Abstract
Interference competition between native and invasive species can be an important driver of the local extirpation of native species; however, extinctions resulting from competition are rare. This study investigates competitive interactions between an invasive and an imperiled species to assess whether competition is an important mechanism behind this species replacement. Freshwater crayfish are one of the most imperiled taxonomic groups in North America, and nonnative crayfish pose a major threat to native crayfishes. Many crayfish have limited distributions, so merely moving crayfish between adjacent drainages can cause species replacements that threaten native species. Here, we examine competitive interactions between the imperiled Black Creek crayfish (BCC; Procambarus pictus), which is endemic to the lower St. Johns River drainage, Florida, and the white tubercled crayfish (WTC; P. spiculifer), an introduced species from a neighboring drainage. We found that WTC grew more rapidly than BCC in common conditions, and when WTC was larger, this species won aggressive interactions and was dominant in shelter competition with the imperiled species. However, when the species were size matched, BCC was more competitive than WTC. These results highlight the importance of size and growth rate for determining the outcome of interference competition. WTC is replacing BCC throughout a substantial portion of its limited range, and our results suggest that size-mediated competition between these species may be an important mechanism for this species replacement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species often alter the structure and composition of ecological communities and cause declines in species diversity at local scales (Fridley et al. 2007; Havel et al. 2015; Gallardo et al. 2016). Whether invasions are a major cause of extinctions, however, is debated (Gurevitch and Padilla 2004; Gilbert and Levine 2013). Most extinctions caused by invasions are the result of predation or parasitism (Warner 1968; Davis 2003; Dueñas et al. 2021). Extinctions resulting from competition between native and invasive species are rare (Davis 2003). Additional research focused on instances in which an invasive species extirpates a native species from much of its range (i.e., when extinction is possible but before it occurs) can provide insight into how and when competition is important for invasive species impacts on diversity. Invasions are more likely to result in extinctions in isolated environments, such as islands (Dueñas et al. 2021). Freshwater species are especially vulnerable to extinction from invasions because many species have limited distributions and occur in isolated stream drainages or lakes (Olden et al. 2010; Haag and Williams 2014; Taylor et al. 2019). Here we investigate the potential for an invasive crayfish to extirpate an imperiled crayfish from its limited range through competitive interactions.
Crustaceans, especially crayfish, are among the most common and impactful freshwater invasive species (Strayer 2010). The southeastern Unites States harbors the greatest diversity of freshwater crayfish in the world and invasive crayfish are one of the leading threats to the conservation of native crayfish (Taylor et al. 2019). In North America, many crayfish invasions are the result of transplants from other drainages within the continent (Strayer 2010), and there are numerous cases of introduced crayfish within North America causing reductions or local extirpations of native crayfish species (Light et al. 1995; Olden et al. 2006; Distefano and Westhoff 2011; Imhoff et al. 2012). Invasive crayfish can also alter other aspects of freshwater ecosystems by affecting organic matter processing, energy flow, and prey-predator relationships (Whitledge and Rabeni 1997; Ficetola et al. 2012; Jackson et al. 2014; Alp et al. 2016). Since freshwater ecosystems contain a higher diversity of species per area than marine or terrestrial ecosystems (Dudgeon et al. 2006), it is crucial to understand the role of invasive species in the local extirpation and/or extinction of native freshwater species.
Invasive crayfish often reach high densities and cause declines the abundance of native crayfish by competing with them for limited resources (Hansen et al. 2013 and Hill and Lodge 1999). Specifically, invasive crayfish often outcompete native crayfish for shelter or high-quality habitat (e.g., habitat with large substrates such as cobbles or boulders where crayfish can shelter in interstitial spaces) which increases predation on the native species (Garvey et al. 1994; Peters and Lodge 2013). In many cases, laboratory behavioral assays that measure aggression level or the ability of a species compete for shelter mirror species replacements observed in the field (Hill and Lodge 1994; Usio et al. 2001; Chucholl et al. 2008; Tricarico and Aquiloni 2016). However, this is not always the case (Larson and Magoulick 2009; Hanshew and Garcia 2012). Larger crayfish also often outcompete smaller conspecifics or heterospecifics (Garvey et al. 1994; Martin and Moore 2007). Therefore, crayfish that outcompete heterospecifics for high quality food resources may also eventually win competitions for shelter as they grow to a larger size (Hill and Lodge 1999). Invasive crayfish can also impact native crayfish populations through hybridization (Perry et al. 2002) or by promoting the spread of disease (Bohman et al. 2006; Holdich et al. 2009).
In northeast Florida, USA, recent stream surveys indicate that the range occupied by the Black Creek Crayfish (BCC, Procambarus pictus) is declining while the range of the White Tubercled Crayfish (WTC, P. spiculifer) is rapidly expanding (Fralick et al. 2021). The imperiled BCC is endemic to tannic, sand-bottom streams in the lower St. John’s River basin (Franz and Franz 1979). This species is state listed as Threatened due to its limited distribution and sensitivity to sedimentation, poor water quality, and urbanization in the surrounding watershed (Franz and Franz 1979). Most records of BCC are from the Black Creek drainage in Duval and Clay counties (Franz et al. 2008; Nelson and Floyd 2011; Fralick et al. 2021). The invasive WTC is native to other drainages in the southeastern USA, including the neighboring Suwannee River drainage in Florida. In 2008, WTC was found at two sites in Bull Creek, which is a tributary to Black Creek (Franz et al. 2008). This species was not detected in the Lower St. John’s watershed (HUC8) prior to 2008 (Franz et al. 2008). WTC has spread rapidly in the past decade, and many of the sites in the Black Creek drainage that were previously occupied by BCC are now occupied by only WTC (Fralick et al. 2021). The introduction pathway for WTC in the Black Creek drainage is unknown, but one plausible mechanism is a bait-bucket introduction (Fralick et al. 2021). Bait-bucket introductions are a common mechanism for crayfish introductions and WTC were first detected in an urbanized area, suggesting a bait-bucket introduction is a likely pathway (DiStefano et al. 2009; Fralick et al. 2021).
