Abstract
Non-native crayfishes can have large impacts on biodiversity and the provisioning of ecosystem services in freshwaters. In 2015 we discovered an established population of the globally widespread red swamp crayfish (Procambarus clarkii) in the North Shore Channel of the Chicago Area Waterway System. This population overlaps with a population of rusty crayfish (Faxonius rusticus), a previous invader that is widely distributed and usually the dominant crayfish species across the Great Lakes region. If P. clarkii continues to spread in the Great Lakes region it will frequently encounter F. rusticus. Factors such as water clarity, competition for food when limited, and susceptibility to predation may alter P. clarkii’s ability to become established and spread. We sampled the overlapping populations and found that P. clarkii are significantly larger than F. rusticus. Next, we conducted lab experiments to examine the outcomes of competition between these species for shelter and food. F. rusticus were significantly more likely to seek shelter when threatened, while P. clarkii were significantly more likely to respond aggressively. P. clarkii won more competitions for food. Finally, we conducted field experiments to investigate rates of predation on each species and found that P. clarkii are predated significantly more often. Our results suggest that P. clarkii is dominant in interactions with F. rusticus but that higher rates of predation, likely occurring because P. clarkii is less likely to flee from threats, mitigate these benefits. We suggest that P. clarkii will dominate crayfish communities in water with low clarity, but not in clear-water habitats where visual predators are more effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spread of non-native invasive species is a globally important driver of ecosystem service and biodiversity loss. Freshwater ecosystems are often strongly impacted, with effects including reduced fisheries, reduced water availability for irrigation and municipal use, impeded navigation, and increased habitat for vectors of human disease (Pimental et al. 2005; Pejchar and Mooney 2009; Oreska and Aldridge 2011; Keller et al. 2018). Invasive freshwater crayfishes can have particularly large ecological and economic impacts. Crayfishes are the largest invertebrates in many freshwater habitats (Lodge et al. 2000) and often act as ecosystem engineers (Charlebois and Lamberti 1996; Statzner et al. 2000; Creed and Reed 2004). The negative impacts of these species include decreased water quality (Souty-Grosset et al. 2016), altered aquatic macroinvertebrate communities (Wilson et al. 2004), reductions in macrophyte biomass and biodiversity (Lodge et al. 1994; Wilson et al. 2004), and extirpation of native crayfish (Hein et al. 2007; Lodge et al. 2000). Crayfish can also be vectors of disease, including the crayfish plague (Aphanomyces astaci), which has been an important agent in the displacement of native European crayfish species by invasive North American species which are immune to the disease (Lodge et al. 2000; Souty-Grosset et al. 2016; Donato et al. 2018).
Much research has been conducted to model the distribution and spatial patterns of non-native freshwater species. This work usually aims to predict future spread of invaders so that such spread can be managed (Clarke Murray et al. 2014). Although models usually predict potential spread as a function of physical factors (e.g., water quality, habitat availability, climate), it is known that interactions between invaders and other species can also be important (Crall et al. 2006; Fletcher 2007; Weis 2010; Behringer and Hart 2017). In particular, previously established species may compete with or predate upon the new arrival, and this can mediate the habitats into which freshwater non-native species can spread (Weis 2010).
The establishment and spread of non-native freshwater crayfishes may be particularly dependent upon their interactions with existing crayfish (Garvey et al. 1994). Crayfish native ranges may not be constrained by just their tolerances of physical factors, but also by their interactions with other species and their ability to access new habitats. When non-native crayfish arrive and spread they often dominate the resulting crayfish community (Garvey et al. 1994). For example, the spread of rusty crayfish (Faxonius rusticus; previously Orconectes rusticus; Crandall and De Grave 2017) across the U.S. Midwest is associated with massive declines in population sizes of the existing crayfish species, including the native virile crayfish (Faxonius virilis), and the northern clear-water crayfish (Faxonius propinquus) (Garvey et al. 1994; Olden et al. 2006).
Faxonius rusticus are now widespread and the dominant crayfish across large areas of the Great Lakes region and Midwest (Peters et al. 2014). Likewise, the invasion of Europe by the North American signal crayfish (Pacifastacus leniusculus) has caused the widespread decline in native species resulting in communities dominated by the invader (Westman et al. 2002; Dunn et al. 2008). In each of these cases the invader has been shown to be competitively dominant for resources such as food and shelter, and this is presumed to be a main mechanism leading to their success (Gherardi and Daniels 2004).
Procambarus clarkii (red swamp crayfish), native to the southern United States, is a globally widespread invader associated with large ecological and economic impacts (Smart et al. 2002; Yue et al. 2010; Taylor et al. 2015). Non-native populations of this species are established in North America, Africa, Asia, and Europe (Donato et al. 2018; Smith et al. 2018). The invasion of P. clarkii in Africa has resulted in the reduction of macrophyte species and damaged shorelines from their burrowing (Smart et al. 2002). In Asia, the species’ burrows have led to damaged irrigation systems resulting in poor crop yields and economic losses (Yue et al. 2010). P. clarkii can also vector the crayfish plague which is lethal to many other crayfishes and has resulted in biodiversity loss in Europe (Lodge et al. 2000; Donato et al. 2018).
