Abstract
Over the last few decades, rainfall has become more variable, with a worldwide increase in the frequency of extreme precipitation events. While increases in rainfall variability are expected to significantly affect plant species, the effects of precipitation dynamics on plant invasions remains understudied. We examined the growth response of Alternanthera philoxeroides to varying water availability at the community level when grown in a monoculture and mixed community with four commonly co-occurring native species. The native species were also grown together as a control to compare to the invasive species communities. Seven water treatments were applied to each community, including a ‘normal’ treatment (based on the average rainfall for the area), drought (water was one third of the normal treatment), flood (water was double the normal treatment), and other simulated extreme events (where water availability was more variable than the normal treatment). All plants were measured for growth (e.g., biomass and stolon length) and competitive traits (i.e., relative competitive dominance and relative interaction indices). Most growth traits in A. philoxeroides were enhanced with increased and/or more variable water availability in both the monoculture and mixed community. In contrast, differences in growth traits in the native plants were mostly non-significant across treatments in each plant community. Compared to the normal treatment, A. philoxeroides had higher relative competitive dominance (RDI) and interaction (RII) indices in response to flooding. Conversely, RII in the native species was not significantly different among treatments. Overall, our results suggest that A. philoxeroides is more responsive to flooding compared to the natives. Our study provides useful insights into growth response and competitiveness of A. philoxeroides to precipitation variability across different plant communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climatic change has been identified as one of the major factors that facilitate the colonization of new areas by invasive species (Chown et al. 2015), and is primarily represented by changes in environmental variability (i.e., alterations in the mean state of climate-dependent environmental conditions, from seasonal changes to variations over a geological timescale, Keyl and Wolff 2008). Such environmental changes can be examined either through an analysis of long-term climatic records or by comparing unusual or extreme climate responses to the typical environmental conditions for a specific ecosystem (Smith 2011). For decades, the field of ecology has used mean values as indicators of climatic site conditions (Jentsch et al. 2007), with previous research often focusing on changes in static average values, and less attention being paid towards dynamic variables (Sisco et al. 2017; Wang et al. 2018). In recent years there has been a worldwide increase in the frequency and magnitude of extreme precipitation events (e.g., very high rainfall and severe droughts, Lehmann et al. 2015). Long-term changes in mean precipitation cause gradual changes in plant establishment and mortality, species composition, and population density (Jin and Goulden 2014); and may result in large-scale alterations in community structure, ecosystem function, and ecotone boundaries (Allen and Breshears 1998; White et al. 2000). In contrast, short-term changes in water availability associated with precipitation variability (including extreme events) can rapidly alter plant physiology, phenology, and leaf growth (Paruelo et al. 1999; Zhang et al. 2005). Precipitation variability may also result in the sudden mortality of populations and extinction of some species (Greenwood et al. 2017).
Changes in precipitation can alter competition for shared resources among native and non-native species (Radford 2013; Schooler et al. 2010). Although much research in invasion biology has focused on the links between environmental variation and invasive species (e.g., Bradley et al. 2010; Parepa et al. 2013), the impacts of precipitation variability (including extreme events) on plant invasions have received less attention (Sardans et al. 2017). Previously it was found that invasive Commelinaceae species displayed higher average performance (e.g., biomass and relative growth rates) compared to native species in the same family for both high nutrients and across water availabilities (Burns 2004). Moreover, Chen et al. (2019) and Wang et al. (2016) found that invasive clonal plants in a native community benefited more from clonal integration under variable water availability compared to consistent watering. Based on these findings, invasive species may be expected to display greater growth and performance under more variable precipitation compared to native species. However, in secondary tropical forests in the Seychelles, only small differences in growth response of native and invasive species to different light and water treatments were found, with the native species performing better under low water availability (Schumacher et al. 2008). Another study revealed that biomass of invasive species did not differ significantly across water treatments (Han et al. 2012). Therefore, further research on how invasive plant species cope with hydrological fluctuations compared to native species is necessary for a more comprehensive understanding of the response of invasive species to varying water availability in native landscapes (Wang et al. 2018). Specifically, research focusing on the impacts of extreme precipitation events (which may result in high levels of disturbance) on invasive plants and co-occurring native species is likely to offer insights into how communities may alter under climate change.
Habitat disturbance is one of the most important variables involved in shaping life-history traits and influences the survival strategies of organisms in ecosystems with highly fluctuating environmental conditions (Gerisch et al. 2012). Disturbance can be defined as an event that disrupts any ecological level, environmental component, and/or organizational status of a biological cycle of organisms (and may include extreme precipitation events, such as floods and severe droughts, Pickett et al. 1989; Battisti et al. 2016). The strength and direction of feedback loops are influenced by external temporal factors, including changes in soil resource availability (e.g., soil moisture) following disturbance (Kardol et al. 2013). For example, increased disturbance is often associated with greater environmental variability (Parepa et al. 2013), and high levels of disturbance have been found to promote biological invasions (Hobbs and Huenneke 1992). The invasibility of a habitat can be enhanced due to disturbance since new sites are cleared and opened up for other species to colonise (Davis et al. 2000; Reznick et al. 2020). Therefore, initial disturbance can promote invasions, which then further disturb the ecosystem. High levels of disturbance can lead to environments becoming increasingly less hospitable for native species (Davidson et al. 2011; Gaertner et al. 2017). Invasive species often display higher phenotypic plasticity than native plants (Liu et al. 2017; Parepa et al. 2013), and as such, disturbance resulting from fluctuating environmental conditions may provide an advantage for non-native species (Gaertner et al. 2017). Consequently, invasive species might be expected to show enhanced competitiveness in response to extreme events. In the current study, we define competition as occurring when individuals of the same or different species (e.g., neighbouring plants) utilize common resources (e.g., light, nutrients, water, space) (Birch 19,757; Milne 1961; Grime 1973). As such, interspecific competition often results in the negative effect of one species on another due to limited shared resources (Birch 1957; Craine 2005), and therefore a good competitor might be one that: (1) exerts a strong negative effect on other species, and (2) has limited response to the presence of other species.