In this study, we conducted a series of experiments to examine competitive interactions between WTC and BCC. Specifically, we examined aggressive interactions between the species and competition for shelter and a high-quality food resource. We hypothesized that WTC would outcompete BCC, reflecting the patterns observed in the Black Creek drainage. In addition, we measured individual growth rates of both species in common conditions in the laboratory to assess whether either species was likely to have a size advantage in competitive interactions in the field. WTC is the largest of the Florida crayfishes (Hobbs 1942), so we expected WTC to grow more rapidly than BCC. We tested competitive interactions between WTC and BCC when the species were matched by size and when WTC had a size advantage to account for the influence of size on these interactions. Overall, these data provide new evidence for the mechanisms responsible for species replacements that result from invasions, and the ways in which invasive species impact native species diversity.
Methods
We conducted competition assays with BCC and WTC from the Black Creek Drainage from December 2020 through September 2021. Initial experiments were conducted in common conditions in the laboratory (December 2020–March 2021), and later experiments were conducted in enclosures in the stream or next to the stream (August 2021–September 2021). Water temperature for laboratory assays ranged from 17.0 to 20.2 °C, and for field assays it ranged from 24.6 to 29.7 °C. These temperatures are within the range of temperatures measured at sites occupied by BCC in the Black Creek Drainage (Franz et al. 2008). Methods were changed from laboratory experiments to field experiments due to the discovery of a microsporidian disease in other BCC and WTC from the drainage and concern that the disease could be spread between individuals collected from different locations during competition assays. We were able to release experimental animals from field experiments back into the stream once experiments were completed since they were not held in laboratory conditions for an extended period or exposed to crayfish from other locations. We investigated the impact of the location of the experiment by including location and the interaction between location and species in statistical models (described in more detail in Statistical Analysis section) to account for these changes.
Collection methods
We used dip nets to hand-collect crayfish for laboratory behavior experiments from wadable streams in the Black Creek drainage located in north central Florida. The two species did not co-occur at these collection locations (we detected either BCC or WTC, but both species were not present). We transported crayfish to the Fisheries and Aquatic Sciences laboratory (Gainesville, FL USA), and housed them in individual perforated deli containers within larger bins of constantly aerated well water. We assigned each individual crayfish an identification number. Once collected, we kept crayfish the laboratory for a minimum of two weeks before experiments began. Crayfish were exposed to a 12:12 h cycle (light:dark), and we fed them three shrimp pellets (OmegaSea, LLC) twice per week. We replaced water in each holding bin within 24 h after feeding to maintain water quality. Crayfish were starved for 24 h prior to the start of each behavioral assay.
We collected crayfish for field behavior experiments from one tributary within the South Fork Black Creek drainage using minnow traps with enlarged openings (5.7 cm) and baited with dog food. Both BCC and WTC were present this area of the South Fork. Therefore, crayfish had probably interacted with individuals of the other species prior to experiments. These crayfish were housed in minnow traps with closed openings with conspecifics for a maximum of 5 days. The minnow traps used for housing crayfish contained window screen and leaf litter to provide shelter and food.
Crayfish growth rate
We measured the carapace length (CL) of crayfish collected for laboratory experiments at the time of their collection using Vernier calipers (to the nearest tenth of a mm). These crayfish were kept in the same laboratory conditions described above for four months and each individual was provided with the same type and quantity of food over this time period. At the end of the four months, we remeasured CL of each crayfish and calculated the daily growth rate as the difference between the initial and final CL divided by the number of days the crayfish was housed in the laboratory. We also measured the blotted wet weight (to the nearest hundredth of a g) of each individual at the end of the growth period to determine whether the relationship between CL and weight was similar across both species. Crayfish that died over the four-month period were not included in the growth rate portion of the study. Overall, we obtained growth rate data for 24 BCC (13 females, 11 males, mean initial CL ± SD = 18.6 ± 4.1 mm) and 40 WTC (18 females, 22 males, 18.1 ± 3.1 mm). All males were form II (non- reproductive form), except for two BCC males that were form I (reproductive form).
Aggressive interactions
We assessed aggressive interactions between BCC and WTC by placing one crayfish of each species together in a bucket and recording the behavior of each crayfish using a GoPro Hero 6. We matched crayfish by sex and reproductive form. We also matched crayfish by size (within 1 mm CL) in some assays (N = 27 total; 21 in the lab and 6 in the field) and gave WTC a size advantage in others (larger by 4–5 mm CL; N = 20 total; 8 in the lab and 12 in the field). WTC has been described as the largest of the Florida crayfishes (Hobbs 1942) and, therefore, it is likely to have a size advantage in natural conditions. In size matched assays, BCC had a mean CL of 18.8 ± 3.1 mm (SD), and WTC had a mean CL of 18.9 ± 3.0 mm (14 females and 13 males of each species). In WTC size advantage assays, BCC had a mean CL of 20.5 ± 3.6 mm, and WTC had a mean CL of 24.9 ± 3.7 mm (10 females and 10 males of each species). All male crayfish used in aggression assays were form II, with the exception of two pairs of males that were form I. To identify crayfish during the experiment, we marked individuals on the carapace using different colors of nail varnish (Sally Hansen). In the field, we marked only WTC and handled BCC to simulate the marking process. We did not mark the imperiled species in field experiments because individuals were released back to the stream following all experiments and marking could potentially make them more visible to predators.
At the start of each assay, we placed crayfish on either side of a perforated plexiglass divider in a 19-L bucket filled with previously aerated well water (laboratory) or stream water (field). We left the crayfish to acclimate on either side of the divider for 15 min, and the divider allowed crayfish to receive visual and chemical cues from the other crayfish during this time period. After acclimation, we lifted the divider and recorded the interactions between crayfish on video for 15 min using a GoPro Hero 6 attached to a frame above the bucket (so researchers did not disturb the experiment). We later scored videos using an ethogram developed by Bergman and Moore (2003). We scored the most aggressive behavior of each crayfish every 5 s (ranging from − 2 for tail-flip retreat to + 5 for unrestrained fighting) and summed the scores for each individual over the 15-min period to obtain an overall aggression score (Reisinger et al. 2015). In addition, we recorded the initiator and winner of each tension contact (head on head encounter between crayfish; Chucholl et al. 2008). The individual that approached the other crayfish was considered the initiator of the contact. The individual that did not retreat or change direction was considered the winner of the contact.