Procambarus clarkii was first recorded in the Great Lakes region in the 1960’s in Sandusky Bay in the western portion of Lake Erie (Peters et al. 2014). More recently populations have been found in Michigan and Wisconsin, although the established populations in Wisconsin have likely been eradicated (Wisconsin DNR; Behm 2009; Bunk and Van Egeren 2014). There is concern that this species will continue to spread in the Great Lakes region (Donato et al. 2018), and a recent species distribution model has shown that it has the potential to become established across a far wider area (Egly et al. 2019).
Our work is focused on the Illinois portion of the southern basin of Lake Michigan where P. clarkii was first recorded in 2001 (USGS 2019). In 2004 a further record of the species was made in the North Shore Channel of the Chicago Area Waterway system (CAWS; Peters et al. 2014; USGS 2019). Neither of these records was confirmed as being of established populations. In 2015 we confirmed an established population in the North Shore Channel that overlaps with a population of invasive F. rusticus, a species that is abundant in the Laurentian Great Lakes region after spreading from the Ohio River drainage (Wilson et al. 2004; Peters and Lodge 2013; Peters et al. 2014). This species has been in the Great Lakes Basin for over 100 years but its spread accelerated in the 1990’s (Peters et al. 2014). F. rusticus has displaced native crayfish species in multiple waterways throughout the U.S. Midwest (Butler and Stein 1985; Gherardi and Daniels 2004) and has large ecosystem impacts including the alteration of whole lake food-webs (Kreps et al. 2014).
Although sampling data in the region prior to 2015 is limited, the invasion histories of these species make it reasonable to infer that F. rusticus was established in the North Shore Channel prior to the arrival of P. clarkii (Peters et al. 2014). To the best of our knowledge this is the first example of these two invaders having overlapping populations (Peters et al. 2014). We note that these species may overlap in the Sandusky Bay area of Lake Erie; however, this has not been studied (Peters et al. 2014).
Here, we have made field observations and conducted lab and field experiments to investigate the potential for competition and predation to affect the persistence and spread of P. clarkii. We have sampled from the overlapping populations to determine size distributions of each species as this is often an indicator of competitive dominance (Rabeni 1985; Klocker and Strayer 2004). Based on sampling results we designed a series of lab experiments to test for dominance between P. clarkii and F. rusticus at accessing limited shelter and food. Results suggested that P. clarkii are more aggressive and less likely to seek shelter when threatened. We hypothesized that this would expose them to greater predation pressure and tested this in a field experiment that covered different habitats. Our work shows that competitive dominance may be associated with higher risk of predation and indicates that these interactions will likely be important mediators of future spread of these species.
Methods
Trapping
The North Shore Channel of the Chicago Area Waterway System (hereafter: the Channel) is a slow moving canal that was constructed between 1907 and 1910 to connect the North Branch of the Chicago River to Lake Michigan (Fig. 1). Its habitat is homogenous, with a maximum depth of 2.9 m, a consistent width of ~ 20 m, and almost entirely soft mucky substrate. The Channel connects to Wilmette Harbor in Lake Michigan at its north end, and to the North Branch of the Chicago River at its south end. It was constructed primarily to deliver water from Lake Michigan to the O’Brien Sewage Treatment plant which is located nearby to where the Channel meets the Chicago River. Additionally, during high-flow events the weir at Wilmette Harbor can be opened to allow water to flow into Lake Michigan, reducing flooding throughout the surrounding urban area (Hill 2000).
Map of crayfish sampling locations in the Chicago area waterway system. The percentage of Procambarus clarkii found at each site is given; all other crayfish were Faxonius rusticus. WH Wilmette Harbor; NS, N North Shore Channel North; NS, S North Shore Channel, South; NS/NB North Shore Channel/North Branch, NB North Branch of the Chicago River
Crayfish populations in the North Shore Channel, North Branch of the Chicago River, and Wilmette Harbor were surveyed during July and August 2015 at five locations (Fig. 1). Subsequent sampling took place during summers 2016 and 2017. In all cases sampling was conducted using standard minnow traps baited with dry dog food. Traps were modified by increasing the openings to ~ 5 cm diameter (Capelli and Magnuson 1983). Crayfish are most active at night, and traps were set 1 day and recovered the next. We recorded species, sex, and carapace length (CL; the length from the tip of the rostrum to the posterior end of the carapace) for all crayfish sampled.
A first observation was that we only found the non-native species F. rusticus and P. clarkii. Populations of these species overlapped, and multiple size classes of both species were found during all 3 years indicating that they are each well established (see below for full sampling results). These observations motivated the competition and predation experiments described in the following.
Competition experiments
To investigate competition between F. rusticus and P. clarkii for food and shelter, we conducted lab experiments at Loyola University Chicago during August–October 2016. All crayfish used in the experiments were collected during this period from the overlapping populations in the Channel. All collected crayfish were acclimatized to lab conditions for at least 1 week in large cattle tanks prior to being used. Individuals were not re-used for any experiments.