Here, we examine the effects of precipitation variability on the growth response of alligator weed (Alternanthera philoxeroides) at the community level. To do this we grew A. philoxeroides in a monoculture and in a mixed community (with four commonly co-occurring species native to China) to compare differences in growth traits among seven water treatments, including drought, flooding, as well as simulated precipitation events (e.g., alternate drought and flood). Additionally, we grew the native species together (to simulate a native species community) and applied the same seven water treatments. As such, we tested the following hypotheses: (1) invasive A. philoxeroides will display enhanced growth in response to precipitation variability, (2) growth trait response of A. philoxeroides and the native species will vary among plant communities (i.e., invasive species only, native species only, and mixed community), and (3) competitiveness of A. philoxeroides will increase in response to higher precipitation variability.
Materials and methods
Study species and plant propagation
Alternanthera philoxeroides is a creeping perennial, stoloniferous, and amphibious herb native to South America (Spencer and Coulson 1976), and has become an aggressive alien invader in 32 countries around the world (Tanveer et al. 2018). The species was introduced to Shanghai as a forage crop in the 1930s and is now widely distributed in over 20 provinces in China (Huang et al. 2017; Yan et al. 2020). Alternanthera philoxeroides disperses rapidly and occurs in aquatic, semi-aquatic, and terrestrial environments, mainly because each stolon consists of nodes that are capable of producing roots and new shoots, which can become new individual plants if disconnected (Xi et al. 2019).
To assess the response of A. philoxeroides to precipitation variability compared to native species from China, we selected four native species to simulate a local community, namely Eleusine indica, Solanum nigrum, Cynodon dactylon, and Youngia japonica (Fig. 1a). During our previous field surveys in Jiangsu Province, we found that these native species often co-exist in the same ecosystem with A. philoxeroides. Moreover, a recent species diversity study based on 59 plots across 10 provinces in China revealed that these four native species co-occur with A. philoxeroides (Wu et al. 2016). Communities where the native species co-occur with A. philoxeroides are primarily associated with aquatic habitats, but can spread into moist terrestrial environments (Schooler et al. 2010).
Experimental design showing: (a) number of species per plant community, and number of replicates in each community and water treatment combination; (b) water treatments; and (c) data analysis design for the effects of water treatment and plant community (i.e., plant pattern). Plant communities in (a) include: P1 (invasive species only), P2 (native species only community), and P3 (mixed plant community comprising both native and invasive species). Water treatments in (b) include: (i) normal (N), (ii) drought (D), (iii) flood (F), (iv) flood-drought interaction (D → F → D → F), (v) drought pulse (N → D → N), (vi) flooded pulse (N → F → N), and (vii) multiple pulses (N → F → N → D → N). Each plot in (b) represents the amount of water (mL) applied every five days during the 65 days of the experiment, with the total amount of water being based on the frequency and amount of rainfall at an interval of five days from 1st October to 5th December between 1950 and 2015 (sourced from the Nanjing weather station). The coefficient of variation (CV) of each treatment (%) is also shown in (b). The data-analysis design in (c), shows a factorial study with dummy variable coding (× 11- × 73) which represents the plant trait values and their corresponding contrasts (contrasts are only between water treatments within communities or between communities within water treatment)
In early autumn (September 2016), healthy and uniform stems of the invasive species (i.e., cuttings) and seeds of the four native species were sampled from natural wetland habitat along the Yangtze River (119°32′E, 32°11′N) and transplanted to a greenhouse at Jiangsu University (119°27′E, 32°12′N, Zhenjiang, Jiangsu Province, China). To investigate differences in plant responses to fluctuations in water supply between the invasive and native plants, 70 similar-sized seedlings per species were selected and three plant communities were established: P1 comprising a monoculture of the invasive species only (A. philoxeroides), P2 which was a native species only community (with four species native to China), and P3 which comprised a mixed community of invasive A. philoxeroides and the four native species (Fig. 1). We transplanted one seedling of each species into plastic pots (30 cm × 20 cm × 8 cm, length × width × height respectively) filled with straw, sand, vermiculite, peat, and fine vermiculite (at a ratio of 1:2:2:3:2). Plants were grown from autumn until early winter (1st October to 5th December) 2016. Due to limited availability of space in the greenhouse, our experiment had to be conducted over a shorter time-period than we initially intended. However, during extreme events, plants are often exposed to higher or lower resource availability at a large magnitude and for short periods of time (Yang et al. 2008). A plant’s fitness can depend on its ability to utilize resources during such events (e.g., flooding, drought) especially in low resource environments (Funk and Zachary 2010). In addition, many studies have demonstrated that invasive species display higher plasticity compared to natives in response to environmental variability on time scales of weeks to months (e.g., Burns and Winn 2006; Muth and Pigliucci 2007). In the case of our study, A. philoxeroides is a fast-growing clonal species, and individuals can become very large when grown under the right conditions. Therefore, given that a major focus of our study is on extreme events and A. philoxeroides is a fast-growing invader, we consider two months an appropriate timeframe for exploring the early growth response of this species to precipitation variability and extreme events.