Shelter affinity and competition
We measured shelter affinity and shelter competition in 19-L buckets with PVC shelters that were scaled to the size of the crayfish so that two individuals could not use the same shelter without being in close contact (51–100 mm diameter PVC pipe cut in half lengthwise). In competition assays, one species may be in shelter more often if it is the dominant competitor or if it has a higher affinity for shelter than the other species. Examining shelter affinity (shelter use without a competitor) allowed us to distinguish between these potential causes of species differences in shelter occupancy in competition trials. We measured shelter affinity in the laboratory (WTC assays: N = 31, 17 females and 14 males; BCC assays: N = 29, 15 females and 14 males). In shelter affinity assays, BCC had a mean CL of 18.4 ± 3.1 mm (SD), and WTC had a mean CL of 20.1 ± 3.8 mm. All males used in shelter affinity assays were reproductive form II. We measured shelter competition in both the laboratory and the field with size matched crayfish or with larger WTC. We conducted the competition assays following the aggression assays, using the same crayfish pairs as for aggression. The sample size, crayfish sex, and crayfish CL for shelter competition are described above in the methods for aggressive interactions, except there was one additional competition assay (size matched, field) that did not have a corresponding aggression assay because of an error with the video recording.
For shelter affinity, we placed each crayfish alone in the bucket with a layer of sand, 5 cm of water, an aerator, and the PVC shelter. We covered buckets with window screen to prevent crayfish escape, and left crayfish to acclimate to the environment overnight. The next day, we recorded the position of the crayfish every hour from 9:00 to 12:00. We classified the crayfish as outside the shelter if all pereopods were visible outside of the PVC pipe. Otherwise, we classified the crayfish as inside the shelter (Reisinger et al. 2015). The proportion of observations in which the crayfish was inside the shelter was considered the shelter affinity for that individual.
We tested shelter competition immediately following crayfish aggression assays in the laboratory and field. Methods for shelter competition in the laboratory were the same as for shelter affinity except we placed crayfish together in the bucket at the start of the acclimation period. In the field, we placed crayfish together in a bucket that was anchored to the stream bed using rebar. Small holes in the bucket allowed water exchange with the stream. We covered each bucket with window screen to prevent crayfish escape and left crayfish to acclimate overnight. The next day, we recorded whether each crayfish was in or out of the shelter every hour from 9:00 to 12:00. The crayfish that was inside the shelter for most observations was considered the winner.
Food competition
We conducted food competition assays using methods similar to those in Reisinger et al. (2020), with paired individuals that were matched by sex and form. In some assays, we also matched crayfish by size (within 1 mm CL; N = 22 total; 16 assays in the lab + 6 in the field), and in others we gave WTC a size advantage (larger by 4–5 mm CL; N = 19 total; 13 assays in the lab and 6 in the field). In size matched assays, BCC had a mean CL of 20.2 ± 3.8 mm (SD), and WTC had a mean CL of 20.3 ± 3.8 mm (13 females and 9 males of each species). In WTC size advantage assays, BCC had a mean CL of 20.2 ± 1.7 mm, and WTC had a mean CL of 24.7 ± 1.9 mm (10 females and 9 males of each species). All males used in food competition assays were reproductive form II. We tested some of the same individuals in both aggression/shelter competition and food competition assays, but we paired individuals with a different competitor, and conducted different assays a minimum of one week apart to remove the potential influence of previous interactions (Seebacher and Wilson 2007). Each individual was only tested once in each type of assay. We used the same methods to mark individual crayfish as we used in the aggression assays (described above).
We conducted food competition assays in wading pools (100 cm diameter) filled with aerated well water (laboratory) or stream water (field). Each wading pool was marked on the bottom so that it was divided into nine segments of equal area (Reisinger et al. 2020), one circular section in the center of the pool and eight sections around the edge of the pool. To start the assay, we placed one crayfish of each species on either side of the pool under a perforated container and left them to acclimate for 15 min. During the acclimation period, we placed a 12–13 mm section of a live earthworm in the center of the pool. After the acclimation period was complete, we gently lifted each perforated container and recorded the behavior of the crayfish for 30 min using a GoPro camera that was attached to a frame above the wading pool. We reviewed videos and recorded the activity level of each crayfish (number of lines crossed in the first 15 min) and which species consumed the worm. We also recorded the initiator and winner of each tension contact.
Statistical analysis
We used ANOVA to assess aggressive interactions and competition between WTC and BCC. We created separate models for the following dependent variables: number of tension contacts initiated, number of tension contacts won, shelter affinity, and activity level. Species, sex, and their interaction were included in each model as independent variables. Prior to analysis, we transformed the number of tension contacts initiated and won using a natural logarithm transformation to meet the assumption of a normal distribution. We also included location (laboratory or field experiment) and the interaction between location and species in each model to assess whether the location of the experiment had an effect on the dependent variable or affected competitive interactions between the species. We also used Kruskal–Wallis tests to assess whether crayfish aggression scores differed between species or locations (laboratory or field). We used Chi-squared tests to assess whether either species was more competitive for shelter or food (i.e., whether the proportion of competitions won by a species differed significantly from 0.5). To test whether there was an effect of species on growth rate, we used ANCOVA with growth per day as the dependent variable, species as the independent variable, and initial CL and sex as covariates. Finally, we used ANCOVA to examine the relationship between wet weight and CL. Both variables were transformed prior to analysis using a natural logarithm transformation to create a linear relationship between them (Rodger and Starks 2020). Species was included as an independent variable in the model. All analyses were conducted in R (version 3.6.2, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Growth rate
The growth rate of WTC was 3 times higher than the growth rate of BCC in common conditions in the laboratory (F1,59 = 67.5, P < 0.001; Fig. 1). The mean growth rate of WTC was 0.039 ± 0.018 (SD) mm/day and the mean growth rate of BCC was 0.013 ± 0.012 mm/day. Initial CL had a significant negative effect on growth rate (F1,59 = 8.4, P = 0.005) indicating that large crayfish grew less than small crayfish over the study, but sex did not have a significant effect on growth rate (P > 0.2).
The relationship between carapace length and wet weight for each species indicated that BCC had a greater wet weight than WTC when the species were matched by carapace length (F1,60 = 66.14, P < 0.001; Fig. 2), especially for larger individuals (> 20 mm CL). Because we chose crayfish for competition assays based on CL, BCC were larger in terms of biomass than WTC in the ‘size matched’ assays.
Aggressive interactions
Aggression scores were similar between BCC and WTC in size matched assays (χ2 = 0.78, P = 0.378) and assays in which WTC had a size advantage (χ2 = 0.82, P = 0.365). In size matched assays, the mean aggression score for BCC was 198 ± 151 (SD) and for WTC it was 189 ± 131. When WTC had a size advantage, the mean aggression score for BCC was 115 ± 104 and for WTC it was 137 ± 99. Location (laboratory vs. field) had a significant effect on aggression score in size matched assays, with higher aggression scores occurring in laboratory assays (209 ± 144 vs. 80 ± 49; χ2 = 10.33, P = 0.001; Table S1). There was no significant effect of location on aggression in WTC size advantage assays (χ2 = 0.02, P = 0.879; Table S1).