Shelter: two species
Shelter competition experiments were used to test which species is dominant for accessing a single shelter when individuals of both species are threatened. These experiments were conducted in ten-gallon aquaria in a closed lab with no movement (apart from what was necessary to simulate fish attacks, see below) visible to the crayfish. Each aquaria was filled with ~ 8 L of water, giving a depth of 15 cm. Our methods followed Alonso and Martínez (2006), and a total of 23 trials were conducted.
For each trial, one crayfish of each species was selected while ensuring that the carapace lengths of the two crayfish differed by no more than 10%. The largest difference between carapace length was 4 mm. Because F. rusticus are smaller we generally used larger individuals from this species and smaller of P. clarkii (but note that a wider range of sizes was used in the field predation experiments, see below). Both chelae of each crayfish were measured prior to each experiment, and only individuals with two functioning chelae were used. We did not match crayfish based upon chelae size or sex. Crayfish were acclimated in the aquaria for 24 h with a divider preventing physical interaction. Each crayfish was provided a length of PVC pipe for shelter.
To begin the experiment the central divider and both shelters were removed from the tank and a single shelter (a 12 cm long, 5.1 cm diameter piece of PVC pipe closed at one end) was added. Trials lasted 20 min, and at 5, 10, and 15 min we used a plastic fish to simulate an attack on the crayfish. Trials were recorded by video to minimize the potential for observers to affect behavior. Videos were later examined to determine the response of each crayfish to the attacks. Additionally, at 10 s intervals, we recorded (a) whether each species was in or out of the shelter; (b) the behavior of each crayfish while out of shelter (active/passive); and (c) the behavior of the crayfish in relation to each other. The behavior of the crayfish in relation to each other was quantified on a scale ranging from − 2 to 5 (Table 1) following Karavanich and Atema (1998). Aggression during the fish attack was shown by raising the chelae and/or grabbing the fish with chelae.
Shelter: single species
Next, we tested the interest of crayfish in shelter when the other species was absent. Identical methods to those just described were followed, with the exception that there was only one crayfish per tank. Twenty-four trials were conducted (i.e., n = 12 trials per species).
Food
To examine competition between the two species for food we conducted 21 feeding trials using similar methods to Hill and Lodge (1999) and Szela and Perry (2013). Crayfish were selected and size matched using the methods described above. No food was available for a minimum of 48 h prior to each trial to encourage crayfish to seek food. To begin the experiment, both crayfish were placed on one side of a divider and a 2 cm piece of earthworm was placed at the opposite end of the tank. The divider was then removed. We recorded the initial winner of the food (i.e., the first individual to access it), the crayfish that ultimately ate the food, and the time elapsed before the food was fully consumed.
Predation
Experiments were conducted in the field to determine relative rates of predation on the two crayfish species in two different habitats. Experiments were conducted in the Channel and in Wilmette Harbor during June and July 2017 (see Fig. 1 for locations) and involved tethering crayfish to weights, leaving them overnight, and checking the next day to see which had been removed. Methods followed those of DiDonato and Lodge (1993) and Childress and Herrnkind (1994). Crayfish were collected from the North Shore Channel and Wilmette Harbor using the sampling methods described above. All individuals used in these experiments were identified to species, measured for carapace length, and sex was determined.
To tether crayfish we cleaned the top of the carapace with 75% alcohol and used superglue to attach a small swivel. This swivel was tied to a 30 cm long piece of four-pound test strength monofilament fishing line, which was in turn tied to a hook in the center of a 15 × 15 cm tile. To ensure that the tethers held, we first tested our methods in the absence of predation in tanks in the lab. Additionally, we included two controls of each species, at each site, each day, where these controls were individuals glued and tethered in the same way but placed inside minnow traps with the openings closed. None of the lab or field crayfish came free of their swivels or line.
Tiles and attached crayfish were placed on the bottom of the habitat at least 5 m apart and left overnight. In Wilmette Harbor the tiles were placed along the edge of the harbor wall, and total of 60 P. clarkii and 41 F. rusticus trials were conducted. Tiles in the Channel were placed in ~ 1.5 m of water along the bank, alternating species, with a total of 63 P. clarkii and 37 F. rusticus trials conducted. Each trial was a 24-h period that an individual was tethered. Secchi depth was recorded daily at three points within each site. Tethered crayfish were checked every day for presence/absence and any that were missing were replaced by new crayfish. Missing crayfish were considered to have been predated. This assumption was supported by the controls described above, and by us frequently finding torn pieces of crayfish still attached to swivels.
All statistical analyses were conducted using the statistical software R v 3.4.4 (R Core Development Team 2015).
Results
Trapping
Procambarus clarkii were found at highest catch per unit effort (CPUE; average number of crayfish per trap) compared to F. rusticus in the southern site on the North Branch of the Chicago River (CPUE = 0.96 from 2015 to 2017; 100% of 53 crayfish collected were P. clarkii). At the junction of the North Branch and North Shore Channel, P. clarkii were 97.44% of the 39 crayfish caught (P. clarkii CPUE = 0.95, F. rusticus CPUE = 0.03), followed by 98.70% of 307 crayfish at the southern site of the Channel (P. clarkii CPUE = 0.86, F. rusticus CPUE = 0.02). P. clarkii were in lowest proportions in the northern most site on the North Shore Channel (18.67% of 332) (P. clarkii CPUE = 0.15, F. rusticus CPUE = 0.93), and in Wilmette Harbor (6.25% of 32) (P. clarkii CPUE = 0.02, F. rusticus CPUE = 0.37).