Precipitation variability and water treatments
One week after transplanting the seedlings, we applied the water treatments. We obtained daily precipitation data from the China Meteorological Administration database (http://data.cma.cn/, accessed September 25, 2016). From this dataset, we selected daily precipitation data from the Nanjing weather station for the growing period of the experiment (autumn to early winter 2016) from 1950 to 2015. Specifically, we focused on the frequency of precipitation at an interval of five days from 1st October to 5th December. We calculated the mean precipitation at five-day intervals based on the frequency of rainfall over the 65 years between 1950 to 2015 (i.e., 1468.92 mL in total, with the following precipitation values determined according to the amount of rainfall over five days: 108.95 mL, 207.55 mL, 186.73 mL, 123.63 mL, 108.41 mL, 164.96 mL, 88.19 mL, 75.33 mL, 74.30 mL, 92.79 mL, 71.22 mL, 51.76 mL, and 115.10 mL) and this was used as the basis for the ‘normal’ water treatment (N) in our study [see (i) in Fig. 1]. For the drought treatment (D), we applied one-fifth of the normal water treatment [see (ii) in Fig. 1]. Plants treated with this amount of water in the D treatment were water-stressed, although the amount of water was sufficient to maintain plant survival. For the flood treatment (F), we used twice the amount of water as the normal water treatment [see (iii) in Fig. 1]. In addition, we had four water treatments that simulated more than one precipitation event. These included a flood and drought interaction, where we applied water simulating alternating drought (D) and flood (F) events (i.e., D → F → D → F); drought pulse treatment, which alternated between normal and drought treatments (N → D → N); flooded pulse treatment, alternating between normal and flood treatments (N → F → N); and multiple pulses treatment (N → F → N → D → N) (iv, v, vi, and vii respectively from Fig. 1). As such, there were seven water treatments in our experiment (see Fig. 1b for specific details). Tap water was used and the water volume was measured out using a measuring cylinder. Water was supplied to the plants through an artificial spray, which was applied 13 times (once every 5-days) during the growing period (on the 3, 8, 13, 18, 23, and 28 October; 3, 8, 13, 18, 23, and 28 November; and 3 Dec 2016). To categorize the degree of variability of the water treatments, we calculated the coefficient of variation (CV) for each treatment (these are given in Fig. 1b).
We used a two-factor study design with 5 replicates per community per treatment. There was a total of 105 pots (3 plant communities × 7 water treatments × 5 replicates) that were arranged in the greenhouse. Pots were spaced at least 20 cm apart from each other to avoid cross-contamination, and pot position was randomized every two weeks to counteract the effects of environmental patchiness within the greenhouse.
Growth trait measurements
All the plants were harvested after 65 days (on the 5th December 2016), which was after the plants had stabilised and displayed vigorous growth. We measured the main stolon length of each plant of A. philoxeroides using a ruler and counted the number of nodes. The diameter of each plant was measured at three points along the stolon (the apex, middle, and terminal points) using a digital Vernier caliper (MNT, Shanghai, China). The root fractions were carefully removed from the soil and thoroughly rinsed using tap water. All plant parts were oven-dried at 60 °C to constant mass and weighed to the nearest 0.01 g (Portela et al. 2019). The four native species were considered together when calculating the native species biomass (and this was measured using the same approach as for A. philoxeroides). Finally, the root to shoot (root/shoot) ratio of plants in each treatment for all species was calculated.
Competition and invasion efficacy in the mixed community
The proportion of invasive plant biomass to total plant biomass can be used as an indicator for invasion efficacy in the vegetation community (Parepa et al. 2013; Wang et al. 2021). We calculated the dominance of A. philoxeroides in only the mixed community using the relative dominance index (RDI) (Myers and Bazely 2003), calculated as follows:
In the case of A. philoxeroides, RDI is the proportion of community biomass represented by A. philoxeroides, and ranges between 0 and 1, with a high RDI (i.e., values closer to 1) indicating that the focal species is highly competitive. In our study, RDI values above 0.6 are considered relatively high (see Zhang et al. 2017). We used the relative interaction index (RII) (Armas et al. 2004; Uddin and Robinson 2018) to characterise interspecific competition (i.e., native versus invasive species in the mixed community) under different precipitation treatments, which was calculated as:
For RII, Bw is the biomass of the focal plant growing with the neighbouring plants, and Bo is the biomass of the focal plant growing in the monoculture. So, when calculating RII for the invasive species, Bw represented the biomass of A. philoxeroides in the mixed community, while Bo was the biomass of A. philoxeroides in the monoculture. For the native species, the four native species were considered together when calculating RII. Therefore, Bw represented the biomass of the native species in the mixed community, while Bo was the biomass of native species in the monoculture. The RII is a metric of interaction intensity, and ranges between −1 and 1. A negative RII indicates that competition prevails, a positive value suggests the prevalence of facilitation, and a value of 0 represents when the net balance of the interaction is neutral (Cavieres et al. 2017). In our study, RII values above 0.1 were considered to be high (see Tirado and Pugnaire 2005; Anthelme et al. 2012; Schöb et al. 2013).