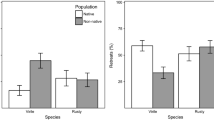
BCC initiated tension contacts more often than WTC when crayfish were size matched (F1, 48 = 19.46, P < 0.001; Fig. 3), but each species initiated a similar number of tension contacts when WTC had a size advantage (F1, 34 = 0.002, P = 0.967; Fig. 3). In size matched assays, BCC initiated a mean of 9 ± 5 (SD) contacts and WTC initiated a mean of 5 ± 3 contacts during the 15-min assay. There was a significant interaction between location and species for these assays (F1, 48 = 4.72, P = 0.035). BCC initiated more contacts than WTC in laboratory assays (10 ± 5 vs. 4 ± 3), but the species initiated similar numbers of contacts in field assays (6 ± 4 for both species). When WTC had a size advantage, BCC initiated a mean of 6 ± 4 contacts, and WTC initiated a mean of 7 ± 6 contacts. There was a trend suggesting there may be an interaction between sex and species in these assays. For male crayfish, BCC tended to initiate more contacts than WTC, but for female crayfish, WTC tended to initiate more contacts than BCC (F1, 34 = 3.26, P = 0.078). Other than those described above, there was no significant effect of any other variable or interaction in either size matched or WTC size advantage assays (variables included sex, sex x species, location, location x species; P > 0.4; Fig. S1).
The proportion of tension contacts initiated (Panels A and B) and won (Panels C and D) by imperiled Black Creek crayfish (BCC) and invasive white tubercled crayfish (WTC) in aggression assays. Panels A and C represent competitions in which the species were matched by size (± 1 mm carapace length) and panels B and D represent competitions in which WTC had a size advantage (larger by 4–5 mm carapace length). Large black points represent the mean. In size matched assays, BCC initiated and won significantly more contacts than WTC. In size advantage assays, WTC won significantly more contacts than BCC
BCC also won more tension contacts than WTC in size matched assays (F1, 48 = 8.01, P = 0.007; Fig. 3). However, WTC won more tension contacts than BCC when WTC had a size advantage (F1, 34 = 7.05, P = 0.012; Fig. 3). In size matched assays, BCC won a mean of 9 ± 7 (SD) contacts during each trial and WTC won a mean of 5 ± 6 contacts. When WTC had a size advantage, BCC won a mean of 4 ± 5 contacts, and WTC a mean of 9 ± 7 contacts. There was a significant interaction between species and sex in assays in which WTC had a size advantage (F1,34 = 6.59, P = 0.015). Both male and female WTC won more contacts than BCC, but this trend was most pronounced for females. There was also a non-significant trend suggesting there may have been an interaction between location and species (F1, 34 = 3.80, P = 0.060). WTC was more likely than BCC to win tension contacts in laboratory assays (11 ± 5 contacts vs. 2 ± 3 contacts), but this trend was not apparent in field assays (5 ± 7 contacts vs. 7 ± 5 contacts; Figure S1). Other than those described above, there was no significant effect of any other variable or interaction in either size matched or WTC size advantage assays (variables included sex, sex x species, location, location x species; P > 0.2).
We also evaluated tension contacts in larger tanks during the food competition assays (100 cm diameter wading pools vs 19-L buckets). When the species were size matched, BCC initiated and won more tension contacts than WTC (F1,38 = 24.70, P < 0.001; F1,38 = 25.52, P < 0.001; Fig. 4). BCC initiated a mean of 14 ± 8 contacts and won a mean of 15 ± 8 contacts. WTC initiated a mean of 5 ± 4 of contacts and won a mean of 4 ± 5 of contacts. Significantly more contacts were won in field assays than laboratory assays, indicating that more tension contacts occurred in these assays (F1,38 = 5.71, P = 0.022). When WTC had a size advantage, WTC initiated and won more tension contacts than BCC (F1,32 = 5.82, P = 0.022; F1,32 = 29.72, P < 0.001; Fig. 4). BCC initiated 9 ± 11 contacts and won 5 ± 11 contacts, and WTC initiated 12 ± 6 contacts and won 16 ± 7 contacts. Other than the effect of location in size matched assays, there was no significant effect of any other variable or interaction in either size matched or WTC size advantage assays (variables included sex, sex x species, location, location x species; P > 0.1; Fig. S2).
The proportion of tension contacts initiated (A and B) and won (C and D) by imperiled Black Creek crayfish (BCC) and invasive white tubercled crayfish (WTC) in food competition assays. A and C represent competitions in which the species were matched by size (± 1 mm carapace length) and B and D represent competitions in which WTC had a size advantage (larger by 4–5 mm carapace length). Large black points represent the mean. In size matched assays, BCC initiated and won significantly more contacts than WTC. In size advantage assays, WTC initiated and won significantly more contacts than BCC
Shelter competition
Both species displayed a high affinity for the shelter, and there was no significant effect of species on shelter affinity (F1,56 = 1.63, P = 0.206; Fig. 5). BCC was in the shelter for 77 ± 26% (SD) of the observations and WTC was in the shelter for 85 ± 22% of the observations (Fig. 5). There was also no effect of sex or interaction between species and sex on shelter affinity (P > 0.2).
Crayfish shelter use for imperiled Black Creek crayfish (BCC, A) and invasive white tubercled crayfish (WTC, B) in different treatments. In the shelter affinity treatment, each crayfish was alone in a bucket with a single shelter. In the size matched treatment, each crayfish was in a bucket with a single shelter and a size-matched opponent of the other species (± 1 mm carapace length), and in the WTC larger treatment, each crayfish was in a bucket with a single shelter and a size-mismatched opponent of the other species (WTC 4–5 mm carapace length larger than BCC). Large black points represent the mean
BCC was more likely to win the competition for shelter in size matched assays (Chi-squared test: χ2 = 5.14, P = 0.023; Fig. 5; Table 1). Specifically, BCC was in the shelter during 59 ± 40% of observations and WTC was in the shelter during 36 ± 39% of observations (Table 1; Fig. 5). WTC was more likely to win the competition for shelter when this species had a size advantage (Chi-squared test: χ2 = 12.8, P < 0.001; Fig. 5). In these competitions, BCC was in the shelter during 19 ± 31% of observations and WTC was in the shelter during 80 ± 34% of observations (Table 1; Fig. 5). Results were similar between assays conducted in the laboratory and those conducted in the field. In the size matched treatment, BCC won 71% of assays in both the lab and field (15 out of 21 and 5 out of 7; Table S2). In the WTC size advantage treatment, BCC won 12% of assays in the lab and 8% of assays in the field (1 out of 8 and 1 out of 12; Table S2).
