Abstract
Biogeographical comparisons of native and non-native populations allow researchers to understand the degree to which traits contributing to invasion success are intrinsic or change during the invasion process. Here, we investigate whether traits underlying interspecific competition change following invasion and whether these alter the impacts of two crayfish congeners that have invaded into each other’s native ranges. Specifically, we compared native and non-native populations of rusty (Faxonius rusticus) and virile crayfish (F. virilis). We compared native and non-native populations of each species using laboratory assays to examine aggression and large mesocosms with the congeners in sympatry to examine growth and survival as well as impacts on lower trophic levels. We found that non-native virile crayfish were more aggressive in response to a threat than native virile crayfish and exhibited greater growth and survival in sympatry with rusty crayfish. These intraspecific differences were large enough to alter coexistence between species in the mesocosm experiment, which is consistent with patterns of coexistence between these species in the field. We did not observe differences in traits between native and non-native rusty crayfish, but rusty crayfish were consistently competitively dominant over virile crayfish in paired laboratory assays. Non-native populations of both species had greater impacts on lower trophic levels than native populations. Taken together, these findings provide new evidence that trait changes during invasions may enhance ecological impacts of invasive animals and their ability to compete with closely related native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased interest in, and awareness of, biological invasions over the last three decades has spurred research aimed at identifying characteristic traits of invasive species (Kolar and Lodge 2001; Hulme et al. 2008; Blackburn et al. 2011). For example, competitive dominance, aggressiveness and faster growth rates than native species have been found to contribute to the invasion success of several invasive species (Hill et al. 1993; Byers 2000; Sanders et al. 2003). However, few studies have taken a comparative biogeographical approach to determine whether the traits that confer competitive dominance over native species are intrinsic to invasive species or are the result of rapid evolution or phenotypic plasticity occurring during the invasion process. This has been particularly the case in the context of animal invasions. Comparing native and non-native populations of known invasive species may allow researchers to tease apart the relative extent to which traits contributing to invasion success are intrinsic or acquired during the invasion process (Lee 2002; Hierro et al. 2005), which may inform predictions and prevention of new invasions and guide management efforts of ongoing ones (Kolar and Lodge 2001; Lodge et al. 2006; Van Kleunen et al. 2010).
Studies that have taken a biogeographical approach to investigate traits of invasive species suggest that trait changes that influence invasion success can occur during the invasion process. For instance, comparisons of native and invasive populations of Argentine ants, Linepithema humile, revealed that a reduction in intraspecific aggression facilitated the formation of super-colonies. This change in behavior has enabled the ants to spread rapidly and have large impacts in their invaded range (Suarez et al. 1999; Tsutsui et al. 2000). While invasive Argentine ants exhibit reduced intraspecific aggression, they exhibit high interspecific aggression toward native ant species and often displace them (Human and Gordon 1999). In European green crabs, Carcinus maenas, larger body size in invasive than native populations, which has been attributed to release from native parasites, enhances invasion success (Torchin et al. 2001; Grosholz and Ruiz 2003). Similarly, studies comparing native and invasive populations of rusty crayfish, Faxonius rusticus (previously Orconectes rusticus; Crandall and De Grave 2017), have documented divergence between native and non-native populations in traits including aggressiveness, boldness and growth rate, which likely contribute to invasion success and increase impacts of this crayfish in its invaded range (Pintor et al. 2008; Pintor and Sih 2009; Sargent and Lodge 2014; Reisinger et al. 2017). These few studies on animal invasions, in combination with the evidence from biogeographical comparisons of native and invasive populations of various plant species (e.g., Bossdorf et al. 2005; Hierro et al. 2005), highlight the potential importance of evolution and phenotypic plasticity in biological invasions. Thus, employing biogeographical comparisons can allow us to test and refine fundamental concepts of invasion ecology, which may yield insights into better invasive species control and management.
Notwithstanding the importance of trait divergence between native and non-native populations, success through each invasion stage (i.e., transport, introduction, establishment, and spread; Blackburn et al. 2011) is the outcome of interactions between the invader’s traits and the abiotic and biotic environment (Shea and Chesson 2002). The presence of native species whose niche overlaps with that of a non-native species may create biotic resistance to invasion by reducing available niche space in an ecosystem (Shea and Chesson 2002; Green et al. 2004; Levine et al. 2004). Yet, a non-native species may overcome this biotic resistance if it is competitively superior to the native species and better able to obtain resources (Hill et al. 1993; Human and Gordon 1996; Sakai et al. 2001). Traits associated with competitive dominance (e.g., aggressiveness and boldness) are often intrinsic traits of non-native species (e.g., Gioria and Osborne 2014), but may also arise during the invasion process through evolution or phenotypic plasticity (Joshi and Vrieling 2005; Pintor and Sih 2009; Sargent and Lodge 2014; Reisinger et al. 2017). Biogeographical comparisons of populations from both their native and invaded ranges offer a powerful approach to disentangle the extent to which traits associated with invasion success are affected by the invasion process.
Here, we had the unique opportunity to use a reciprocal invasion of two crayfish species (i.e., invasions into each other’s native ranges) to examine whether traits associated with competitive ability change following invasion. Specifically, we compared behavioral traits, growth and mortality of native and non-native populations of rusty crayfish, F. rusticus, and virile crayfish, Faxonius virilis (previously Orconectes virilis; Crandall and De Grave 2017). Rusty crayfish (native to the Ohio River Drainage) and virile crayfish (native to much of the Midwest and parts of Canada) have both been widely introduced across the United States and have competitively displaced native crayfish following introduction (Lodge et al. 1986; Olden et al. 2006; Kilian et al. 2010; Swecker et al. 2010). In the upper Midwest, invasive rusty crayfish typically displace native virile crayfish by outcompeting this species for food and shelter, which makes virile crayfish more susceptible to predation (Hill et al. 1993; Garvey et al. 1994; Hill and Lodge 1999). The ability of invasive rusty crayfish to displace native virile crayfish may be enhanced by the former’s higher level of aggression (Garvey et al. 1994). These same traits are thought to underlie competitive displacement of native crayfishes by invasive virile crayfish (Swecker et al. 2010), but this has yet to be empirically demonstrated. The recent introduction of the virile crayfish into the native range of rusty crayfish provided the opportunity to examine whether traits that contribute to the invasion success of rusty crayfish were inherent to this species or whether the invasion process selects for traits that increase competitive ability in non-native crayfishes in general.