Data analysis
For all growth and competitive traits, differences across treatments were explored using multivariate analysis of variance (MANOVA). Since many of the response variables we measured are likely to be highly correlated, MANOVA was considered appropriate for investigating treatment level differences on all growth traits (as MANOVA enables all response variables to be considered simultaneously, Quinn and Keough 2002). A one-way MANOVA was performed to investigate differences in growth traits across water treatments for each plant community (i.e., A. philoxeroides only community, native species only community, and mixed community). A two-way analysis of variance (ANOVA) was used to examine the effects of water treatment, plant community and their interactions on plant growth traits. In our study, water treatment and plant community were independent variables, while plant growth traits were dependent variables. To further examine competitiveness in A. philoxeroides, we used a one-way MANOVA to investigate differences in RDI and RII across water treatments (N = 5 each for RDI and RII). Tukey’s honest significant difference (HSD) test was applied for multiple comparisons at 0.05 level of significance among the treatments. To determine whether the data met the assumptions of ANOVA, we tested all data for normality using the Shapiro-Wilks test and QQ plots, and for equality of variance with Levene’s test. We used Box's M test of equality of covariance to test whether the variance–covariance matrices assumptions of MANOVA were met. We found that all traits met the normality and variance assumptions of parametric tests. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY, USA).
Results
To compare the level of variability across water treatments, we determined the coefficient of variation (CV) of each treatment (Fig. 1b). The drought treatment was the least variable of all the treatments (CV = 49.26%), followed by the normal and flood treatments (CV values were 49.97% and 50.06% respectively, Fig. 1b). The flood-drought interaction was the most variable of all the treatments (CV = 85.03%), followed by the multiple pulse (CV = 83.07%). Overall, our analyses revealed that for each plant community, growth traits were significantly different across water treatments (p < 0.01, see Table 1 for outputs from one-way MANOVA). We also found that competitive traits significantly varied across the water treatments (p < 0.005, one-way MANOVA, Table 2). For invasive A. philoxeroides, all growth traits were significantly affected by water treatment (p < 0.01, two-way ANOVA, Table 3). In contrast, only stolon length and root/shoot ratio in A. philoxeroides were significantly affected by plant community (p < 0.01, two-way ANOVA, Table 3). For the native species, total biomass was significantly affected by plant community, while root/shoot ratio was significantly affected by water treatment (p < 0.05, two-way ANOVA, Table 3). The interaction between water treatment and plant community had a significant effect on stolon length in A. philoxeroides and root/shoot ratio in the native species (p < 0.05, two-way ANOVA, Table 3).
Effects of water treatments and plant community on growth response of invasive and native species
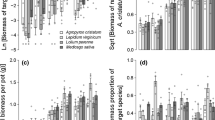
When grown in the monoculture, only biomass, node number and root/shoot ratio of A. philoxeroides significantly varied among water treatments (p < 0.05, Tukey’s HSD test, Fig. 2c, f and g). Conversely, in the mixed community, all growth traits of A. philoxeroides differed significantly among water treatments (p < 0.05, Tukey’s HSD test, Fig. 2). In the mixed community, compared to the normal treatment, stolon length of A. philoxeroides was 37% shorter in the drought treatment; and 32.5%, 27.5%, and 47.5% longer in the flood, flooded pulse and multiple pulse treatments respectively (Fig. 2a). Stolon diameter was significantly wider (p < 0.05, Tukey’s HSD test) in the drought pulse treatment compared with the normal and drought treatments (21.3% and 25% wider respectively, Fig. 2b). Root/shoot ratio in A. philoxeroides was lowest in the flood treatment and highest in the drought treatment irrespective of plant community (Fig. 2g).
Effects of water treatment on growth traits in Alternanthera philoxeroides and native species (Eleusine indica, Solanum nigrum, Cynodon dactylon, and Youngia japonica) across plant communities (N = 5). Significant differences (p < 0.05) among treatments are denoted by different letters above and below the data points (upper case letters are used for the mixed culture, while lower case letters are used for the monoculture). Codes for water treatments (i–vii) are given in Fig. 1
We found that the total biomass of native species was not significantly different across treatments in either the native species only or mixed communities (p > 0.05, Tukey’s HSD test, Fig. 2d). Similarly, root/shoot ratio of native species did not significantly vary among treatments in the mixed culture (Fig. 2e). In the monoculture, root/shoot ratio of native species in the flood-drought interaction, drought pulse and flooded pulse treatments were significantly higher than in the normal and flood treatments (p < 0.05, Tukey’s HSD test, Fig. 2e).
Competition between A. philoxeroides and native species across water treatments
The highest RDI of A. philoxeroides was in the flood treatment, which was significantly greater than the normal and drought treatments (p < 0.05, Tukey’s HSD test, Fig. 3). RII of A. philoxeroides was highest in the flood pulse treatment, which was significantly greater than in the normal and drought treatments (p < 0.05, Tukey’s HSD test, Fig. 3). In contrast, RII of the native species was not significantly different among treatments (Fig. 3). Thus, our findings suggest that while RII of the native species was not influenced by water availability, RII of A. philoxeroides was affected by changes in water level.
Competitive traits in the mixed community (comprising both native and invasive species) showing a the relative dominance index (RDI) or proportion of community biomass of Alternanthera philoxeroides, b relative interaction index (RII) of the native species, and c RII of A. philoxeroides. Codes for water treatments (i–vii) are given in Fig. 1. Significant differences (p < 0.05) among treatments are denoted by different letters above the data points. Values represent the mean ± standard error (SE) (N = 5)
Discussion
Improving our understanding of plant invasions is critical not only because of its importance to invasion management, but also because it may offer insights into ecological patterns and processes (Guo et al. 2015). In the current study, our approach of subjecting different plant communities (invasive species only, native species only and mixed community) to a wide range of water treatments provided important information on growth, competitiveness and resource allocation strategy of A. philoxeroides. Our study offered insights into the effects of invasion from A. philoxeroides on biomass, competitiveness and resource allocation of the native species community. The findings from this study have implications for the response and impacts of invasive A. philoxeroides to precipitation variability under climate change, particularly extreme events, at the community level.