Food competition
There was no effect of species on whether or not the individual consumed the worm during both size matched assays and assays in which WTC had a size advantage (Chi-squared tests: χ2 = 0.05, P = 0.808; χ2 = 0.11, P = 0.739). In size matched assays, BCC consumed the worm in 41% of assays and WTC consumed the worm in 36% of assays. When WTC had a size advantage, BCC consumed the worm in 21% of assays and WTC consumed the worm in 26% of assays. The remainder of the time, no crayfish consumed the worm. Overall, a crayfish consumed the worm in 50% of laboratory assays and 47% of field assays (Table S3).
Activity level
There was no effect of species on crayfish activity level during both size matched assays and assays in which WTC had a size advantage (number of lines crossed; F1,38 = 0.88, P = 0.355; F1,32 = 0.01, P = 0.935). In size matched assays, BCC crossed a mean of 61 ± 21 (SD) lines during the 15-min period, and WTC crossed a mean of 55 ± 25 lines. Crayfish were significantly more active in field assays than laboratory assays (F1,38 = 14.36, P < 0.001). When WTC had a size advantage, BCC crossed a mean of 67 ± 27 lines and WTC crossed a mean of 68 ± 28 lines. Crayfish were significantly more active in field assays than laboratory assays (F1,32 = 18.56, P < 0.001) and females were significantly more active than males (F1,32 = 6.12, P = 0.019). Other than those described above, there was no significant effect of any other variable or interaction in either size matched or WTC size advantage assays (variables included sex, sex x species, location, location x species; P > 0.1).
Discussion
Our initial hypothesis that invasive WTC would consistently outcompete imperiled BCC, reflecting patterns of species replacement in the Black Creek Drainage, was not supported by the data. When the species were matched by size, BCC won more aggressive interactions and competitions for shelter than WTC. Our findings indicate, however, that the rapid growth and larger maximum size of WTC is an important factor in competitive interactions with BCC. Larger WTC won aggressive interactions and shelter competitions against BCC, which likely gives this species an advantage in the field. Analysis of length weight relationships in these species indicates that BCC has a greater mass when the species are matched by CL. Since we paired crayfish based on CL, our results are conservative (i.e., WTC may have won more size matched competitions if it was matched with BCC based on mass rather than CL). Chelae size and pinching force may also play a role in crayfish competitive interactions (Parvulescu et al. 2021) but were not measured in this study. Overall, interference competition and the larger body size of the invader may be key mechanisms governing the impacts of this invasive crayfish on the imperiled crayfish species.
Interference competition can be important for species replacements in invasions (Matheson and Gagnon 2012; Champneys et al. 2021), and body size plays a role in interference competition across a variety of taxa (Orpwood et al. 2003; Harris et al. 2020; Edeline and Loeuille 2021). In crayfish, in particular, body size often affects aggressive interactions and the outcome of shelter competitions (Garvey et al. 1994; Vorburger and Ribi 1999; Hudina et al. 2011). In our study, WTC had a substantially faster growth rate than BCC (3 × greater), which may allow it to reach a larger size. It is possible that the growth rates we measured in the laboratory differ from those in natural streams, as factors such as food availability, food quality, and water temperature could differ between these environments. However, water temperatures in the laboratory (17–20 °C) were similar to those previously measured in the Black Creek drainage. Franz et al. (2008) measured spring water temperatures at several sites in the Black Creek Drainage that were occupied by BCC. Temperatures measured at each site ranged from 17.9 to 24.8 °C in March, 16.8 to 26.2 °C in April, and 20.5 to 29.4 °C in May. Other data also support WTC reaching a larger size in streams than BCC. As part of another study, we collected crayfish with dip nets from nine sites in the Black Creek Drainage (174 BCC and 225 WTC; Reisinger unpublished data). The CL of the largest crayfish collected was 43 mm for WTC and 27 mm for BCC, and the 95th percentile for CL was 28 mm for WTC and 23 mm for BCC (using the 95th percentile ensures that results are not driven by a single large crayfish). Further, WTC has previously been described as the largest of the Florida crayfish (Hobbs 1942). Overall, the data indicate that WTC grow more rapidly and reach a larger maximum size than BCC, and our results provide additional evidence that crayfish body size is important for determining the outcome of interference competition between native and invasive crayfish species.
WTC grew more rapidly than BCC when the crayfish were housed in individual containers and fed a standard amount of food, but we did not observe any differences between these species in feeding behavior in the food competition assays. It is unclear whether this reflects similar rates of food acquisition by these species in the field. Food acquisition could be affected by habitat use, and larger, more aggressive invasive crayfish may displace the imperiled crayfish species from high quality habitat, which has been observed in other invasions (e.g., Peters and Lodge 2013). In addition, there were many assays in which neither individual consumed the food item, possibly because crayfish were prioritizing other behaviors such as exploring the novel environment or interacting with the competitor. Longer assays that include a wider variety of food items could provide additional insight into food competition between these species.
Crayfish behavior was measured across a variety of contexts in our study, and the outcome of competition was typically consistent across contexts (assays conducted in buckets or wading pools and assays conducted in the field or laboratory). Specifically, when the species were size matched, BCC won more aggressive interactions than WTC in both small (bucket) and large (wading pool) arenas, and when WTC had a size advantage, WTC won more aggressive interactions than BCC across both of these contexts. The species that was dominant in aggressive interactions was also dominant in shelter competition. In both laboratory and field contexts, BCC won more shelter competitions in size-matched assays, and WTC won more shelter competitions in size-advantaged assays. Our findings are similar to those from other studies that demonstrate crayfish that win aggressive interactions are typically also dominant in shelter competition (Capelli and Munjal 1982; Vorburger and Ribi 1999; Usio et al. 2001; Chucholl et al. 2008).