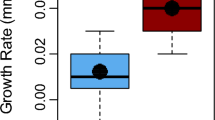
Across all individuals trapped P. clarkii were significantly larger (n = 360; average CL = 50.8 mm) than F. rusticus (n = 157; 43.0 mm; t test, p = ≪ 0.001; Fig. 2). Males were captured at higher rates for both P. clarkii (65% of all captured) and F. rusticus (60%).
Competition experiments
Shelter
Procambarus clarkii responded to fish attacks with aggression at least once during more trials (23/23) than F. rusticus (9/23) (X2 with Yates correction, p = ≪ 0.001). Fifty-six percent of F. rusticus sought shelter during the trial, usually immediately after fish attacks. F. rusticus were significantly more likely (X2 with Yates correction, p = 0.015) to spend time in the shelter (n = 13/23) than P. clarkii (n = 4/23). However, once inside P. clarkii stayed in the shelter longer (t test, p = 0.017). The four P. clarkii that entered the shelter spent an average of 16.01 min of the 20-min trial inside, while the 13 F. rusticus that entered shelter spent an average of 7.67 min inside.
Interaction between P. clarkii and F. rusticus was recorded every 10 s during the entire trial (Table 1). P. clarkii displayed aggressive behaviors [1–5; Table 1] significantly more often than F. rusticus (Wilcoxon signed rank test with paired data, p = 0.042). F. rusticus displayed submissive behaviors [− 1, − 2; Table 1] significantly more often than P. clarkii (p = 0.049). When competition was eliminated and trials with one crayfish per tank were conducted, F. rusticus entered the shelter in 41% of the trials and P. clarkii in 25%.
Food
Procambarus clarkii ate the food in two-thirds of the food competition trials (n = 14/21), though this result is only marginally significant (X2 with Yates correction, p = 0.064, see supplementary tables). When P. clarkii ate the food, they did so more quickly than F. rusticus (average time to consumption of 58.2 min for P. clarkii vs. 81.4 min for F. rusticus; Mann–Whitney U, W = 35, p = 0.322), though this result is not significant. There was no significant difference in the gender of the crayfish that won the food when trials were mixed sex (4/7 mixed sex trials were won by females; X2, p = 0.593; see supplementary data). In all trials examining food competition the two crayfish showed aggressive behavior toward each other, indicating that competition was taking place.
Predation experiment
We report predation rates as the percent of 24-h trials after which we found crayfish had been removed from their tethers. P. clarkii were predated at a significantly higher rate (32%) than F. rusticus in (17%; X2 with Yates correction, p = 0.049) when both habitats were combined (Fig. 3). This trend was also significant in the North Shore Channel, where 29% of P. clarkii were predated versus 8% of F. rusticus (X2 with Yates correction, p = 0.030). Differences in predation rate were not significant when only Wilmette Harbor was considered (35% of P. clarkii predated vs. 24% F. rusticus; X2 with Yates correction, p = 0.360). Although not significant, F. rusticus were predated at higher rates in Wilmette Harbor than the Channel, and no significant difference was seen between the two sites for P. clarkii (X2 with Yates correction, F. rusticus: p = 0.105; P. clarkii: p = 0.567).
There was no effect of gender on predation rate for either species (X2, P. clarkii p = 0.734; F. rusticus p = 0.309). Water clarity in Wilmette Harbor was higher (average 207 cm Secchi depth) than in the North Shore Channel (81 cm Secchi depth). In three of our 18 Secchi measurements at Wilmette Harbor the bottom could be seen. For these measurements, the water depth was used as a proxy for Secchi depth, although we acknowledge this was a low estimate.
Crayfish sizes for experiments
Crayfish size is known to affect competitive interactions (Issa et al. 1999; see supplementary material for sizes of all crayfish used in all experiments). There was no significant difference in the carapace length of the crayfish used in the shelter trials and the food trials (t test, p = 0.528). Additionally, there was no significant difference in the carapace size of P. clarkii used among competition experiments and the predation experiment (ANOVA, p = 0.416). There was no significant difference in F. rusticus used in the shelter and food trials (t test, p = 0.721), but the F. rusticus used in the predation trials were significantly smaller (ANOVA, p = 0.001) that those used in the competition experiments. This difference arose because we used a full range of F. rusticus sizes in the predation experiments, whereas our size matching meant that we could only use larger individuals for competition experiments.
In 88% of the competition trials the P. clarkii individual was larger (but never by more than 10%) and in 73% of the trials the F. rusticus had larger chelae (see supplementary data). The difference in chelae length varied from 1 to 20 mm with an average of 8.8 mm. Although the F. rusticus were often smaller (by CL) than P. clarkii they had significantly larger chelae in the shelter and food competition experiments (t test, p = 0.002).