Growth of A. philoxeroides was enhanced in response to precipitation variability and extreme events
We found that precipitation variability and extreme events (e.g., flooding) significantly increased most growth traits of A. philoxeroides compared to the normal treatment, particularly in the mixed community (Fig. 2). Thus, our first hypothesis that this invasive species would display enhanced growth in response to precipitation variability was supported. Stolon length is positively associated with water fluctuations, and increases in this trait may enable plants to escape from canopy shading, thereby facilitating greater light resource acquisition (Chen et al. 2019). Increases in stolon length and node number can result in greater clonal propagation (Xi et al. 2019), which likely provides clonal species with a competitive advantage. Additionally, increases in stolon diameter can boost buoyancy in clonal plants, enhancing their ability to survive in aquatic environments (He et al. 1999), ensuring photosynthesis is maintained during flooding (Ayi et al. 2016). Alternanthera philoxeroides has been found to have higher tolerance and plasticity in response to water fluctuations than native Ludwigia adscendens (Chen et al. 2016). It is likely that under conditions where water availability is variable, the ability of A. philoxeroides to invade a new habitat may be enhanced as the species can increase stolon growth and clonal propagation in response to a range of precipitation events. This notion agrees with Chen et al. (2019), who found that the positive effect of clonal integration on biomass and ramets of A. philoxeroides was stronger under a variable watering regime compared to constant watering.
In contrast to A. philoxeroides, total biomass of the native species from the current study did not vary in response to precipitation variability or extreme events in either plant community (Fig. 2). Our findings for the native species agrees with Chen et al. (2019) who revealed that water variability had a limited effect on biomass in a native plant community. These findings have implications for restoration purposes with climate change impacts and provide a good avenue for future research. Differences in response across native species may account for the limited effects of water variability on total biomass of native plant communities. For the native species, root/shoot ratio was significantly affected by water treatment (Table 3, Fig. 2). Water availability is one of the major limiting factors for plant performance and strongly affects plant allocation strategy (and root/shoot ratio) (Viciedo et al. 2021). Our findings for the native species here are consistent with predications of resource-limitation whereby plants should allocate biomass to structures that help them acquire more of the most limiting resource (Qi et al. 2022).
Growth of invasive and native species and resource allocation under varying precipitation
Our study revealed that while all growth traits in A. philoxeroides were significantly affected by water treatment, fewer traits were significantly affected by plant community (Table 3). Similarly, although native species biomass was significantly different between the mixed- and monoculture, differences in root/shoot ratio of native species between plant communities was not significant (Table 3). Consequently, our second hypothesis that growth trait response of the invasive and native species will vary among plant communities was supported to a lesser extent. Unlike in the mixed community, stolon length and diameter of A. philoxeroides did not significantly vary among water treatments when the species was grown in a monoculture (Fig. 2). In addition, compared to the monoculture, both stolon length and diameter were often greater in the mixed community under the more variable water treatments. Thus, our results suggest that interspecific competition in combination with highly variable water availability affects growth response in A. philoxeroides in mixed communities. This finding agrees with previous research which suggest that the success of an invasive species is dependent on its ability to persist in competition with other species (Bando 2006; Ullah et al. 2021; Wang et al. 2021). The role of interspecific competition with native co-occurring species on the growth performance of invasive A. philoxeroides in response to increasingly variable precipitation provides a promising avenue for future studies.
Plants tend to enhance root growth when soil nutrients and water are limited and allocate less to their root systems when aboveground resources are limiting, maximizing their acquisition of limiting resources (Gill and Finzi 2016; Qi et al. 2022). In our study, we found a significant increase in the root/shoot ratio in response to the drought and flooded pulse treatments compared to the normal treatment in A. philoxeroides in the mixed community (Fig. 2). The higher root/shoot ratios of A. philoxeroides in these treatments in the mixed community suggest that this invasive species is able to alter its biomass allocation to obtain moisture under limiting and variable water availability (Freschet et al. 2018). In contrast, although the species in the natives only community had a significantly higher root/shoot ratio in the flood-drought interaction, drought pulse and flooded pulse treatments than in the normal treatment, in the mixed community this trait did not significantly differ across water treatments (Fig. 2). Our findings are consistent with the results of Wang et al. (2018) which revealed that invasive A. philoxeroides displayed higher root allocation than co-occurring native species in disturbed habitats. It appears that some exotic plants have a superior resource allocation strategy under environmental stress compared to native species. The findings from our study indicate that precipitation variability may favour A. philoxeroides (through an enhanced root system in response to water stress), making it more competitive than the native species used in this study (Gill and Finzi 2016). Our results therefore support the hypothesis that resource allocation strategies can play a major role in the success of this invasive species in limited or disturbed resource environments (Portela et al. 2019; Ren et al. 2019).
Competitive traits and the role of facilitation
Our findings revealed that competitive traits in A. philoxeroides in the mixed community were influenced by variations in water level (Fig. 3). As such, our third hypothesis that competitiveness of A. philoxeroides will increase in response to increased precipitation variability was met. Overall, our results suggest that water variability can improve competitiveness of A. philoxeroides which may mitigate the inhibitory effects from neighbouring species. In contrast, the RII of native species in the mixed community remained unchanged in response to water variation (Fig. 3). This finding could be interpreted in multiple ways. For example, it may indicate that species diversity is equivalent to trait diversity, leading to low overall community response. The result could also suggest that the native species were equally unaffected by the treatments. Growth response measurements collated from each species would offer further insights into the competitive ability of the natives. The mixed community was affected by the presence of invasive A. philoxeroides and previous studies suggest that there is a complex set of interactions between invasive species, native species, and the environment when resources are limiting or variable (Sardans et al. 2017).