While many aspects of behavior and competition were consistent across contexts, we did observe some differences between assays conducted in the laboratory and those conducted in the field. Key variables that differed across these contexts included water temperature (warmer water temperature in field assays than laboratory assays) and crayfish collection methods. Crayfish for field assays were collected from a site where both species were present using baited traps and crayfish for laboratory assays were collected from sites where the other crayfish species was absent using handheld nets. Both species of crayfish had higher activity levels in the field than in the laboratory, and in some treatments, there were also more tension contacts between the species in the field than in the laboratory (in size matched assays in wading pools). This may be due to water temperature, which has a strong influence on the activity levels of ectotherms. Alternatively, using baited traps to collect crayfish may have selected for more active individuals than using nets. In addition to differences in activity, both species had higher aggression scores in laboratory assays compared to those conducted in the field, and in some treatments, BCC initiated more contacts with WTC in laboratory assays compared to field assays (in size matched assays in buckets). These differences could be related to housing conditions, as crayfish in laboratory experiments were isolated from one another prior to trials. It is also possible that the higher aggression levels observed in the laboratory are a result of the lack of prior experience of each species with the competitor. However, these results differ from the findings of other studies that suggest that prior experience can lead to increased aggression between native and invasive crayfish (Hayes et al. 2009; Pintor and Sih 2009). Crayfish behavioral traits, including aggression, can vary substantially among populations of the same species for other reasons (e.g., environmental variables such as resource availability; Pintor et al. 2008). So, prior experience with the competitor is only one potential driver of these behavioral differences.
In addition to differences in activity and aggression levels, we also found some evidence that WTC was less likely to win aggressive interactions in field assays compared to laboratory assays (in WTC size advantage assays in buckets), which could indicate that the experienced population of BCC has a greater ability to compete with WTC than the naïve population. Examining this relationship across a greater number of populations would bolster this evidence. Evidence from the invasion of a different species (rusty crayfish, Faxonius rusticus) indicates that native crayfish that have coexisted with the invader are better able to compete with this species than individuals from naïve populations, but in this case the difference in competitive ability is not substantial enough to mitigate the negative impacts of the invader on native populations (Hayes et al. 2009). While prior experience may also benefit the native species in the WTC invasion, the shelter competition results from our study suggest that the benefit is not substantial enough to mitigate the negative impacts of WTC on BCC.
Shelter competition is often important for interspecific interactions among crayfish, but shelter may not be a limiting resource for crayfish in some instances. For example, species that often construct burrows for shelter may not be limited by competition for shelter. WTC has occasionally been collected from shallow burrows in the stream bank, but BCC is not known to burrow (Hobbs 1942, Franz and Frans 1979, Hobbs 1981). Although WTC can construct burrows, both of these species are most abundant in locations where the in-stream habitat contains shelter (e.g., woody debris, undercut banks, aquatic vegetation, or piles of detritus) and they are commonly observed using these shelters in the stream (Hobbs 1942, Franz and Frans 1979, Hobbs 1981). Therefore, we expect that shelter is a critical resource for both BCC and WTC.
The invasion of the WTC may have ecological impacts outside the extirpation of the BCC. Crayfish can have significant impacts on freshwater ecosystems and crayfish species replacements can have far reaching ecological effects (Wilson et al. 2004; Twardochleb et al. 2013). Although the specific ecological effects of WTC and BCC have not yet been researched, changes in the size distribution of individuals in a population can alter key ecosystem functions including rates of nutrient recycling, since larger individuals excrete nutrients at a lower rate per body mass than smaller individuals (Fritschie and Olden 2016). Thus, the replacement of a crayfish with a smaller body size (BCC) with a crayfish with a larger body size (WTC) is likely to affect stream ecosystems. Successful invasive species are also typically found at higher densities compared to native populations (Hansen et al. 2013), so crayfish abundance, as well as crayfish size, could also be impacted by this invasion. Both WTC and BCC are found in well-aerated, cool, sand-bottom lotic habitats (Hobbs and Hart 1959). Therefore, the sites currently occupied by BCC are likely to also be suitable for WTC and this species may continue to expand its range and replace BCC throughout the Black Creek Drainage.
Overall, this study adds to the growing body of evidence that crayfish species replacements are often mediated by interference competition and the relative ability of each species to compete for shelter. Crayfish are among the most imperiled taxonomic groups both at a global scale and in the USA (which harbors most of the world’s crayfish diversity), and invasive crayfish are a major threat to native crayfish (Collier et al. 2016; Taylor et al. 2019). Therefore, understanding the mechanisms responsible for species replacements in this group is important for conservation. Shelter is a key resource for crayfish because it reduces predation (Garvey et al. 1994), and several other studies have found that invasive crayfish that outcompete native crayfish for shelter displace those species in freshwater ecosystems (Hill and Lodge 1994; Usio et al. 2001; Chucholl et al. 2008). In our study, the invasive species won more aggressive interactions and competitions for shelter, but only when it had a size advantage. The size dependency of these interactions could have implications for management. Intensive trapping has been used as a mechanism to control other invasive crayfish populations, and traps often select for large individuals (Hein et al. 2007; Gherardi et al. 2011). Therefore, trapping may remove those individuals that are most likely to negatively affect the imperiled species. Overall, our results suggest that differences in growth rate, and therefore size, mediate competitive interactions between these species, which is likely to be a key mechanism by which invasive WTC are replacing imperiled BCC throughout a substantial portion of its range.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alp M, Cucherousset J, Buoro M, Lecerf A (2016) Phenological response of a key ecosystem function to biological invasion. Ecol Lett 19:519–527. https://doi.org/10.1111/ele.12585