In this study, we used a series of laboratory experiments to quantify and compare behavioral traits of one native and one non-native population of both rusty and virile crayfish. We also used a mesocosm experiment to examine the growth and survival of each population in sympatry with the other congener to determine whether trait differences between populations affect competitive outcomes and resulted in different impacts on food resources (periphyton and benthic macroinvertebrates). Our overarching hypothesis was that native and non-native populations of both rusty and virile crayfish differ in traits associated with competitive ability and in their impacts on food resources. Specifically, we predicted that: (1) non-native populations of both species would exhibit more aggressive behaviors and higher growth and survival than native populations and that (2) differences between native and non-native populations of virile crayfish would be large enough to alter the outcome of their competitive interactions with rusty crayfish. Finally, we predicted that non-native populations of both species would have larger negative effects on periphyton and macroinvertebrates than native populations.
Methods
Animal collection and housing
We collected native rusty crayfish and non-native virile crayfish by hand in streams in Wayne County and Franklin County, Indiana between May 7th and 14th, 2016. Specifically, we caught native rusty crayfish from Butler Creek (N39°44′12″, W85°02′56″) and Salt Creek (N39°23′3″, W85°13′7″), and non-native virile crayfish in Butler Creek. Both crayfish species were present at both sites; however, virile crayfish were more common in the upper reaches of Butler Creek.
We collected invasive rusty crayfish and native virile crayfish by hand while snorkeling in Vilas county, Wisconsin between May 15th and May 20th, 2016. Specifically, we caught invasive rusty crayfish in Star Lake (N46°01′29″, W89°28′08″) and native virile crayfish in Forest Lake (N46°09′23″, W89°22′38″). Forest Lake was previously considered uninvaded by rusty crayfish; however, we found a small number of non-native rusty crayfish during our sampling of Forest Lake, suggesting that these crayfish are in the process of invading. We did not encounter any virile crayfish in Star Lake.
We transported crayfish to either a laboratory at The Ohio State University or the Central Michigan University Mesocosm Facility on Beaver Island in Lake Michigan, where we placed each one in an individual, lidded and perforated 16 oz plastic container inside of species- and population-specific plastic pools with constant aeration. We fed each crayfish two to three commercial sinking shrimp pellets twice a week and performed complete water changes the day after each feeding. We gave the crayfish a minimum of 1 week to acclimate to laboratory conditions prior to using them in experiments. We checked for molts daily and, if a crayfish molted within 1 week of having been used in a behavioral experiment (threat response or aggression assay), we excluded its data from the analysis. Likewise, we waited a minimum of 1 week following a molt before using a crayfish in behavioral experiments, and only used freshly molted crayfish once their exoskeletons were hard enough to allow them to be handled. Lastly, male crayfish of the family Cambaridae alternate between a reproductively active form I and reproductively inactive form II. Form I males have consistently larger chelae and are more aggressive than form II males (Stein 1976; Bergman et al. 2003), so we accounted for this dimorphism throughout our experiments.
Chelae size
Chela size is an important determiner of success in agonistic encounters between crayfish; therefore, we measured the length and width of all form I male crayfish used in our behavioral experiments (n = 69). We used the methods outlined by Usio et al. (2016) to approximate chela area as chela length * chela width * 0.5, then divided chela area by the crayfish’s CL to standardize this measurement to the size of each crayfish (hereafter referred to as “standardized chela area”).
Threat response assay
Crayfish exhibit a stereotyped set of behavioral responses when threatened, either raising their chelae (meral spread), retreating, or not responding. A meral spread is considered to be a more aggressive response than retreating (Bergman and Moore 2003). To compare the threat response of native and non-native populations of the rusty and virile crayfish, we conducted threat response assays in plastic pools with a bottom diameter of 100 cm and filled to approximately 13 cm depth with fresh, dechlorinated tap water (approximately 100 L). We allowed the crayfish to acclimate to the pool for 10 min prior to the start of the assay and observed behavior from behind a black curtain to prevent the crayfish from being influenced by the observer. We used both female and form I male crayfish (n = 115), which we tested individually within the pool. We used a replica blue heron skull (Ardea herodias) to simulate the attack of a common predator of crayfish (Englund and Krupa 2000). The heron skull was held by the experimenter and introduced into the testing arena through a small opening in the black curtain. An attack was simulated by quickly approaching the crayfish with the heron skull at a 45° angle, but not making direct contact with the crayfish, and then pulling the heron skull out of the arena. We repeated this for an additional two times with a twenty-second pause in between each attack (three attacks total). We recorded the crayfish’s immediate response to the attack as either a defense (moving towards the beak with claws raised and open), neutral (no movement in response to beak), or retreat (walking, running or tail-flipping away from the beak or crouching down against the bottom of the pool).
Paired agonistic assay
We conducted paired assays to compare and quantify the agonistic behavior of native and non-native crayfish toward congeners. Specifically, we compared agonistic behavior of crayfish in different contexts by creating three treatments (Table 1): (1) Indiana: native rusty versus non-native virile crayfish, (2) Wisconsin: native virile versus invasive rusty crayfish, and (3) native: native rusty and native virile crayfish. To test our main hypothesis that native and non-native populations differ in behavior, we used the native treatment as a control to compare against the Wisconsin and Indiana treatments. In other words, the native treatment allowed us to test for differences between the native and non-native populations of each species while holding the identity of the competing species constant. In all cases, we used female or form 1 male crayfish (all matchups were intra-sex; n = 106) that were size-matched to within 1 mm carapace length (CL: distance from the tip of the rostrum to the posteriomedian edge of the carapace). We used a small number of individuals in more than one assay, but only did so after waiting a minimum of 1 week to remove the possible influence of previous interactions with other individuals (Seebacher and Wilson 2007). We conducted assays within a 26 cm diameter arena filled with 8 L of dechlorinated tap water placed behind a black curtain to minimize interference by the experimenter. Prior to the start of the experiment, we placed a crayfish pair within the arena separated from each other by a transparent, perforated divider. We allowed crayfish to acclimate to the arena for 10 min, following which we removed the divider and began the assay. We recorded all assays using a video camera then used slow-motion playback to assign an aggression score to both crayfish at 5 s intervals. Aggression scoring was based on an ethogram with point values ranging from − 2 for a tail-flip retreat to + 5 for unrestrained fighting (Bruski and Dunham 1987; Bergman and Moore 2003).