Discussion
Procambarus clarkii is an internationally well-known invader of freshwaters, with populations established in Africa, Asia, Europe, and elsewhere in North America (Yue et al. 2010; Smith et al. 2018). It has been shown to outcompete native species in many regions (Yue et al. 2010; Smith et al. 2018). There is much concern about the continued spread of this species in the Great Lakes region, and if this occurs it will likely frequently come into contact with established populations of F. rusticus (Egly et al. 2019; Smith et al. 2018). Our results from the Channel demonstrate that P. clarkii are larger than F. rusticus and are more often behave aggressively when threatened. We followed our field observations of crayfish size with lab experiments to test competition between these species for two resources that have been found by other studies to be important predictors of dominance and survival (Figler et al. 1999; Gherardi and Daniels 2004).
Although the food competition results were only marginally significant, P. clarkii ate the food in two-thirds of all trials when individuals were size-matched. Our size matching likely gave an advantage to the smaller species (F. rusticus) relative to what would be experienced in the field because we chose larger F. rusticus and smaller P. clarkii. Our results are consistent with previous work showing that body size is a determinant of dominance in crayfish (Pavey and Fielder 1996; Issa et al. 1999), with larger individuals most often winning competitive interactions for resources (Butler and Stein 1985; Mazlum 2007).
Our field observations of crayfish size combine with our competition experiments to indicate that P. clarkii is likely able to outcompete F. rusticus for food. We note, however, that it is unknown whether food is limiting for these populations, and that the omnivorous behavior of crayfish (Lodge et al. 1994) may make it unlikely. Multi-week lab studies have shown that there may be an effect on long term growth and survival when crayfish are stocked with and without other invasive crayfish, even in a system with ample food (Hill and Lodge 1999). The behavior of crayfishes in our system may be altered based on the presence of an invasive crayfish species, and this may include feeding behaviors. Thus, although food as a resource may not be limited the aggressive behavior of P. clarkii may lead to worse growth outcomes for F. rusticus. P. clarkii showed significantly greater aggression in direct response to F. rusticus, and in response to our simulated fish attack.
During our shelter experiments we found that once inside a shelter P. clarkii stayed there for a significantly longer time than F. rusticus. We do not have a good explanation for this but suggest that it may be an underlying behavioral trait of unknown importance to competition. P. clarkii are a burrowing crayfish species (Correia and Ferreira 1995) and remaining in the shelter may have been akin to remaining in a burrow once one is found.
Based on our field sampling and results of the competition trials we hypothesized that the higher levels of aggression shown by P. clarkii may come at the cost of higher predation. Specifically, responding aggressively may be useful when interacting with another crayfish, especially one from a species that is generally smaller. In contrast, this behavior may be detrimental when the threat is a much larger predator such as largemouth bass (Garvey et al. 1994). Further, we hypothesized that the detrimental effects of aggression would be greater in clearer water where visual predators—such as fishes—are more effective (Crowl 1987). Although fish tend to predate large crayfish less often, we believed that the aggression of P. clarkii would increase the rates at which they were predated (Stein 1977, Garvey et al. 1994, Kershner and Lodge 1995).
To test this we looked at differential predation on P. clarkii and F. rusticus in two habitats, one of which (Wilmette Harbor) has significantly greater water clarity. Our results support our hypotheses. Specifically, P. clarkii were predated at significantly higher rates across both habitats, and this was also true in just the Channel habitat. Both species were predated more often in Wilmette Harbor than the Channel but this trend was not significant. Although the 30-cm tether line would have limited the ability to flee from a threat, the experiments were conducted during late summer when macrophyte cover was plentiful in both habitats. Thus, we believe it likely that predation rates may have been somewhat inflated over what would occur without tethering, but that the difference between the species is indicative of true susceptibility to predation. Previous studies in habitats with high macrophyte cover have found that crayfish with smaller carapace lengths are predated at significantly higher rates compared to larger crayfish (Garvey et al. 1994). Given that F. rusticus are smaller in general, and that the individuals used in our tethering were smaller, this provides further support for our contention that the behavior of P. clarkii exposes it to greater risk.
Additional support for our hypotheses comes from our sampling results which show that although P. clarkii are found throughout the North Shore Channel they are rarely found in Wilmette Harbor of Lake Michigan. These habitats are separated by a weir, but given the propensity of P. clarkii to travel overland (Ramalho and Anastácio 2015; Smith et al. 2018) we doubt that this is a serious barrier to movement, particularly given that the weir is occasionally opened to allow water to flow from the Channel into the Harbor. Our results suggest the possibility that P. clarkii have access to Wilmette Harbor, which has a similar substrate to the Channel, but cannot persist there due to higher predation. An alternative explanation for the distribution patterns is that the population of P. clarkii is still spreading within the Channel and will eventually move into the Harbor and Lake Michigan. We consider this unlikely because of the high population of P. clarkii in much of the Channel and right up to the weir that separates the Channel and Harbor, but further sampling over subsequent years will be needed to determine whether this is the case.