Bergman DA, Moore PA (2003) Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol Bull 205:26–35. https://doi.org/10.2307/1543442
Bohman P, Nordwall F, Edsman L (2006) The effect of the large-scale introduction of signal crayfish on the spread of crayfish plague in Sweden. Bull Fr Pêche Piscic 380:1291–1302. https://doi.org/10.1051/kmae:2006026
Capelli GM, Munjal BL (1982) Aggressive interactions and resource competition in relation to species displacement among crayfish of the genus Orconectes. J Crustac Biol 2:486–492. https://doi.org/10.2307/1548090
Champneys T, Genner MJ, Ioannou CC (2021) Invasive Nile tilapia dominates a threatened indigenous tilapia in competition over shelter. Hydrobiologia 848:3747–3762. https://doi.org/10.1007/s10750-020-04341-8
Chucholl C, Stich HB, Maier G (2008) Aggressive interactions and competition for shelter between a recently introduced and an established invasive crayfish: Orconectes immunis vs. O. Limosus. Fundam Appl Limnol 172:27–36. https://doi.org/10.1127/1863-9135/2008/0172-0027
Collier KJ, Probert PK, Jeffries M (2016) Conservation of aquatic invertebrates: concerns, challenges and conundrums. Aquat Conserv Mar Freshw Ecosyst 26:817–837. https://doi.org/10.1002/aqc.2710
Davis MA (2003) Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 53:481–489. https://doi.org/10.1641/0006-3568(2003)053[0481:BGDCFI]2.0.CO;2
DiStefano RJ, Litvan ME, Horner PT (2009) The bait industry as a potential vector for alien crayfish introductions: problem recognition by fisheries agencies and a Missouri evaluation. Fisheries 34:586–597. https://doi.org/10.1577/1548-8446-34.12.586
Distefano RJ, Westhoff JT (2011) Range expansion by an invasive crayfish and subsequent range contraction of imperiled endemic crayfish in Missouri (USA) Ozark streams. Freshw Crayfish 18:37–44
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. https://doi.org/10.1017/S1464793105006950
Dueñas MA, Hemming DJ, Roberts A, Diaz-Soltero H (2021) The threat of invasive species to IUCN-listed critically endangered species: a systematic review. Glob Ecol Conserv 26:e01476. https://doi.org/10.1016/j.gecco.2021.e01476
Edeline E, Loeuille N (2021) Size-dependent eco-evolutionary feedbacks in harvested systems. Oikos 130:1636–1649. https://doi.org/10.1111/oik.08592
Ficetola GF, Siesa ME, De Bernardi F, Padoa-Schioppa E (2012) Complex impact of an invasive crayfish on freshwater food webs. Biodivers Conserv 21:2641–2651. https://doi.org/10.1007/s10531-012-0323-1
Fralick K, Warren G, Tripp N, et al (2021) Evaluation of Black Creek crayfish (Procambarus pictus) population status, 1976 – 2021. Florida Fish and Wildlife Conservation Commission Report.
Franz R, Smith H, Hallman A (2008) Survey for black creek crayfish (Procambarus pictus) at Jennings State Forest and Camp Blanding Joint Training Center, Clay and Duval Counties, Florida. Florida Fish and Wildlife Conservation Commission Report
Franz R, Franz LM (1979) Distribution, habitat preference and status of populations of the Black Creek crayfish, Procambarus (Ortmannicus) pictus (Decapoda: Cambaridae). Florida Sci 42:13–17
Fridley JD, Stachowicz JJ, Naeem S et al (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17. https://doi.org/10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2
Fritschie KJ, Olden JD (2016) Disentangling the influences of mean body size and size structure on ecosystem functioning: an example of nutrient recycling by a non-native crayfish. Ecol Evol 6:159–169. https://doi.org/10.1002/ece3.1852
Gallardo B, Clavero M, Sánchez MI, Vilà M (2016) Global ecological impacts of invasive species in aquatic ecosystems. Glob Chang Biol 22:151–163. https://doi.org/10.1111/gcb.13004
Garvey JE, Stein RA, Thomas HM (1994) Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75:532–547. https://doi.org/10.2307/1939556
Gherardi F, Aquiloni L, Diéguez-Uribeondo J, Tricarico E (2011) Managing invasive crayfish: Is there a hope? Aquat Sci 73:185–200. https://doi.org/10.1007/s00027-011-0181-z
Gilbert B, Levine JM (2013) Plant invasions and extinction debts. Proc Natl Acad Sci 110:1744–1749. https://doi.org/10.1073/pnas.1212375110
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474. https://doi.org/10.1016/j.tree.2004.07.005
Haag WR, Williams JD (2014) Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735:45–60. https://doi.org/10.1007/s10750-013-1524-7
Hansen GJA, Vander Zanden MJ, Blum MJ et al (2013) Commonly rare and rarely common: comparing population abundance of invasive and native aquatic species. PLoS ONE 8:e77415. https://doi.org/10.1371/journal.pone.0077415
Hanshew BA, Garcia TS (2012) Invasion of the shelter snatchers: behavioural plasticity in invasive red swamp crayfish, Procambarus clarkii. Freshw Biol 57:2285–2296. https://doi.org/10.1111/fwb.12002
Harris MH, Womble KI, Alford JB (2020) Size-specific advantage in shelter competition between the mountain madtom and crayfishes. J Fish Wildl Manag 11:401–409. https://doi.org/10.3996/042019-JFWM-023
Havel JE, Kovalenko KE, Thomaz SM et al (2015) Aquatic invasive species: challenges for the future. Hydrobiologia 750:147–170. https://doi.org/10.1007/s10750-014-2166-0
Hayes NM, Butkas KJ, Olden JD, Vander Zanden MJ (2009) Behavioural and growth differences between experienced and naïve populations of a native crayfish in the presence of invasive rusty crayfish. Freshw Biol 54:1876–1887. https://doi.org/10.1111/j.1365-2427.2009.02237.x
Hein CL, Vander Zanden MJ, Magnuson JJ (2007) Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshw Biol 52:1134–1146. https://doi.org/10.1111/j.1365-2427.2007.01741.x
Hill AM, Lodge DM (1994) Diel changes in resource demand: competition and predation in species replacement among crayfishes. Ecology 75:2118–2126. https://doi.org/10.2307/1941615
Hill AM, Lodge DM (1999) Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Appl 9:678–690. https://doi.org/10.1890/1051-0761(1999)009[0678:RORCBA]2.0.CO;2
Hobbs HH (1942) The crayfishes of Florida. University of Florida publication, Gainesville
Hobbs HH (1981) The crayfishes of Georgia. Smothsonian Institution Press, Washington D.C