Biotic interactions are important drivers of biological invasions (Simberloff and Von Holle 1999), with many studies demonstrating the biotic containment of invaders by native species and greater competitive ability of invasive species (e.g., Levine et al. 2004; Callaway and Aschehoug 2000). Yet, other studies have shown that there can be positive interactions between native and invasive species and these interactions may promote plant invasions (Mitchell et al. 2006; Bulleri et al. 2008). Such biotic interactions can be strongly influenced by changes in environmental conditions (Good et al. 2014). In our study, positive RII values were found for A. philoxeroides under increasing precipitation variability indicating facilitation. Specifically, the highest RII was found for A. philoxeroides in response to the flooded pulse treatment (RII = 0.17, Fig. 3). Positive RII values were also found for the native species in the flooded pulse and multiple pulse treatments (Fig. 3). Thus our findings suggest that the native species are facilitating the invader under variable precipitation. This result is consistent with a previous study where it was revealed that invasive Acer negundo in a floodplain was facilitated in a native Salix community (Saccone et al. 2010). As such, while competition may be important under favourable environmental conditions, facilitation may be more important in more stressful conditions with resident native species facilitating rather than competing with invasive species (Badano et al. 2007; Saccone et al. 2010). Facilitation can be an important driver of plant community structure (Brooker et al. 2008), and facilitative interactions among plants can result in increases in species abundance and diversity (Callaway 2007). In the case of stressful environments, facilitation may be a very important mechanism with established plants providing a beneficial microclimate underneath their foliage and protecting other plants from stressors (Callaway et al. 2002; Good et al. 2014). For example, facilitation in flooded environments may involve some plants ameliorating the anoxic rhizosphere for neighbouring plants via oxygen leakage (Luo et al. 2010). Therefore, facilitation among species may promote biological invasions through changes in environmental conditions (Flory and Bauer 2014), and positive interactions among species may result in an invasive species facilitating further invasions (Simberloff and Von Holle 1999).
Conclusion
We found that invasive A. philoxeroides displayed enhanced growth and competitiveness, and optimised biomass allocation in response to precipitation variability and extreme precipitation events (e.g., flooding). Consequently, A. philoxeroides can increase clonal growth and invest more resources into roots when water availability is variable or under extreme conditions, which likely confers a competitive advantage over native species in the wild. A limitation of our study was that only biomass was measured for the native species (and this measure was pooled for all species). Another limitation of this study is that it was conducted over a relatively short period of time. Future work should involve collating growth trait measurements of each individual native species to enable a species-level comparison, to provide further insights into the invasion of alligator weed in native communities. In addition, further work should apply treatments over a longer time period, include repeat trials, and involve altering water regimes for the extreme events in order to demonstrate clear effects of disturbance on these species. Specifically, conducting an experiment over an entire growing season or over several years may provide information on the demographic impacts of competition and variable water availability. Overall, the findings from our study suggest that highly variable precipitation could facilitate the future spread of A. philoxeroides to new regions.
Data availability
All data generated or analysed during this study are included in this article.
References
Allen CD, Breshears DD (1998) Drought-induced shift of a forest-woodland ecotone: rapid landscape response to climate variation. Proc Natl Acad Sci USA 95:14839–14842
Anthelme F, Buendia B, Mazoyer C, Dangles O (2012) Unexpected mechanisms sustain the stress gradient hypothesis in a tropical alpine environment. J Veg Sci 23:62–72
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Ayi Q, Zeng B, Liu J, Li S, van Bodegom PM, Cornelissen JHC (2016) Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann Bot 118:675–683
Badano EI, Villarroel E, Bustamante RO, Marquet PA, Cavieres LA (2007) Ecosystem engineering facilitates invasions by exotic plants in high-Andean ecosystems. J Ecol 95:682–688
Bando KJ (2006) The roles of competition and disturbance in a marine invasion. Biol Invasions 8:755–763
Battisti C, Poeta G, Fanelli G (2016) (eds) The concept of disturbance. An introduction to disturbance ecology. Springer, Cham, pp 7–12
Birch LC (1957) The meanings of competition. Am Nat 91:5–18
Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH (2010) Predicting plant invasions in an era of global change. Trends Ecol Evol 25:310–318
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JM, Anthelme F, Armas C (2008) Facilitation in plant communities: the past, the present and the future. J Ecol 1:18–34
Bulleri F, Bruno JF, Benedetti-Cecchi L (2008) Beyond competition: incorporating positive interactions between species to predict ecosystem invasibility. PLoS Biol 6:e162
Burns JH (2004) A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers Distrib 10:387–397
Burns JH, Winn AA (2006) A comparison of plastic responses to competition by invasive and non-invasive congeners in the Commelinaceae. Biol Invasions 8:797–807
