Altitudinal distribution of native and alien plant species in roadside communities from central Argentina. Austral Ecol 36:176–184
Papierowska E, Szatyłowicz J, Ruta M, Łachacz A, Gnatowski T, Stańczyk T (2020) Water repellency of soils on unpaved roads in coniferous forests. CATENA 195:104784
Parendes LA, Jones JA (2000) Role of light availability and dispersal in exotic plant invasion along roads and streams in the H.J. andrews experimental forest. Oregon Conserv Biol 14:64–75
Paschke MW, DeLeo C, Redente EF (2000) Revegetation of roadcut slopes in mesa verde national park, USA. Restor Ecol 8:276–282
Phillips BB, Navaratnam A, Hooper J, Bullock JM, Osborne JL, Gaston KJ (2021) Road verge extent and habitat composition across Great Britain. Landsc Urban Plan 214:104159
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Pierce S, Luzzaro A, Caccianiga M, Ceriani RM, Cerabolini B (2007) Disturbance is the principal α-scale filter determining niche differentiation, coexistence and biodiversity in alpine community. J Ecol 95:698–706
Poland TM, Patel-Weynand T, Finch DM, Miniat CF, Hayes DC, Lopez VM (2021) Invasive species in forests and rangelands of the United States: a comprehensive science synthesis for the United States forest sector. Springer Verlag, Cham
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rahbarisisakht S, Moayeri MH, Hayati E, Sadeghi SMM, Kepfer-Rojas S, Pahlavani MH, Kappel Schmidt I, Borz SA (2021) Changes in soil’s chemical and biochemical properties induced by road geometry in the hyrcanian temperate forests. Forests 12(12): 1805.
Raju VS, Krishna PG, Suthari S (2014) Environmental assessment of climate of a habitat through floristic life-form spectra, a case study of Warangal north forest division, Telangana, India. J Nat Sci 2:77–93
Raunkiaer C (1934) The life forms of plants and statistical plant geography; being the collected papers of C. Raunkiaer, London
Rayment GE, Lyons DJ (2011) Soil chemical methods: Australasia, vol 3. CSIRO publishing, Clayton, Australia
Rechinger KH (1963) Flora Iranica. Akademisch Druck-U Verlagsanstalt, Graz
Rentch JS, Fortney RH, Stephenson SL, Adams HS, Grafton WN, Anderson JT (2005) Vegetation–site relationships of roadside plant communities in West Virginia, USA. J Appl Ecol 42:129–138
Rumpf SB, Hülber K, Klonner G, Moser D, Schütz M, Wessely J, Willner W, Zimmermann NE, Dullinger S (2018) Range dynamics of mountain plants decrease with elevation. Proc Natl Acad Sci 115:1848–1853
Salinitro M, Alessandrini A, Zappi A, Melucci D, Tassoni A (2018) Floristic diversity in different urban ecological niches of a southern European city. Sci Rep 8:1–10
Sang W (2009) Plant diversity patterns and their relationships with soil and climatic factors along an altitudinal gradient in the middle Tianshan Mountain area, Xinjiang, China. Environ Res 24:303–314
Scharnweber T, Rietschel M, Manthey M (2007) Degradation stages of the Hyrcanian forests in southern Azerbaijan. Archiv Für Naturschutz Und Landschafts Forschung 46:133–156
Sefidi K, Copenheaver CA, Sadeghi SMM (2022) Anthropogenic pressures decrease structural complexity in Caucasian forests of Iran. Écoscience 29:199–209
Shakeri Z, Simberloff D, Bernhardt-Römermann M, Eckstein RL (2021) The impact of livestock grazing and canopy gaps on species pool and functional diversity of ground flora in the Caspian beech forests of Iran. Appl Veg Sci 24(3):12592
Sharma GP, Raghubanshi AS (2009) Plant invasions along roads: a case study from central highlands. India Environ Monit Assess 157:191–198
Siadati S, Moradi H, Attar F, Etemad V, Hamzeh HHB, Naqinezhad A (2010) Botanical diversity of Hyrcanian forests; a case study of a transect in the Kheyrud protected lowland mountain forests in northern Iran. Phytotaxa 7:1–18
Skrindo AB, Pedersen PA (2004) Natural revegetation of indigenous roadside vegetation by propagules from topsoil. Urban Urban Green 3:29–37
Solgi A, Naghdi R, Zenner EK, Hemmati V, Keivan Behjou F, Masumian A (2021) Evaluating the effectiveness of mulching for reducing soil erosion in cut slope and fill slope of forest roads in Hyrcanian forests. Croatian J Forest Eng 42(2):259–268
Son D, Alday JG, Chu Y, Lee EJ, Park S, Lee H (2020) Plant species colonization in newly created road habitats of South Korea: Insights for more effective restoration. Sci Total Environ 719:137476
Sukopp H, Blume HP, Kunick W (1979) The soil, flora, and vegetation of Berlin’s waste lands. Wiley, Chichester
Talebi KS, Sajedi T, Pourhashemi M (2014) Forests of Iran: a treasure from the past, a hope for the future. Springer, Netherlands
Taylor K, Brummer T, Taper ML, Wing A, Rew LJ (2012) Human mediated long distance dispersal: an empirical evaluation of seed dispersal by vehicles. Divers Distrib 18:942–951
Tilk M, Tullus T, Ots K (2017) Effects of Environmental Factors on the species richness, composition and community horizontal structure of vascular plants in scots pine forests on fixed sand dunes. 51.