Hobbs H, Hart CW (1959) The freshwater decapod crustaceans of the Apalachicola drainage system in Florida, Southern Alabama, and Georgia. Bull Florida State Museum Biol Sci 4:145–191
Holdich DMM, Reynolds JDD, Souty-Grosset C, Sibley PJJ (2009) A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl Manag Aquat Ecosyst 11(1–46):11. https://doi.org/10.1051/kmae/2009025
Hudina S, Galić N, Roessink I, Hock K (2011) Competitive interactions between co-occurring invaders: identifying asymmetries between two invasive crayfish species. Biol Invasions 13:1791–1803. https://doi.org/10.1007/s10530-010-9933-2
Imhoff EM, Moore MJ, DiStefano RJ (2012) Introduced alien ringed crayfish (Orconectes neglectus neglectus [Faxon, 1885]) threaten imperiled coldwater crayfish (Orconectes eupunctus Williams, 1952) in the Eleven Point River drainage, Missouri, USA. Aquat Invasions 7:129–134. https://doi.org/10.3391/ai.2012.7.1.014
Jackson MC, Jones T, Milligan M et al (2014) Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw Biol 59:1123–1135. https://doi.org/10.1111/fwb.12333
Larson ER, Magoulick DD (2009) Does juvenile competition explain displacement of a native crayfish by an introduced crayfish? Biol Invasions 11:725–735. https://doi.org/10.1007/s10530-008-9286-2
Light T, Erman DC, Myrick C, Clarke J (1995) Decline of the shasta crayfish (Pacifastacus fortis Faxon) of Northeastern California. Conserv Biol 9:1567–1577. https://doi.org/10.1046/j.1523-1739.1995.09061567.x
Martin AL, Moore PA (2007) Field observations of agonism in the crayfish, Orconectes rusticus: shelter use in a natural environment. Ethology 113:1192–1201. https://doi.org/10.1111/j.1439-0310.2007.01429.x
Matheson K, Gagnon P (2012) Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus Say) and recently introduced green crab (Carcinus maenas L.). J Exp Mar Bio Ecol 428:49–56. https://doi.org/10.1016/j.jembe.2012.06.003
Nelson EB, Floyd MR (2011) Black creek crayfish baseline survey at camp blanding joint training center. Dep Mil Aff Environ Div Rep
Olden JD, McCarthy JM, Maxted JT et al (2006) The rapid spread of rusty crayfish (Orconectes rusticus) with observations on native crayfish declines in Wisconsin (U.S.A.) over the past 130 years. Biol Invasions 8:1621–1628. https://doi.org/10.1007/s10530-005-7854-2
Olden JD, Kennard MJ, Leprieur F et al (2010) Conservation biogeography of freshwater fishes: recent progress and future challenges. Divers Distrib 16:496–513. https://doi.org/10.1111/j.1472-4642.2010.00655.x
Orpwood JE, Griffiths SW, Armstrong JD (2003) Effects of body size on sympatric shelter use in over-wintering juvenile salmonids. J Fish Biol 63:166–173. https://doi.org/10.1111/j.1095-8649.2003.00206.x
Pârvulescu L, Stoia DI, Miok K, Ion MC, Puha AE, Sterie M, Vereş M, Marcu I, Muntean MD, Aburel OM (2021) Force and boldness: cumulative assets of a successful crayfish invader. Front Ecol Evol 9:581247. https://doi.org/10.3389/fevo.2021.581247
Perry WL, Lodge DM, Feder JL (2002) Importance of hybridization between indigenous and nonindigenous freshwater species: an overlooked threat to North American biodiversity. Syst Biol 51:255–275. https://doi.org/10.1080/10635150252899761
Peters JA, Lodge DM (2013) Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biol Invasions 15:2489–2502. https://doi.org/10.1007/s10530-013-0468-1
Pintor LM, Sih A (2009) Differences in growth and foraging behavior of native and introduced populations of an invasive crayfish. Biol Invasions 11:1895–1902. https://doi.org/10.1007/s10530-008-9367-2
Pintor LM, Sih A, Bauer ML (2008) Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117:1629–1636. https://doi.org/10.1111/j.1600-0706.2008.16578.x
Reisinger LS, Petersen I, Hing JS et al (2015) Infection with a trematode parasite differentially alters competitive interactions and antipredator behaviour in native and invasive crayfish. Freshw Biol 60:1581–1595
Reisinger LS, Glon MG, Pintor LM (2020) Divergence in foraging and predator avoidance behavior across the geographic range of native and non-native crayfish. Hydrobiologia 847:803–818. https://doi.org/10.1007/s10750-019-04139-3
Rodger AW, Starks TA (2020) Length-weight and morphological relationships for ecological studies involving ringed crayfish (Faxonius neglectus neglectus): an extraregional invader. Southeast Nat 19:637–648. https://doi.org/10.1656/058.019.0403
Seebacher F, Wilson RS (2007) Individual recognition in crayfish (Cherax dispar): the roles of strength and experience in deciding aggressive encounters. Biol Lett 3:471–474
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174. https://doi.org/10.1111/j.1365-2427.2009.02380.x
Taylor CA, DiStefano RJ, Larson ER, Stoeckel J (2019) Towards a cohesive strategy for the conservation of the United States’ diverse and highly endemic crayfish fauna. Hydrobiologia 846:39–58. https://doi.org/10.1007/s10750-019-04066-3
Tricarico E, Aquiloni L (2016) How behaviour has helped invasive crayfish to conquer freshwater ecosystems. In: Weis J, Sol D (eds) Biological invasions and animal behaviour. Cambridge University Press, New York, pp 291–308
Twardochleb LA, Olden JD, Larson ER (2013) A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382. https://doi.org/10.1899/12-203.1
Usio N, Konishi M, Nakano S (2001) Species displacement between an introduced and a ‘vulnerable’ crayfish: the role of aggressive interactions and shelter competition. Biol Invasions 3:179–185. https://doi.org/10.1023/A:1014573915464
Vorburger C, Ribi G (1999) Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshw Biol 42:111–119
Warner RE (1968) The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70:101–120. https://doi.org/10.2307/1365954
Whitledge GW, Rabeni CF (1997) Energy sources and ecological role of crayfishes in an Ozark stream: insights from stable isotopes and gut analysis. Can J Fish Aquat Sci 54:2555–2563. https://doi.org/10.1139/cjfas-54-11-2555
Wilson KA, Magnuson JJ, Lodge DM et al (2004) A long-term rusty crayfish (Orconectes rusticus) invasion: dispersal patterns and community change in a north temperate lake. Can J Fish Aquat Sci 61:2255–2266. https://doi.org/10.1139/F04-170
Acknowledgements
We would like to thank Kasey Fralick, Gary Warren, Danielle Drumheller, and Savannah Cantrell for assistance with field research.
Funding
Funding for this research was provided by the Florida Fish and Wildlife Conservation Commission and U.S. Fish and Wildlife Service Sect. 6 funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tripp, N., VanBuren, H. & Reisinger, L.S. Size-mediated competitive interactions between an invasive and an imperiled crayfish may explain extirpation of the imperiled species. Biol Invasions 26, 1091–1104 (2024). https://doi.org/10.1007/s10530-023-03231-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03231-z