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Berlin
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Cavieres LA, Sanhueza AK, Torres-Mellado G, Casanova-Katny A (2017) Competition between native Antarctic vascular plants and invasive Poa annua changes with temperature and soil nitrogen availability. Biol Invasions 20:1597–1610
Chen XW, Yu D, Liu CH (2016) Effect of water level fluctuation frequency on Alternanthera philoxeroides, Myriophyllum aquaticum and Ludwigia adscendens in autumn. Chin J Plant Ecol 40:493–501
Chen D, Xiong H, Lin CG, He W, Zhang ZW, Wang H, Wang YJ (2019) Clonal integration benefits invasive alien plants under water variability in a native community. J Plant Ecol 12:574–582
Chown SL, Hodgins KA, Griffin PC, Oakeshott JG, Byrne M, Hoffmann AA (2015) Biological invasions, climate change and genomics. Evol Appl 8:23–46
Craine JM (2005) Reconciling plant strategy theories of Grime and Tilman. J Ecol 93:1041–1052
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Flory SL, Bauer JT (2014) Experimental evidence for indirect facilitation among invasive plants. J Ecol 102:12–18
Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F (2018) Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytol 219:1338–1352
Funk JL, Zachary VA (2010) Physiological responses to short-term water and light stress in native and invasive plant species in southern California. Biol Invasions 12:1685–1694
Gaertner M, Le Maitre DC, Esler KJ (2017) Impact of biological invasions on ecosystem services. Springer, Cham, United States
Gerisch M, Agostinelli V, Henle K, Dziock F (2012) More species, but all do the same: contrasting effects of flood disturbance on ground beetle functional and species diversity. Oikos 121:508–515
Gill AL, Finzi AC (2016) Belowground carbon flux links biogeochemical cycles and resource-use efficiency at the global scale. Ecol Lett 19:1419–1428
Good MK, Clarke PJ, Price JN, Reid N (2014) Seasonality and facilitation drive tree establishment in a semi-arid floodplain savanna. Oecologia 175:261–271
Greenwood S, Ruiz-Benito P, Martinez-Vilalta J, Lloret F, Kitzberger T, Allen CD, Fensham R, Laughlin DC, Kattge J, Bönisch G, Kraft NJ (2017) Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol Lett 20:539–553
Grime JP (1973) Competition and diversity in herbaceous vegetation (reply). Nature 244:311
Guo QF, Fei SL, Dukes JS, Oswalt CM, Iannone BV III, Potter KM (2015) A unified approach for quantifying invasibility and degree of invasion. Ecology 96:2613–2621
Han Y, Buckley YM, Firn J (2012) An invasive grass shows colonization advantages over native grasses under conditions of low resource availability. Plant Ecol 213:1117–1130
He JB, BoGemann GM, Steeg HMVD, Rijnders JGHM, Voesenek LACJ, Blom CWPM (1999) Survival tactics of Ranunculus species in river floodplains. Oecologia 118:1–8
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity and invasion: implications for conservation. Conserv Biol 6:324–337
Huang Y, Ge Y, Wang Q, Zhou H, Liu W, Christie P (2017) Allelopathic effects of aqueous extracts of Alternanthera philoxeroides on the growth of Zoysia matrella. Pol J Environ Stud 26:97–105
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374
Jin Y, Goulden ML (2014) Ecological consequences of variation in precipitation: separating short-versus long-term effects using satellite data. Glob Ecol Biogeogr 23:358–370
Kardol P, De Deyn GB, Laliberté E, Mariotte P, Hawkes CV (2013) Biotic plant–soil feedbacks across temporal scales. J Ecol 101:309–315
Keyl F, Wolff M (2008) Environmental variability and fisheries: what can models do? Rev Fish Biol Fish 18:273–299
Lehmann J, Coumou D, Frieler K (2015) Increased record-breaking precipitation events under global warming. Clim Change 132:501–515
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to toxic plant invasions. Ecol Lett 7:975–989
Liu Y, Oduor AM, Zhang Z, Manea A, Tooth IM, Leishman MR, Xu X, van Kleunen M (2017) Do invasive alien plants benefit more from global environmental change than native plants? Glob Chang Biol 23:3363–3370
Luo W, Xie Y, Chen X, Li F, Qin X (2010) Competition and facilitation in three marsh plants in response to a water-level gradient. Wetlands 30:525–530
Milne A (1961) Definition of competition among animals. In: Milnethorpe FL (ed) Mechanisms in biological competition. University Press, Cambridge
Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, Maron JL, Morris WF, Parker IM, Power AG, Seabloom EW (2006) Biot interact plant invasions. Ecol Lett 9:726–740
Muth NZ, Pigliucci M (2007) Implementation of a novel framework for assessing species plasticity in biological invasions: responses of Centaurea and Crepis to phosphorus and water availability. J Ecol 95:1001–1013
Myers JH, Bazely R (2003) Appendix-Some tools for studying plant populations. In: Collinge SK (ed) Ecology and control of introduced plants. Cambridge University Press, Cambridge, p 255
Parepa M, Fischer M, Bossdorf O (2013) Environmental variability promotes plant invasion. Nat Commun 4:1–4
Paruelo JM, Lauenroth WK, Burke IC, Sala OE (1999) Grassland precipitation-use efficiency varies across a resource gradient. Ecosystems 2:64–68
Pickett ST, Kolasa J, Armesto JJ, Collins SL (1989) The ecological concept of disturbance and its expression at various hierarchical levels. Oikos 1:129–136
Portela R, Barreiro R, Roiloa SR (2019) Biomass partitioning in response to resources availability: a comparison between native and invaded ranges in the clonal invader Carpobrotus edulis. Plant Species Biol 34:11–18
Qi SS, Rutherford S, He FR, Dong BC, Zhu B, Dai ZC, Fu WG, Mao HP, Du DL (2022) Opposing effects of plant growth regulators via clonal integration on apical and basal performance in alligator weed. J Plant Ecol 15:650–662