Tiţă GC, Marcu MV, Ignea G, Borz SA (2019) Near the forest road: Small changes in air temperature and relative humidity in mixed temperate mountainous forests. Transp Res D Transp Environ. https://doi.org/10.1016/j.trd.2019.07.016
Transportation Research Board and National Research Council (2005) Assessing and managing the ecological impacts of paved roads. The National Academies Press, Washington
Truscott AM, Palmer SCF, McGowan GM, Cape JN, Smart S (2005) Vegetation composition of roadside verges in Scotland: the effects of nitrogen deposition, disturbance and management. Environ Pollut 136:109–118
Vanneste T, Govaert S, De Kesel W, Van Den Berge S, Vangansbeke P, Meeussen C, Brunet J, Cousins SA, Decocq G, Diekmann M, Graae BJ (2020) Plant diversity in hedgerows and road verges across Europe. J Appl Ecol 57:1244–1257
Venanzi R, Picchio R, Spinelli R, Grigolato S (2020) Soil disturbance and recovery after coppicing a Mediterranean Oak stand: The effects of silviculture and technology. Sustainability 12:4074
Viskari EL, Holopainen T, Karenlampi L (2000) Responses of spruce seedlings (Picea abies) to exhaust gas under laboratory conditions e II e ultrastructural changes and stomatal behaviour. Environ Pollut 107:99e107
Wagner SA, Fraterrigo JM (2015) Positive feedbacks between fire and non-native grass invasion in temperate deciduous forests. For Ecol Manag 354:170–176
Yang T, Chen C, Wang X, Xie S (2021) impact analysis of highway system on Wetland vegetation in Northern China. In: proceedings of the 2nd international conference on green energy, environment and sustainable development (GEESD2021), IOS Press. 3–14
Yu F, Chen Y, Ming D (2002) Clonal integration enhances survival and performance of Potentilla anserina, suffering from partial sand burial on Ordos Plateau, China. Ecology and Evolutionary Biology of Clonal Plants. Springer, Dordrecht, pp 81–96
Zohary M (1973) Geobotanical foundations of the Middle East. Gustav Fischer Verlag Stuttgar.
Acknowledgements
This work was supported by the “Iran National Science Foundation (INSF)” under grant number 99016122. Azade Deljouei’s research at the Transilvania University of Brasov, Romania, has been supported by the program “Transilvania Fellowship for Postdoctoral Research/Young Researchers.”
Funding
This work was supported by the “Iran National Science Foundation (INSF)” under grant number 99016122.
Author information
Authors and Affiliations
Contributions
SK, ZS, and EA were involved in conceptualization; SK, AD, and AL-L performed the formal analysis and were involved in writing—original draft preparation; SK, EA, and AD were involved in methodology; EA and VE were involved in resources; SK and VE were involved in investigation; EA was involved in supervision; EA, AD, GN. E, and SAB were involved in writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by Eric R. Labelle.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1.
Appendix 1.
Supporting material.
See Table
3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keybondori, S., Abdi, E., Deljouei, A. et al. Effect of forest roadside on vegetation characteristics in the Hyrcanian temperate forest. Eur J Forest Res 142, 455–473 (2023). https://doi.org/10.1007/s10342-023-01535-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01535-2