Lower rates of predation on F. rusticus are consistent with past studies that have found this species to be superior at avoiding predators compared to native crayfish species (DiDonato and Lodge 1993). F. rusticus were found to have lower mortality rates compared to Faxonius propinquus and Faxonius virilis (Hill and Lodge 1999), both of which are native in our study area (Peters et al. 2014), when in tanks in the presence of a largemouth bass. In our predation experiment the average chelae size of F. rusticus was significantly greater than for P. clarkii which may have aided in their defense against predation. Chelae size has been found to be positively associated with success in encounters with fish and other crustaceans in both lobsters (Scrivner 1971) and crayfish (Stein 1976). Overall our results indicate that shelter seeking by F. rusticus, and potentially their larger chelae, significantly increased their likelihood of survival compared to P. clarkii. This occurred despite the P. clarkii in the experiment having significantly larger carapace size which in other cases has been associated with lower predation.
Male P. clarkii and F. rusticus alternate during the year between form I and form II, which are reproductively active and inactive respectively. Form I crayfish are generally more aggressive (Tierney et al. 2008), which may affect competition and predation outcomes. A previous study showed that form I males were less likely to be predated than form II and female crayfish, with the latter two equally likely to be predated (Stein 1977). We used males and females of each species in our experiments but did not record form. To test whether changes in form may have affected our results we compared males to females, the latter of which should have stable behaviors (note that no berried [= with eggs] females were included in our experiments). Although the behaviors of form I males, form II males, and females has been found to be different in the past, we found that males and females of each species did not differ significantly in the rates at which they were predated. Further, the outcomes for males and females of each species in the competition experiments were not different. Thus, we do not see evidence that the form of male crayfish affected our results.
If there is a trade-off between competitive advantage and exposure to predation that explains the distribution patterns observed then we would expect P. clarkii to continue their spread into habitats that either have low water clarity, or that are clear but have few predators of crayfish. This would make many rivers and wetlands across the Great Lakes region susceptible. While much of the Great Lakes themselves may be too clear, there are large and productive areas that have secchi depths similar to those observed in the Channel (GLEC 2006; Qualls et al. 2013). These include Lake Michigan’s Green Bay (Qualls et al. 2013) and Lake Huron’s Saginaw Bay (GLEC 2006). The only known populations of P. clarkii that have existed in the Great Lakes region over a long period are in wetlands connected to Sandusky Bay in the western basin of Lake Erie (USGS 2019). P. clarkii were first recorded at the Winous Bay Shooting Club there in 1967, and the Resthaven Wildlife Area in 1982 (Nagy et al. 2018). Both populations persist in the wetlands, but in neither case is there evidence that P. clarkii have moved into the western areas of Lake Erie, which tend to be clearer (USGS 2019). Based on our results from the predation experiments, this lack of spread could be partially attributed to water clarity and predation, with habitat type perhaps also playing a role.
Procambarus clarkii is a recent and spreading crayfish invader of freshwaters in the Laurentian Great Lakes region (Nagy et al. 2018). There is much concern about its potential impacts and a desire to prevent its further spread. If it does continue to spread it will come into contact and competition with established crayfishes, and often this will be the widely established and currently dominant F. rusticus (Peters et al. 2014). A recent study has suggested that the potential range of P. clarkii in the Great Lakes is much larger than its current range and includes areas such as Saginaw Bay in Lake Huron, Green Bay in Lake Michigan, and Henderson Bay in Lake Ontario (Egly et al. 2019). Our work shows that P. clarkii are larger and more aggressive than F. rusticus, and that when threatened they are less likely to seek shelter. A consequence of this aggression, however, is that P. clarkii respond to threats—such as predators—by aggressively displaying their chelae rather than fleeing. Our experiments and observations offer a mechanistic explanation for patterns of distribution of P. clarkii and can be used to aid predictions of future spread.
References
Alonso F, Martínez R (2006) Shelter competition between two invasive crayfish species: a laboratory study. Bulletin François de la Peche et de la Pisciculture 380–381:1121–1132
Behm J (2009) Bleach set to eradicate Germantown’s invasive crayfish. Milwaukee, Wisconsin Journal Sentential
Behringer DC, Hart JE (2017) Competition with stone crabs drives juvenile spiny lobster abundance and distribution. Oecologia 184:205–218