Mesocosm experiment
To compare growth and survival between native and non-native populations of rusty and virile crayfish in sympatry, we conducted an experiment in 800 L mesocosms (1.27 m2 surface area, 1.27 m diameter, 0.66 m depth) at Central Michigan University’s Biological Station on Beaver Island, Michigan, USA. We applied one of the three population treatments used in the behavioral assays described above to each of 12 mesocosms (i.e., Indiana, Wisconsin and native; n = 4 per treatment; n = 12 total), then chose five crayfish of each species (10 crayfish per mesocosm; approximately 8 crayfish per m2) to add to each mesocosm based on these treatments. As in the paired agonistic assay, to test our main hypothesis that native and non-native populations differ, the native treatment served as a control to compare against the Wisconsin and Indiana treatments. Before adding the crayfish to the mesocosms, we marked each one with a unique identifier by pushing a pin through its telson or one of its uropods, permitting us to track individual crayfish throughout the experiment. We also measured initial CL and blotted wet weight of each crayfish. Initial mean CL of rusty crayfish was 29.7 ± 0.5 mm (range = 22.5 to 35.5 mm), and mean CL of virile crayfish was 30.2 ± 0.6 mm (range = 21.8 to 36.7 mm). Each mesocosm contained a range of sizes of each species to represent a population with multiple age classes (mean CL size range per mesocosm = 11.0 ± 0.2 mm). Mean crayfish CL across population treatments varied by less than 3 mm, and the difference in mean CL of rusty and virile crayfish in each mesocosm was less than 3 mm (mean difference = 1.2 mm). All crayfish in this experiment were form II males except for a small subset of native virile crayfish males which were form I. Thus, in each of the mesocosms with native virile crayfish, one of the virile crayfish was form I and the other four were form II. Over 1 month (August 3rd–September 3rd and 4th, 2016), we examined the growth and survival of these crayfish as well as their impacts on benthic macroinvertebrates and periphyton in these mesocosms. In order to avoid disrupting the experiment and because cannibalism is common amongst wild crayfishes, we did not monitor crayfish survival or attempt to replace dead crayfish in the mesocosms while the experiment was running.
Throughout the experiment, fresh water from Lake Michigan flowed continuously through each mesocosm (approximately 0.05 L/s), and we monitored temperature using Onset Hobo Data Loggers (Bourne, MA, USA) placed in each mesocosm (range: 19.3–25.8 °C; mean = 23.3 °C). We conducted the experiment under a 12 h light, 12 h dark photoperiod. Each mesocosm contained a thin layer of gravel over its entire bottom, and also contained cobbles and boulders over 1/3 of the tank to provide shelter for crayfish. We collected all gravel, cobble and boulders from Lake Michigan to mimic natural conditions as closely as possible. To provide food for crayfish, we added 39 snails (1 Physidae, 11 Lymnaeidae, 2 Planorbidae, 23 Pleuroceridae, 2 Viviparidae) to each mesocosm. Halfway through the experiment, we added additional food to each mesocosm including snails (3 Physidae, 11 Lymnaeidae, 5 Planorbidae, 3 Viviparidae) and other macroinvertebrates (2 Chironomids, 6 Amphipods, 1 Odonate). We also added 250 ± 4 g of homogenized detritus collected from Font Lake on Beaver Island halfway through the experiment. Finally, to assess crayfish impacts on benthic algal growth, we placed four 4.85 cm2 unglazed tiles in each mesocosm spaced evenly across the open gravel section at the beginning of the experiment.
At the end of the experiment, we gently removed all cobbles and boulders and scrubbed them over a bucket filled with water to remove attached macroinvertebrates. We poured the water in the bucket through a sieve and preserved the macroinvertebrates in 70% ethanol and later identified them to order (Ephemeroptera, Trichoptera, Diptera, Odonata, Amphipoda, Isopoda) or class (Gastropoda, Hirudinea). We then removed all crayfish from each mesocosm, identified individuals via their individual mark, and recorded their CL and blotted wet weight. Following the experiment, we extracted chlorophyll a from the algae on the unglazed tiles using 95% ethanol, and measured the concentration on a spectrophotometer using standard methods (Wetzel and Likens 2000).
Threat response assay
To examine threat response behavior, we calculated the proportion of defenses and retreats in response to the three attacks for each crayfish used in this assay. Size has been shown to influence crayfish behavior, with larger individuals generally being more dominant and aggressive (Rubenstein and Hazlett 1974; Bergman and Moore 2003); we therefore measured each crayfish’s carapace length (CL) and included it as a covariate in this and subsequent behavioral analyses. Additionally, we observed that the temperature of the tap water that we used varied with time of day and date, so we also included water temperature as a covariate in this and subsequent behavioral analyses. In cases when CL or temperature were not statistically significant, we excluded them from the model and reran the analysis. We used a general linear model to assess the effects of crayfish species, range (native vs. non-native), sex, and their interactions as well as CL and temperature on the proportion of retreats and defenses of the crayfish (we used a logit transformation because the response variables are non-binomial proportions; Warton and Hui 2011).
Paired agonistic assay
To examine aggression in the paired agonistic assay, we used a general linear mixed-effects model to examine the effects of population treatment (i.e., Indiana, Wisconsin, or native), species, and their interaction, as well as sex, CL and temperature on the aggression scores (log transformed to meet assumption of normality) of individual crayfish. We included the trial as a random effect in the model. To test our main hypothesis that native and non-native populations differ in behavior, we ran post hoc Tukey’s range tests to compare aggression scores between native and non-native populations of rusty and virile crayfish. We also used an analysis of variance (ANOVA) to assess the effects of crayfish species, range, and their interaction on standardized chela area.
Mesocosm experiment
Within the mesocosm experiment, we used mixed effects logistic regression models with mesocosm included as a random factor and initial CL included as a covariate to examine the effect of species, population treatment, and their interaction on mortality in each species. We used likelihood ratio tests to examine the influence of independent variables in mixed effects logistic regressions. To examine the effect of species, population treatment, and their interaction on growth in terms of change in CL, we used mixed effects models with mesocosm as a random effect and initial CL as a covariate. We also assessed the effect of population treatment on growth in terms of change in weight (log transformed to meet assumption of normality) using mixed effects models with the same independent variables, except these models contained initial weight as a covariate. We removed initial CL and weight from our models in cases when they were not statistically significant and reran models. To test our main hypothesis that native and non-native populations differ in growth, we ran post hoc Tukey’s range tests to compare growth and survival between native and non-native populations of rusty and virile crayfish.
In addition, within the mesocosm experiment, we used ANOVA to test the effect of population treatment on mean benthic chlorophyll a concentration at the mesocosm level. We included the number of rusty and virile crayfish that survived as covariates and used Tukey’s multiple comparison test for pairwise comparisons. We used ANOVA with the same independent variables to examine the effect of population treatment on the number of benthic macroinvertebrates that were collected from mesocosms at the end of the experiment.
We performed our analyses and created plots using the packages ggplot2 (Wickham 2009), lme4 (Bates et al. 2014), lmerTest (Kuznetsova et al. 2016), lsmeans (Lenth 2016), MASS (Venables and Ripley 2002) in R statistical software (R Core Team 2016).