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, United Kingdom
Radford IJ (2013) Fluctuating resources, disturbance and plant strategies: diverse mechanisms underlying plant invasions. J Arid Land 5:284–297
Ren GQ, Li Q, Li Y, Li J, Adomako MO, Dai ZC, Li GL, Wan LY, Zhang B, Zou CB, Ran Q, Du DL (2019) The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 261:151486
Reznick DN, Bona SD, López-Sepulcre A, Torres M, Bassar RD, Benzen P, Travis J (2020) Experimental study of species invasion: early population dynamics and role of disturbance in invasion success. Ecol Monogr 90:e01413
Saccone P, Pagès JP, Girel J, Brun JJ, Michalet R (2010) Acer negundo invasion along a successional gradient: early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytol 187:831–842
Sardans J, Bartrons M, Margalef O, Gargallo-Garriga A, Janssens IA, Ciais P, Obersteiner M, Sigurdsson BD, Chen HY, Penuelas J (2017) Plant invasion is associated with higher plant-soil nutrient concentrations in nutrient-poor environments. Glob Chang Biol 23:1282–1291
Schöb C, Armas C, Pugnaire FI (2013) Direct and indirect interactions co-determine species composition in nurse plant systems. Oikos 122:1371–1379
Schooler SS, Cook T, Prichard G, Yeates AG (2010) Disturbance-mediated competition: the interacting roles of inundation regime and mechanical and herbicidal control in determining native and invasive plant abundance. Biol Invasions 12:3289–3298
Schumacher E, Kueffer C, Tobler M, Gmür V, Edwards PJ, Dietz H (2008) Influence of drought and shade on seedling growth of native and invasive trees in the Seychelles. Biotropica 40:543–549
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Sisco MR, Bosetti V, Weber EU (2017) When do extreme weather events generate attention to climate change? Clim Change 143:227–241
Smith MD (2011) An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J Ecol 99:656–663
Spencer NR, Coulson JR (1976) The biological control of alligatorweed, Alternanthera philoxeroides, in the United States of America. Aquat Bot 2:177–190
Tanveer A, Ali HH, Manalil S, Raza A, Chauhan BS (2018) Eco-biology and management of Alligator Weed [Alternanthera philoxeroides)(Mart.) Griseb.]: a review. Wetlands 38:1067–1079
Tirado R, Pugnaire FI (2005) Community structure and positive interactions in constraining environments. Oikos 111(3):437–444
Uddin MN, Robinson RW (2018) Can nutrient enrichment influence the invasion of Phragmites australis? Sci Total Environ 613–614:1449–1459
Ullah MS, Sun J, Rutherford S, Ullah I, Javed Q, Rasool G, Ajmal M, Du D (2021) Evaluation of the allelopathic effects of leachate from an invasive species (Wedelia trilobata) on its own growth and performance and those of a native congener (W. chinensis). Biol Invasions 12:1–5
Viciedo DO, Prado RdM, Martinez CA, Habermann E, Piccolo MdC, Hurtado AC, Barreto RF, Calzada KP (2021) Changes in soil water availability and air-temperature impact biomass allocation and C:N: P stoichiometry in different organs of Stylosanthes capitata Vogel. J Environ Manage 278:111540
Wang YJ, Bai YF, Zeng SQ, Yao B, Wang W, Luo FL (2016) Heterogeneous water supply affects growth and benefits of clonal integration between co-existing invasive and native Hydrocotyle species. Sci Rep 6:29420
Wang T, Hu JT, Wang RQ, Liu Ch, Yu D (2018) Tolerance and resistance facilitate the invasion success of Alternanthera philoxeroides in disturbed habitats: a reconsideration of the disturbance hypothesis in the light of phenotypic variation. Environ Exp Bot 153:135–142
Wang CY, Cheng HY, Wang S, Wei M, Du DL (2021) Plant community and the influence of plant taxonomic diversity on community stability and invasibility: a case study based on Solidago canadensis L. Sci Total Environ 768:144518
White TA, Campbell BD, Kemp PD, Hunt CL (2000) Sensitivity of three grassland communities to simulated extreme temperature and rainfall events. Glob Chang Biol 6:671–684
Wu H, Carrillo J, Ding J (2016) Invasion by alligator weed, Alternanthera philoxeroides is associated with decreased species diversity across the latitudinal gradient in China. J Plant Ecol 9:311–319
Xi DG, You WH, Hu AA, Huang P, Du DL (2019) Developmentally programmed division of labor in the aquatic invader Alternanthera philoxeroides under homogeneous soil nutrients. Front Plant Sci 10:485
Yan H, Feng L, Zhao Y, Feng L, Wu D, Zhu C (2020) Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob Ecol Conserv 21:e00856
Yang LH, Bastow JL, Spence KO, Wright AN (2008) What can we learn from resource pulses? Ecology 89:621–634
Zhang X, Friedl MA, Schaaf CB, Strahler AH, Liu Z (2005) Monitoring the response of vegetation phenology to precipitation in Africa by coupling MODIS and TRMM instruments. J Geophys Res Atmos 110:D12103
Zhang H, Chang R, Guo X, Liang X, Wang R, Liu J (2017) Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ Exp Bot 135:118–125
Acknowledgements
We thank Prof. Justin SH Wan for advice on this manuscript. This work was supported by the National Natural Science Foundation of China (32071521), Jiangsu Planned Projects for Postdoctoral Research Funds (2021K384C), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). S. Rutherford is supported by the National Natural Science Foundation of China (32001087) and Jiangsu University Research Foundation (20JDG055). We are grateful to two anonymous reviewers whose comments enabled us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, G., Du, Y., Yang, B. et al. Influence of precipitation dynamics on plant invasions: response of alligator weed (Alternanthera philoxeroides) and co-occurring native species to varying water availability across plant communities. Biol Invasions 25, 519–532 (2023). https://doi.org/10.1007/s10530-022-02931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02931-2