Bunk H, Van Egeren S (2014) Containment. In: Control and eradication of ambitious architect: Procambarus clarkii, The Red Swamp Crayfish
Butler M, Stein R (1985) An analysis of the mechanisms governing species replacement in crayfish. Oecologia 66(2):168–177
Capelli GM, Magnuson JJ (1983) Morphoedaphic and biogeographic analysis of crayfish distribution in Northern Wisconsin. J Crustac Biol 3(4):548–564
Charlebois PM, Lamberti GA (1996) Invading crayfish in a Michigan stream: direct and indirect effects of periphyton and macroinvertabrates. J N Am Benthol Soc 15(4):551–563
Childress MJ, Herrnkind WF (1994) The behavior of Caribbean juvenile spiny lobster in Florida Bay: seasonality, ontogeny and sociality. Bull Mar Sci 54(3):819–827
Clarke Murray CC, Gartner H, Gregr EJ, Chan K, Pakhomov E, Therriault TW (2014) Spatial distribution of marine invasive species: environmental, demographic and vector drivers. Divers Distrib 20:824–836
Correia AM, Ferreira O (1995) Burrowing behavior of the introduced red swamp crayfish Procambarus clarkii (Decapoda: Cambaridae) in Portugal. J Crustac Biol 15(2):248–257
Crall AW, Newman GJ, Stohlgren TJ, Jarnevich CS, Evangelista P, Guenther D (2006) Evaluating dominance as a component of non-native species invasions. Divers Distrib 12:195–204
Crandall KA, De Grave S (2017) An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J Crustac Biol 37(5):615–653
Creed RP Jr, Reed JM (2004) Ecosystem engineering by crayfish in a headwater stream community. J N Am Benthol Soc 23(2):224–236
Crowl TA (1987) Effects of crayfish size, orientation, and movement on the reactive distance of largemouth bass foraging in clear and turbid water. Hydrobiologia 183(2):133–140
DiDonato GT, Lodge DM (1993) Species replacements among Orconectes crayfishes in Wisconsin lakes: the role of predation by fish. Can J Fish Aquat Sci 50:1484–1488
Donato R, Rollandin M, Favaro L, Ferrarese A, Pessani D, Ghia D (2018) Habitat use and population structure of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852) in a protected area in northern Italy. Knowl Manag Aquat Ecosyst 419:12
Dunn JC, McClymont HE, Christmas M, Dunn AM (2008) Competition and parasitism in the native white clawed crayfish Austropotamobius pallipes and the invasive signal crayfish Pacifastacus leniusculus in the UK. Biol Invasions 11(2):315–324
Egly RM, Annis GM, Chadderton WL, Peters JA, Larson ER (2019) Predicting the potential distribution of the non-native red swamp crayfish Procambarus clarkii in the Laurentian Great Lakes. J Great Lakes Res 45(1):150–159
Figler MH, Cheverton HM, Blank GS (1999) Shelter competition in juvenile red swamp crayfish (Procambarus clarkii): the influences of sex differences, relative size, and prior residence. Aquaculture 178(1–2):63–75
Fletcher RJ Jr (2007) Species interactions and population density mediate the use of social cues for habitat selection. J Anim Ecol 76(3):598–606
Garvey JE, Stein RA, Thomas TM (1994) Assessing how fish predation and interspecific prey competition influence crayfish assemblage. Ecology 75(2):532–547
Gherardi F, Daniels WH (2004) Agonism and shelter competition between invasive and indigenous crayfish species. Can J Zool 82:1923–1932
[GLEC] Great Lakes Environment Center (2006) Water quality monitoring of Saginaw and Grand Traverse Bays. Michigan Department of Environmental Quality report #MI/DEQ/WB-06/096. https://www.michigan.gov/documents/deq/wrd-swas-9304bayreport_445627_7.pdf
Hein CL, Vander Zanden MJ, Magnuson JJ (2007) Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshw Biol 52:1134–1146
Hill L (2000) The Chicago river: a natural and unnatural history. Southern Illinois University Press, Carbondale
Hill AM, Lodge DM (1999) Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Appl 9(2):678–690
Issa FA, Adamson DJ, Edwards DH (1999) Dominance hierarchy formation in juvenile crayfish Procambarus clarkii. J Exp Biol 202:3497–3506
Karavanich C, Atema J (1998) Individual recognition and memory in lobster dominance. Anim Behav 56:1553–1560
Keller RP, Masoodi A, Shackleton RT (2018) The impact of invasive aquatic plants on ecosystem services and human well-being in Wular Lake, India. Reg Environ Change 18:847–857
Kershner MW, Lodge DM (1995) Effects of littoral habitat and fish predation on the distribution of an exotic crayfish, Orconectes rusticus. Freshw Sci 14(3):414–422
Klocker CA, Strayer DL (2004) Interactions among an invasive crayfish (Orconectes rusticus), a native crayfish (Orconectes limosus), and native bivalves (Sphaeriidae and Unionidae). Northeast Nat 11(2):167–178
Kreps TA, Larson ER, Lodge DM (2014) Do invasive rusty crayfish (Orconectes rusticus) decouple littoral and pelagic energy flows in lake food webs? Freshw Sci 35(1):103–113
Lodge DM, Kershner MW, Aloi JE, Covich AP (1994) Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food source. Ecology 75(5):1265–1281
Lodge DM, Taylor CA, Holdich DM, Skurdal J (2000) Nonindigenous crayfishes threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25(8):7–20
Mazlum Y (2007) Effects of temperature on the survival and growth of two cambarid crayfish juveniles. Crustaceana 80(8):947–954
Nagy R, Fusaro A, Conard W (2018) Procambarus clarkii (Girard, 1852). U.S Geological Survey, Nonindigenous Aquatic Species Database, Gainesvill, FL