Results
Threat response: defense
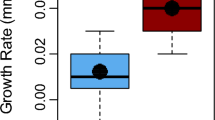
Defense behavior differed between native and non-native virile crayfish populations (Fig. 1). We found a significant interaction between species and range (F1,106 = 5.20, p = 0.03) on the proportion of defense responses. Non-native virile crayfish (44.83 ± 7.07%) defended themselves more frequently than their native counterparts (16.67 ± 4.72%; p < 0.01), however, there was no difference between non-native (26.67 ± 6.47%) and native (28.21 ± 7.32%) rusty crayfish (p = 0.99). On average, non-native crayfish defended themselves more frequently than native crayfish (F1,106 = 4.63, p = 0.03), but this was dependent on sex (F1,106 = 9.93, p < 0.01). Non-native males (50.57 ± 7.32%) defended themselves more than native males (16.67 ± 6.20%; p < 0.001), but there was no difference between non-native (21.11 ± 5.42%) and native (26.67 ± 5.85%) females (p = 0.95). There was also a significant effect of CL (F1,106 = 7.25, p < 0.01; estimated beta coefficient = 0.03), in which larger crayfish displayed a greater proportion of defenses. We did not find significant effects of species (F1,106 = 0.27, p = 0.6), sex (F1,106 = 3.91, p = 0.06), the interaction between species and sex (F1,106 = 0.98, p = 0.32), or the interaction between species, range, and sex (F1,106 = 0.07, p = 0.79) on the proportion of defenses.
Threat response: retreat
Retreat behavior also differed between virile crayfish populations (Fig. 1). There was a significant interaction between species and range on the proportion of retreats (F1,107 = 5.75, p = 0.02). The proportion of retreats did not differ between non-native (57.7 ± 5.97%) and native (51.29 ± 6.73%) rusty crayfish (p = 0.94); however, non-native virile crayfish (33.3 ± 5.49%) retreated less frequently than native virile crayfish (58.89 ± 4.97%; p = 0.04). There was no overall effect of range (F1,107 = 1.91, p = 0.17), species (F1,107 = 3.37, p = 0.07), or sex (F1,107 = 0.54, p = 0.46) on the proportion of retreats. There was also no interaction between species and sex (F1,107 = 0.98, p = 0.32), range and sex (F1,107 = 1.42, p = 0.23), or three-way interaction between species, range, and sex (F1,107 = 0.57, p = 0.45) on the proportion of retreats.
Paired agonistic assay
For both rusty and virile crayfish, crayfish had significantly higher aggression scores in the native treatment (162.97 ± 19.33) than in the Wisconsin treatment (85.62 ± 23.52; F2,98 = 3.99, p = 0.02; Fig. 2), but aggression scores were similar between other population treatments (p > 0.2 for all comparisons). There was no interaction between population treatment and species (F1,99 = 0.259, p = 0.77). Across treatments, rusty crayfish (143.94 ± 16.37) had higher aggression scores than virile crayfish (97.34 ± 16.37; F1,99 = 55.30, p < 0.001) and males (163.48 ± 33.09) had higher aggression scores than females (77.80 ± 15.04; F1,99 = 4.56, p = 0.04). In addition, CL had a positive effect on aggression score (estimated beta coefficient = 7.23; F1,99 = 5.01, p = 0.03). Finally, despite differences in behavior between native and non-native virile crayfish, rusty crayfish won 85.71% (12/14) of the Indiana treatment matchups, 87.50% (14/16) of the Wisconsin treatment matchups, and 82.61% (19/23) of the native treatment matchups.
Chelae size
Rusty crayfish had significantly greater standardized chela areas (7.65 ± 0.37) than virile crayfish (5.44 ± 0.20) (F1,65 = 30.78, p < 0.001). We did not find effects of range (F1,65 = 1.70, p = 0.20) or the interaction between species and range (F1,65 = 0.01, p = 0.92) on standardized chela area.
Mesocosm experiment: survival
We found a significant interaction between species and population treatment on survival (likelihood ratio test: χ2 = 11.70, N = 120, p < 0.01; Fig. 3). There was no difference in survival between native and non-native populations of rusty crayfish (p = 0.3). However, non-native virile crayfish in the Indiana treatment (85 ± 10%) had higher survival than those in the native treatment (15 ± 10%; p < 0.001), but there were no significant differences between the other population treatments (p > 0.2 for all comparisons). Rusty crayfish had higher survival than virile crayfish in the native treatment (85 ± 10% and 15 ± 10%, respectively; p < 0.01) and tended to in the Wisconsin treatment (95 ± 5% and 45 ± 15%; p = 0.06), but survival was similar between native rusty and non-native virile crayfish in the Indiana treatment (80 ± 0% and 85 ± 10%; p = 1.00). Rusty crayfish had significantly higher survival during the experiment (87 ± 4%), compared to virile crayfish (48 ± 11%) (χ2 = 36.87, N = 120, p < 0.001). Further, there was a significant overall effect of population treatment on survival (χ2 = 19.74, N = 120, p < 0.001). On average, crayfish in the Indiana treatment (83 ± 5%) tended to have higher survival than those in the native treatment (50 ± 15%; p = 0.06). There were no differences in survival among the other population treatments (p > 0.1 for all comparisons). Lastly, size increased the likelihood of crayfish survival (χ2 = 5.27, N = 120, p = 0.02).
Mesocosm experiment: growth (CL)
We found a significant interaction between species and population treatment on growth (F2,74 = 9.98, p < 0.001; Fig. 4). There was no significant difference in growth between native and non-native populations of rusty crayfish across the three population treatments (p > 0.4 for all pairwise comparisons). However, non-native virile crayfish from the Indiana treatment (e.g., non-native population) (1.32 ± 0.2 mm CL) grew more than native virile crayfish from the two population treatments with native virile crayfish (native: − 0.45 ± 0.45 mm CL, Wisconsin: 0.11 ± 0.26 mm CL, p < 0.01). Rusty crayfish had greater growth than virile crayfish in the native and Wisconsin treatments (rusty: 1.63 ± 0.19 mm CL, virile − 0.45 ± 0.45 mm CL, p < 0.001 and rusty: 1.21 ± 0.18 mm CL, virile: 0.11 ± 0.26 mm CL, p = 0.01, respectively), but growth was similar between the species in the Indiana treatment (native rusty: 1.11 ± 0.20 mm CL, non-native virile: 1.32 ± 0.20 mm CL, p = 0.9760). Rusty crayfish (1.32 ± 0.11 mm CL) grew more than virile crayfish (0.33 ± 0.19 mm CL) (F1,74 = 20.82, p < 0.001) over the course of the experiment. Further, there was a significant overall effect of population treatment on growth (F2,74 = 4.54, p = 0.01). Specifically, crayfish in the Indiana treatment (1.21 ± 0.14 mm CL) grew significantly more than those in the Wisconsin treatment (0.66 ± 0.16 mm CL; pairwise comparison: p = 0.03) and grew more on average than those in the native treatment (0.59 ± 0.25 mm CL; p = 0.08). Crayfish in the Wisconsin and native treatments had similar growth (p = 0.97). Finally, on average, smaller crayfish grew more than large crayfish (F1,74 = 23.50, p < 0.001).
Mesocosm experiment: growth (g)
We found a significant interaction between species and population treatment on weight gain (F2,75 = 3.35, p = 0.04). There were no significant differences in weight gain among rusty crayfish in different population treatments (p > 0.4). There was a non-significant trend suggesting that non-native virile crayfish from the Indiana treatment (0.59 ± 0.22 g) gained more weight than virile crayfish from the Wisconsin treatment (− 0.18 ± 0.30 g, p = 0.05), but there were no significant differences in weight gain between virile crayfish in the other population treatments (p > 0.8 in both cases). Rusty crayfish gained more weight than virile crayfish in the native and Wisconsin treatments (rusty: 2.10 ± 0.22 g, virile: 0.05 ± 0.52 g, p = 0.01 and rusty: 1.40 ± 0.20 g, virile: − 0.18 ± 0.30 g, p < 0.001, respectively), but weight gain was not significantly different between the congeners in the Indiana treatment (rusty: 1.34 ± 0.22 g, virile: 0.59 ± 0.22 g, p = 0.17). Rusty crayfish (1.60 ± 0.15 g) gaining significantly more weight than virile crayfish (0.15 ± 0.21 g) over the course of the experiment (F1,75 = 38.13, p < 0.001).
Mesocosm experiment: chlorophyll a concentration
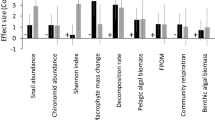
Population treatment had a significant effect on benthic chlorophyll a concentration at the end of the experiment after controlling for survival of virile and rusty crayfish (F2,7 = 15.55, p < 0.01; Fig. 5). Chlorophyll a concentrations were higher in the native treatment (0.10 ± 0.006 mg/cm2) than in either of the treatments containing non-native species (Indiana: 0.08 ± 0.006 mg/cm2, Wisconsin: 0.08 ± 0.007 mg/cm2; Tukey’s HSD: p < 0.01). Virile crayfish survival, but not rusty crayfish survival, was a significant covariate in the model (F1,7 = 37.65, p < 0.001 and F1,7 = 3.19, p = 0.12, respectively).
Mesocosm experiment: benthic macroinvertebrates
There was no effect of population treatment on benthic macroinvertebrate abundance after controlling for survival of virile and rusty crayfish (F2,7 = 0.07, p = 0.93). Rusty crayfish survival, but not virile crayfish survival was a significant covariate in the model (F1,7 = 7.38, p = 0.03, and F1,7 = 0.75, p = 0.41, respectively).
Discussion
In order to investigate divergence during invasions, we used a comparative biogeographical approach to examine trait differences between native and non-native populations of two crayfish species, the rusty crayfish and the virile crayfish, which have independently been introduced into each other’s native ranges. We also tested whether trait differences were strong enough to alter competitive interactions between these different species and their impacts on lower trophic levels. Most of the divergence we observed in behavior, growth and survival was between native and non-native virile crayfish populations, and these intraspecific trait differences were large enough to alter coexistence between virile and rusty crayfish in our mesocosm experiment. These mesocosm results are consistent with patterns of coexistence across the ranges of these two species as virile crayfish are often replaced by rusty crayfish in lakes in the upper Midwest (Capelli and Munjal 1982; Olsen et al. 1991), but we found apparently healthy populations of virile crayfish in sympatry with rusty crayfish in Indiana. Further, differences between native and non-native populations were strong enough to affect the ecological impact of both crayfish species on lower trophic levels. Specifically, benthic chlorophyll a concentration was lower in mesocosms that contained non-native populations of either rusty or virile crayfish compared to mesocosms containing only native populations. Thus, the traits that determine the impacts of these invasive species are not necessarily intrinsic to each species, but instead can vary between native and non-native populations and may arise during the invasion process.
Differences between native and non-native virile crayfish populations were consistent with selection for traits that enhance competition with closely related native species occurring during the invasion process. For example, in response to a predator threat, non-native virile crayfish were more likely to move towards the threat and less likely to retreat than their native counterparts, suggesting that they were bolder and more aggressive in this context. Furthermore, the growth and survival of non-native virile crayfish was significantly higher than that of native virile crayfish and similar to that of the native rusty crayfish when directly competing with them for resources (e.g., Indiana treatment). This is consistent with previous research on non-native crayfish that demonstrated that non-native populations are generally bolder, more aggressive and have higher growth rates, than their native counterparts (Pintor and Sih 2009; Sargent and Lodge 2014; Reisinger et al. 2017). Boldness, aggressiveness and faster growth leading to a larger body size are particularly important traits that underlie the outcome of both intra- and interspecific competition (e.g., more aggressive species are competitively dominant) and are likely key to successfully invading a system with native crayfish (Hill et al. 1993; Pintor and Sih 2009; Reisinger et al. 2017). Previous research in other invasive species suggests that aggression may be important for invaders to replace native species (Holway and Suarez 1999), and aggression can change during the invasion process (Suarez et al. 1999). In addition, boldness may correlate with dispersal ability (Rehage and Sih 2004) and may be selected for during the spread phase of an invasion (Phillips et al. 2010). Here, differences in behavior and growth between native and non-native populations of virile crayfish suggest that the invasion process may have selected for bolder and more aggressive individuals that were better able to compete for resources with native rusty crayfish. Thus, the differences we observed between native and non-native virile crayfish in combination with results from previous studies suggest that traits that facilitate invasion success and competitive displacement of congeners may be selected for during the invasion process (Pintor et al. 2008; Pintor and Sih 2009; Sargent and Lodge 2014; Reisinger et al. 2017).
In contrast, we did not detect any differences in behavior and growth between native and non-native rusty crayfish. This is contrary to previous research suggesting that non-native populations of rusty crayfish have faster growth rates than native populations of this species (Pintor and Sih 2009), and that there is likely a genetic basis for this trait difference (Sargent and Lodge 2014). Our ability to detect differences in growth may have been affected by differences in water temperature, food availability, time of year, or initial crayfish size between this and previous studies. Alternatively, the populations used in this study are different than those used in Pintor and Sih (2009) and Sargent and Lodge (2014), so it is possible that the particular native and non-native rusty crayfish populations we examined here do not differ in growth rate regardless of condition. However, differences in chlorophyll a concentrations between treatments with native and non-native rusty crayfish suggest that there may be some difference in traits related to foraging between native and non-native rusty crayfish that we were unable to detect in this experiment. Overall, these results combined with those from previous research suggest that some native and non-native populations of rusty crayfish may also differ in traits that are likely to enhance invasion success and impacts in the invaded range.