Olden JD, McCarthy JM, Maxted JT, Fetzer MW, Vander Zanden MJ (2006) The rapid spread of rusty crayfish (Orconectes rusticus) with observations on native crayfish declines in Wisconsin (U.S.A.) over the past 130 years. Biol Invasions 8(8):1621–1628
Oreska MPJ, Aldridge DC (2011) Estimating the financial costs of freshwater invasive species in Great Britain: a standardized approach to invasive species costing. Biol Invasions 13:305–319
Pavey CR, Fielder DR (1996) The influence of size differential on agonistic behaviour in the freshwater crayfish, Cherax cuspidatus (Decapoda: Parastacidae). J Zool 238:445–457
Pejchar L, Mooney HA (2009) Invasive species, ecosystem services and human well-being. Trends Ecol Evol 24(9):497–504
Peters JA, Lodge DM (2013) Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biol Invasions 15:2489–2502
Peters JA, Cooper MJ, Creque SM, Kornis MS, Maxted JT, Pretty WL, Schueler FW, Simon TP, Taylor CA, Thoma RF, Uzarski DG, Lodge DM (2014) Historical changes and current status of crayfish diversity and distribution in the Laurentian Great Lakes. J Great Lakes Res 40(1):35–46
Pimental D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52(3):273–288
Qualls T, Harris HJ, Harris V (2013) The state of the Bay: the condition of the Bay of Green Bay/Lake Michigan 2013. University of Wisconsin Sea Grant Institute. http://www.seagrant.wisc.edu/home/Portals/0/Files/Habitats%20and%20Ecosystems/SotBReport.pdf
Rabeni CF (1985) Resource partitioning by stream-dwelling crayfish: the influence of body size. Am Midl Nat 113(1):20–29
Ramalho RO, Anastácio P (2015) Factors inducing overland movement of invasive crayfish (Procambarus clarkii) in a ricefield habitat. Hydrobiologia 746(1):135–146
R Core Development Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Scrivner JCE (1971) Agonistic behavior of the American lobster Homarus americanus (Milne-Edwards). Fish Res Board Can Tech 235:128
Smart AC, Harper DM, Malaisse F, Schmitz S, Coley S, Gouder de Beauregard AC (2002) Feeding of the exotic Lousiana red swamp crayfish, Procambarus clarkii (Crustacea, Decapoda), in an African tropical lake: Lake Naivasha, Kenya. Hydrobiologia 488:129–142
Smith K, Roth BM, Herbst SJ, Thoma RF, Popoff N, Hayes DB, Jones ML (2018) Assessment of invasion risks for red swamp crayfish (Procambarus clarkii) in Michigan. Management of Biological Invasions, USA, p 9
Souty-Grosset C, Anastácio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E (2016) The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica 58:78–93
Statzner B, Fièvet E, Champagne J, Morel R, Herouin E (2000) Crayfish as geomorphic agents and ecosystem engineers: biological behavior affects sand and gravel erosion in experimental streams. Limnol Oceanogr 45:1030–1040
Stein RA (1976) Sexual dimorphism in crayfish chelae: functional significance linked to reproductive activities. Can J Zool 54(2):220–227
Stein RA (1977) Selective predation, optimal foraging and the predator-prety interaction between fish and crayfish. Ecology 58:1237–1253
Szela K, Perry WL (2013) Laboratory competition hierarchies between potentially invasive rusty crayfish (Orconectes rusticus) and native crayfishes of conservation concern. Am Midl Nat 169(2):345–353
Taylor CA, Schuster GA, Wylie DB (2015) Field guide to crayfishes of the Midwest. Manual 15. Illinois Natural History Survey, Champaign, IL
Tierney AJ, Gunaratne C, Jennison K, Monroy V, Donnelly L (2008) Behavioral correlates of alternate male forms (form I and form II) in the crayfish Orconectes rusticus. J Crustac Biol 28(4):596–600
[USGS] United States Geological Society, Nonindigenous aquatic species (2019) Crustaceans, crayfish map
Weis JS (2010) The role of behavior in the success of invasive crustaceans. Mar Freshw Behav Physiol 43(2):83–98
Westman K, Savolainen R, Julkunen M (2002) Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a small, enclosed Finnish lake: a 30-year study. Ecography 25(1):53–73
Wilson KA, Magnuson JJ, Lodge DM, Hill AM, Kratz TK, Perry WL, Willis TV (2004) A long-term rusty crayfish (Orconectes rusticus) invasion: dispersal patterns and community change in a north temperate lake. Can J Aquat Sci 61:2255–2266
Wisconsin Division of Natural Resources (WIDNR). Aquatic invasive species, red swamp crayfish. https://dnr.wi.gov/lakes/invasives/AISLists.aspx?species=RED_SWAMP_CRAYF
Yue GH, Li J, Bai Z, Wang CM, Feng F (2010) Genetic diversity and population structure of the invasive alien red swamp crayfish. Biol Invasions 12:2697–2706
Acknowledgements
The authors wish to thank the following for field and lab assistance: Jonathon Brenner, Shehla Chowdhury, Gabriel Habeeb, Trent Henry, Alexander Papaioannou, Jenny Par, and John Zink. Martin Berg and Victoria Prescott provided statistical analysis assistance. The Illinois Department of Natural Resources (Grant No. F16AP00241) and US Fish and Wildlife Service provided funding for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Shaughnessey, E.M., Keller, R.P. When invaders collide: competition, aggression, and predators affect outcomes in overlapping populations of red swamp (Procambarus clarkii) and rusty (Faxonius rusticus) crayfishes. Biol Invasions 21, 3671–3683 (2019). https://doi.org/10.1007/s10530-019-02079-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02079-6