Aggression scores in the native treatment of the paired assay were unexpectedly high, but this may have been an artifact of our experiment. For example, while aggression scores were generally higher in the native treatment than the Wisconsin treatment, when we paired native rusty crayfish with non-native virile crayfish (i.e., Indiana treatment), the native rusty crayfish exhibited a similar level of aggression as their non-native counterparts in the Wisconsin treatment. It is unclear why this occurred, but the native treatment represents a combination of populations that do not naturally co-occur. It is possible that crayfish are more aggressive towards unknown competitors or that the stereotyped behaviors of crayfish from these two populations are mismatched, resulting in escalation of the interaction above that observed in the other two treatments. Another possible explanation involves the mechanism by which crayfish establish and maintain dominance hierarchies. Crayfish communicate dominance status through the use of chemicals present in their urine, a mechanism which is thought to prevent unnecessary energy expenditure and injury in agonistic encounters (Schneider et al. 2001; Moore and Bergman 2005, Bergman et al. 2006). The specific chemical cues used to relay dominance status may vary between populations, and this may have reduced the efficiency with which dominance status was transmitted between individuals, leading to heightened aggression in the native crayfish treatment. Previous studies have shown that if perception of sensory information (and therefore status recognition) in crayfish is reduced or inhibited, it leads to longer, more aggressive agonistic encounters (Bruski and Dunham 1987; Schneider et al. 2001). Despite differences in aggression between different population treatments, rusty crayfish won a similar number of matchups across treatments, so increased aggression in the native treatment did not benefit either congener.
Non-native populations of both crayfish species had greater impacts on lower trophic levels than did native populations, suggesting that trait changes during invasions may contribute to ecological impacts caused by invasive species. Specifically, we found that benthic chlorophyll a concentrations were lower in mesocosms that contained non-native populations of either rusty or virile crayfish compared to mesocosms containing only native populations. Crayfish are generalist omnivores that readily graze on benthic algae (Charlebois and Lamberti 1996). Crayfish also consume and thus inhibit the grazing behavior of snails, which may indirectly affect benthic algae. Although we cannot ultimately ascertain whether differences in chlorophyll a concentrations were primarily due to direct or indirect effects, macroinvertebrate (e.g., snails) abundance remained the same across population treatments suggesting that these differences were more a consequence of direct algal consumption by crayfish. This mechanism is also supported by the finding that virile crayfish survival was negatively related to chlorophyll a concentration. Chlorophyll a concentration was not associated with rusty crayfish survival, which may have been due to the invariably high survival rate of rusty crayfish survival across mesocosms (i.e., there was insufficient variation in rusty crayfish survival to detect a relationship with chlorophyll a concentrations, see Fig. 3). We did not detect differences in growth or survival between native and non-native rusty crayfish in mesocosms, but differences in chlorophyll a concentration between treatments suggest that non-native crayfish of both species were consuming more benthic algae than their native counterparts and therefore having greater ecological impacts. Conversely, the reduced impact of crayfish in the native treatment on chlorophyll a concentration may be the result of the same increased competition intensity that we observed in the paired assays. Other studies (e.g., Berger-Tal et al. 2015) have found that interference competition can reduce foraging time and efficiency, so if native rusty and virile crayfish spent more time fighting with each other than either would have against a non-native congener, they may have had less opportunity to graze on periphyton.
In contrast to the chlorophyll a results, benthic invertebrate abundance was similar among the different population treatments, but there was a negative relationship between rusty crayfish survival and macroinvertebrate abundance. This finding corroborates previous research showing large impacts of rusty crayfish on macroinvertebrate communities (e.g., Charlebois and Lamberti 1996; Perry et al. 2000; McCarthy et al. 2006). In contrast, there was no association between virile crayfish survival and macroinvertebrate abundance. This may be due to exploitative competition by rusty crayfish leading to resource partitioning between these species (i.e., rusty crayfish fed on macroinvertebrates and virile crayfish fed on benthic algae). In a similarly designed experiment, Glon et al. (2017) housed non-native rusty and native virile crayfish in sympatry and examined their respective abilities to exploit invasive dreissenid mussels and benthic algae associated with the mussels. In the experiment, rusty crayfish growth increased with the addition of dreissenid mussels, but virile crayfish growth was not affected, suggesting that rusty crayfish had a greater ability to exploit the mussels and associated algae as a food resource than virile crayfish. Similar to the results in our study, Glon et al. (2017) also found that native virile crayfish had low growth and survival in sympatry with non-native rusty crayfish. Overall, our results suggest that non-native virile crayfish growth and survival was likely enhanced by greater consumption of algae, but not macroinvertebrates.
Despite evidence that non-native virile crayfish were bolder and more aggressive in response to a threat and had higher survival and growth than their native counterparts, they remained competitively inferior to both native and non-native rusty crayfish in the paired agonistic assay. This result suggests that rusty crayfish may inherently be a better competitor than virile crayfish. One trait that might contribute to their competitive dominance may be the larger chelae of the rusty crayfish. Chelae are used by crayfish for numerous activities including mating, feeding, and predator defense (e.g., Stein 1976), and chela size is a key determiner of success in competitive interactions (Rutherford et al. 1995; Gherardi et al. 1999; Moore 2007). Indeed, the consistently larger chelae of rusty crayfish relative to those of virile crayfish are thought to have contributed to the former’s invasion success in Wisconsin lakes, both by increasing success of rusty crayfish in competitive interactions with virile crayfish and by leading to disproportionate predation of fish on virile crayfish due to their smaller chelae (Garvey and Stein 1993; Garvey et al. 1994).
Our experimental design does not enable us to definitively tie the differences in growth and behavior between native and non-native virile crayfish to a specific causal mechanism. Because we collected crayfish from their natal environments rather than raising them from birth in the lab, we are unable to determine whether the differences we observed are due to genetic differences or to phenotypic plasticity. The differences we observed are consistent with the hypothesis that the invasion process selects for traits that allow non-native species to overcome biotic resistance. However, genetic differences between populations could also be due to chance events such as a founder effect or genetic drift. In addition, differences in the environment (e.g., predator abundance, water temperature, food availability) and habitat types (e.g., stream vs. lake) in Indiana and Wisconsin could select for different crayfish traits, but we might have expected to see similar changes in rusty crayfish traits if the environment or habitat was the primary driver of differences in virile crayfish traits. Another possible explanation is that the source population for the Indiana (non-native) virile crayfish was different than the native population we tested and individuals already possessed different traits upon introduction. Lastly, time since introduction has been shown to play a role in invasion success (e.g., Pyšek et al. 2009). Unfortunately, we have not been able to obtain data tracking the invasion of virile crayfish in Indiana, so we were unable to consider the possible influence of this mechanism in our study. Future research comparing additional native and non-native crayfish populations could help elucidate the mechanism responsible for the divergence we observed between populations. Regardless of the mechanism, the intraspecific differences we measured were large enough to alter the impacts of both of crayfish species on lower trophic levels and the ability of virile crayfish to persist in sympatry with rusty crayfish in mesocosms.
The results of our study highlight the need to consider how evolution and/or plasticity may alter ecologically important traits of organisms during an introduction. Even though we do not know the mechanism behind the divergence we observed, the differences between native and non-native crayfish populations were important enough to meaningfully alter ecological interactions. Given that virile crayfish are often either entirely extirpated or at least excluded from high quality habitat by rusty crayfish in lakes in the upper Midwest (Capelli and Munjal 1982; Olsen et al. 1991; Peters and Lodge 2013), one might not expect virile crayfish to become established in the native range of rusty crayfish and coexist with the latter, yet this scenario is currently occurring in the streams that we sampled in southern Indiana. Our findings set the stage for further investigations into trait differences between different native and non-native populations, which could provide evidence for the causal mechanism behind the trait differences we observed and identify traits that are important for invasion success in locations with ecologically similar native species. Biological invasions are an ongoing global threat causing ecological and economic impacts, and our results suggest that including the potential for traits to change during the invasion process may be essential for predicting the effects of invasions, and thus should be taken into account when weighing the costs and benefits of moving species.
References
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv 1406.5823
Berger-Tal O, Embar K, Kotler BP, Saltz D (2015) Everybody loses: intraspecific competition induces tragedy of the commons in Allenby’s gerbils. Ecology 96:54–61. https://doi.org/10.1890/14-0130.1
Bergman D, Moore PA (2003) Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol Bull 205:26–35. https://doi.org/10.2307/1543442
Bergman DA, Kozlowski CP, McIntyre JC, Huber R, Daws AG, Moore PA (2003) Temporal dynamics and communication of winner-effects in the crayfish, Orconectes rusticus. Behaviour 140(6): 805–825
Bergman DA, Redman CN, Fero KC, Simon JL, Moore PA (2006) The impacts of flow on chemical communication strategies and fight dynamics of crayfish. Mar Freshw Behav Physiol 39:245–258. https://doi.org/10.1080/10236240600980608
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JR, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339. https://doi.org/10.1016/j.tree.2011.03.023
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11. https://doi.org/10.1007/s00442-005-0070-z
Bruski CA, Dunham DW (1987) The importance of vision in agonistic communication of the crayfish Orconectes rusticus. I: an analysis of bout dynamics. Behaviour 103:83–107. https://doi.org/10.1163/156853987X00288
Byers JE (2000) Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81:1225–1239. https://doi.org/10.1890/0012-9658(2000)081[1225:CBTESI]2.0.CO;2
Capelli G, Munjal BL (1982) Aggressive interactions and resource competition in relation to species displacement among crayfish of the genus Orconectes. J Crustac Biol 2:486–492. https://doi.org/10.1163/1937240X82X00176
Charlebois PM, Lamberti GA (1996) Invading crayfish in a Michigan stream: direct and indirect effects on periphyton and macroinvertebrates. J N Am Benthol Soc 15:551–563. https://doi.org/10.2307/1467806
Crandall KA, De Grave S (2017) An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J Crustac Biol 37:615–653. https://doi.org/10.1093/jcbiol/rux070
Englund G, Krupa JJ (2000) Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshw Biol 43:75–83. https://doi.org/10.1046/j.1365-2427.2000.00524.x
Garvey JE, Stein RA (1993) Evaluating how chela size influences the invasion potential of an introduced crayfish (Orconectes rusticus). Am Midl Nat 129:172–181. https://doi.org/10.2307/2426446
Garvey JE, Stein RA, Thomas HM (1994) Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75:532–547. https://doi.org/10.2307/1939556
Gherardi F, Barbaresi S, Raddi A (1999) The agonistic behaviour in the red swamp crayfish, Procambarus clarkii: functions of the chelae. Freshw Crayfish 12:233–243
Gioria M, Osborne BA (2014) Resource competition in plant invasions: emerging patterns and research needs. Front Plant Sci 5:1–21. https://doi.org/10.3389/fpls.2014.00501
Glon MG, Larson ER, Reisinger LS, Pangle KL (2017) Invasive dreissenid mussels benefit invasive crayfish but not native crayfish in the Laurentian Great Lakes. J Great Lakes Res 43:289–297. https://doi.org/10.1016/j.jglr.2017.01.011
Green PT, Lake PS, O’dowd DJ (2004) Resistance of island rainforest to invasion by alien plants: influence of microhabitat and herbivory on seedling performance. Biol Invasions 6:1–9. https://doi.org/10.1023/B:BINV.0000010144.12808.cb
Grosholz ED, Ruiz GM (2003) Biological invasions drive size increases in marine and estuarine invertebrates. Ecol Lett 6:700–705. https://doi.org/10.1046/j.1461-0248.2003.00495.x
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15. https://doi.org/10.1111/j.0022-0477.2004.00953.x
Hill AM, Lodge DM (1999) Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Appl 9:678–690. https://doi.org/10.1890/1051-0761(1999)009[0678:RORCBA]2.0.CO;2
Hill AM, Sinars DM, Lodge DM (1993) Invasion of an occupied niche by the crayfish Orconectes rusticus: potential importance of growth and mortality. Oecologia 94:303–306. https://doi.org/10.1007/BF00317102
Holway DA, Suarez AV (1999) Animal behavior: an essential component of invasion biology. Trends Ecol Evol 14:328–330. https://doi.org/10.1016/S0169-5347(99)01636-5
Hulme PE, Bacher S, Kenis M, Klotz S, Kühn I, Minchin D, Nentwig W, Olenin S, Panov V, Pergl J, Pyšek P, Roques A, Sol D, Solarz W, Vilà M (2008) Grasping at the routes of biological invasions: a framework for integrating pathways into policy. J Appl Ecol 45:403–414. https://doi.org/10.1111/j.1365-2664.2007.01442.x
Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105:405–412. https://doi.org/10.1007/BF00328744
Human KG, Gordon DM (1999) Behavioral interactions of the invasive Argentine ant with native ant species. Insectes Soc 46:159–163. https://doi.org/10.1007/s000400050
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714. https://doi.org/10.1111/j.1461-0248.2005.00769.x
Kilian JV, Becker AJ, Stranko SA, Ashton M, Klauda RJ, Gerber J, Hurd M (2010) The status and distribution of Maryland crayfishes. Southeast Nat 9:11–32. https://doi.org/10.1656/058.009.s302
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: tests in linear mixed effects models. R package version 2.0-33
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391. https://doi.org/10.1016/S0169-5347(02)02554-5
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Lodge DM, Kratz TK, Capelli GM (1986) Long-term dynamics of three crayfish species in Trout Lake, Wisconsin. Can J Fish Aquat Sci 43:993–998. https://doi.org/10.1139/f86-122
Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, Carlton JT, McMichael A (2006) Biological invasions: recommendations for US policy and management. Ecol Appl 16:2035–2054. https://doi.org/10.1890/1051-0761(2006)016[2035:BIRFUP]2.0.CO;2
Mccarthy JM, Hein CL, Olden JD, Jake Vander Zanden M (2006) Coupling long-term studies with meta-analysis to investigate impacts of non-native crayfish on zoobenthic communities. Freshw Biol 51:224–235. https://doi.org/10.1111/j.1365-2427.2005.01485.x
Moore PA (2007) Agonistic behavior in freshwater crayfish: the influence of intrinsic and extrinsic factors on aggressive behavior and dominance. In: Emmett DJ, Thiel M (eds) Evolutionary ecology of social and sexual systems: crustacea as model organisms. Oxford University Press, Oxford, pp 90–114
Moore PA, Bergman DA (2005) The smell of success and failure: the role of intrinsic and extrinsic chemical signals on the social behavior of crayfish. Integr Comp Biol 45:650–657. https://doi.org/10.1093/icb/45.4.650
Olden JD, McCarthy JM, Maxted JT, Fetzer WW, Vander Zanden MJ (2006) The rapid spread of rusty crayfish (Orconectes rusticus) with observations on native crayfish declines in Wisconsin (USA) over the past 130 years. Biol Invasions 8:1621–1628. https://doi.org/10.1007/s10530-005-7854-2
Olsen TM, Lodge DM, Capelli GM, Houlihan RJ (1991) Mechanisms of impact of an introduced crayfish (Orconectes rusticus) on littoral congeners, snails, and macrophytes. Can J Fish Aquat Sci 48:1853–1861. https://doi.org/10.1139/f91-219
Perry WL, Lodge DM, Lamberti GA (2000) Crayfish (Orconectes rusticus) impacts on zebra mussel (Dreissena polymorpha) recruitment, other macroinvertebrates and algal biomass in a lake-outlet stream. Am Midl Nat 144:308–316. https://doi.org/10.1674/0003-0031(2000)144[0308:CORIOZ]2.0.CO;2
Peters JA, Lodge DM (2013) Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biol Invasions 15:2489–2502. https://doi.org/10.1007/s10530-013-0468-1
Phillips BL, Brown GP, Shine R (2010) Life-history evolution in range-shifting populations. Ecology 91:1617–1627. https://doi.org/10.1890/09-0910.1
Pintor LM, Sih A (2009) Differences in growth and foraging behavior of native and introduced populations of an invasive crayfish. Biol Invasions 11:1895–1902. https://doi.org/10.1007/s10530-008-9367-2
Pintor LM, Sih A, Bauer ML (2008) Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117:1629–1636. https://doi.org/10.1111/j.1600-0706.2008.16578.x
Pyšek P, Křivánek M, Jarošík V (2009) Planting intensity, residence time, and species traits determine invasion success of alien woody species. Ecology 90:2734–2744. https://doi.org/10.1890/08-0857.1
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 1 May 2016
Rehage JS, Sih A (2004) Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions 6:379–391. https://doi.org/10.1023/B:BINV.0000034618.93140.a5
Reisinger LS, Elgin AK, Towle KM, Chan DJ, Lodge DM (2017) The influence of evolution and plasticity on the behavior of an invasive crayfish. Biol Invasions 19:815–830. https://doi.org/10.1007/s10530-016-1346-4
Rubenstein DI, Hazlett BA (1974) Examination of the agonistic behaviour of the crayfish Orconectes virilis by character analysis. Behaviour 50:193–215. https://doi.org/10.1163/156853974X00453
Rutherford PL, Dunham DW, Allison V (1995) Winning agonistic encounters by male crayfish Orconectes rusticus (Girard) (Decapoda, Cambaridae): chela size matters but chela symmetry does not. Crustaceana 68:526–529
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Evol Syst 32:305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Natl Acad Sci USA 100:2474–2477. https://doi.org/10.1073/pnas.0437913100
Sargent LW, Lodge DM (2014) Evolution of invasive traits in nonindigenous species: increased survival and faster growth in invasive populations of rusty crayfish (Orconectes rusticus). Evol Appl 7:949–961. https://doi.org/10.1111/eva.12198
Schneider RAZ, Huber R, Moore PA (2001) Individual and status recognition in the crayfish, Orconectes rusticus: the effects of urine release on fight dynamics. Behaviour 138:137–153. https://doi.org/10.1163/15685390151074348
Seebacher F, Wilson RS (2007) Individual recognition in crayfish (Cherax dispar): the roles of strength and experience in deciding aggressive encounters. Biol Lett 3:471–474. https://doi.org/10.1098/rsbl.2007.0289
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176. https://doi.org/10.1016/S0169-5347(02)02495-3
Stein RA (1976) Sexual dimorphism in crayfish chelae: functional significance linked to reproductive activities. Can J Zool 54:220–227. https://doi.org/10.1139/z76-024
Suarez AV, Tsutsui ND, Holway DA, Case TJ (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invasions 1:43–53. https://doi.org/10.1023/A:1010038413690
Swecker CD, Jones TG, Donahue K II, McKinney D, Smith GD (2010) The extirpation of Orconectes limosus (Spinycheek crayfish) populations in West Virginia. Southeast Nat 9:155–164. https://doi.org/10.1656/058.009.s306
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345. https://doi.org/10.1023/A:1015855019360
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA 97:5948–5953. https://doi.org/10.1073/pnas.100110397
Usio N, Azuma N, Larson ER, Abbott CL, Olden JD, Akanuma H, Takamura K, Takamura N (2016) Phylogeographic insights into the invasion history and secondary spread of the signal crayfish in Japan. Ecol Evol 6:5366–5382. https://doi.org/10.1002/ece3.2286
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. https://doi.org/10.1890/10-0340.1
Wetzel RG, Likens GE (2000) Limnological analysis, 3rd edn. Springer, New York
Wickham H (2009) Ggplot2: elegant graphics for data analysis. Springer, New York
Acknowledgements
We thank the 2016 Central Michigan University (CMU) Great Lakes Summer Research Program students, students in the Pintor Lab at The Ohio State University (OSU), the University of Wisconsin-Madison’s Trout Lake Station Staff, Ying Guy and Rongyi Zhu for help with crayfish collection and husbandry, the CMU Biological Station staff for assistance with mesocosm setup and maintenance, Ray Clark of CMU for assistance with fabrication of experimental equipment, and two anonymous reviewers for their help in preparing this manuscript for publication. Funding provided to LMP and MGG by OSU’s School of Environment and Natural Resources and the Ohio Agricultural Research and Development Center and to LSR by CMU’s Institute for Great Lakes Research. This is contribution 100 of the Central Michigan University Institute for Great Lakes Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Glon, M.G., Reisinger, L.S. & Pintor, L.M. Biogeographic differences between native and non-native populations of crayfish alter species coexistence and trophic interactions in mesocosms. Biol Invasions 20, 3475–3490 (2018). https://doi.org/10.1007/s10530-018-1788-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1788-y