Abstract
As climate variability increases in low-resource environments, the ability of native and invasive species to tolerate stress and respond to large, ephemeral resource pulses will strongly influence plant fitness and, consequently, competitive outcomes. We examined how native and invasive species occurring in arid coastal sage scrub communities in southern California responded to water and high-light stress. We also examined how plants responded to irrigation following short-term water stress. While species responded differently to water and light treatments, no general pattern emerged between native and invasive species. Photosynthetic function of Ricinus communis (invasive) and Salvia mellifera (native) was most robust to water stress and most responsive to irrigation following water stress. Leaf transpiration data suggested that Ricinus and Salvia maintained photosynthetic function by high water use efficiency rather than higher water status via large root biomass. Brassica nigra (invasive) and Encelia californica (native) were more resistant to photoinhibition in response to high-light stress than Ricinus, Salvia, Artemesia californica (native) or Nicotiana glauca (invasive). Our data suggest that native and invasive species in these arid systems display a range of physiological responses to stress and that strategies for invasive species control or native ecosystem restoration based on plant responses to stress may require species-specific approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most widely accepted hypotheses explaining the success of invasive plant species is the fluctuating resource hypothesis, which posits that invasion is facilitated by high resource availability resulting from disturbance or low resource uptake by the native plant community (Davis et al. 2000). Invasive species generally possess physiological, morphological or life history traits that allow them to quickly colonize disturbed areas (e.g., copious small seeds) or grow rapidly in response to high resource availability (e.g., high growth rate, leaf area ratio, photosynthetic rate; Grotkopp et al. 2002; Hamilton et al. 2005; Rejmanek et al. 2005), thereby outperforming native species. However, numerous invasive species inhabit low-resource systems, which are characterized by extreme limitation of light, water or nutrient availability, and few studies have examined the mechanisms by which invasive species succeed in these systems (Baruch and Goldstein 1999; Funk 2008; Funk and Vitousek 2007; Krueger-Mangold et al. 2006; Leishman and Thomson 2005; Muth and Pigliucci 2007; Reed et al. 2005).
Low-resource systems are often temporally or spatially variable with plants receiving resource pulses, defined as episodes of increased resource availability that combine low frequency, large magnitude and short duration (Yang et al. 2008). Plant fitness can depend on utilization of resources during these pulses. For example, it is thought that a large percentage of annual carbon assimilation in desert plants occurs during the few days following each large precipitation event (Loik 2007). A rapid response to water availability is advantageous given the threat of immediate evaporation (particularly from shallow soil layers) and competition for water from other plant species (Chesson et al. 2004; Loik 2007; Noy-Meir 1973). Thus, the ability of species occurring in low-resource environments to respond to resource pulses at physiological (e.g., photosynthesis) and morphological (e.g., increased root growth) levels and on short time scales will strongly influence plant fitness and, ultimately, community composition (Chesson et al. 2004).
Several studies have shown that invasive species are more phenotypically plastic than natives in response to changes in environmental factors on time scales of weeks to months (Burns and Winn 2006; Funk 2008; Gleason and Ares 2004; Muth and Pigliucci 2007; Niinemets et al. 2003; Padgett and Allen 1999; Williams and Black 1994). Fewer studies have examined how native and invasive species respond to shorter-term changes (days) in environmental factors (Durand and Goldstein 2001; Yamashita et al. 2000) and many of these studies have focused on grasses (Huxman et al. 2004; Ignace et al. 2007; Resco et al. 2008). In this study, we conducted two experiments to investigate stress and stress-recovery of native and invasive species over a seven day period. In the first experiment, we examined how species responded to the resumption of irrigation following moderate drought. In the second experiment, we examined how shade-acclimated plants coped with high-light stress. We examined physiological responses indicative of carbon assimilation and stress in plant species that are native to or invasive in southern California coastal sage scrub communities. Sage scrub communities occur throughout California in semi-arid habitats receiving no more than 300 mm of annual precipitation (Rundel and Gustafson 2005). These communities are dominated by short-statured, shallow-rooted shrubs that drop their leaves during the summer drought season, which typically lasts for six months or more.
Our objective was to determine if native species in this arid system would be more stress tolerant than invasive species by maintaining higher rates of carbon assimilation in response to both water stress and high-light stress. Species growing in arid systems commonly experience simultaneous water and high-light stress. As stomata close to reduce transpiration loss, lower intercellular CO2 concentration decreases the activity of the Calvin cycle and, consequently, demand for products of the light-harvesting photosystems and electron transport chain. These compounds have very negative redox potentials and their accumulation in chloroplasts causes photoinhibition as they react with cellular components (proteins, lipids) associated with photosynthesis (Lawlor and Tezara 2009). Additionally, a large proportion of annual rainfall is delivered during multi-day winter and spring storms (http://cdec.water.ca.gov) where light availability can fall below 25% of full sunlight (JL Funk, unpublished data). Thus, the ability to minimize photoinhibition during conditions of high soil water availability following storms is likely to improve plant fitness. Our previous work in arid regions of Hawaii demonstrated that invasive species maintained high carbon assimilation despite low water availability, which may allow them to persist in these low-resource systems; however, tolerance of high-light stress was not examined (Funk and Vitousek 2007). Based on our previous work in Hawaii, we predicted that invasive and native species in this arid system would be similarly tolerant to both water and light stress.
Materials and methods
In February 2008, seedlings of three species native to southern California coastal sage scrub communities (Encelia californica, Artemesia californica, Salvia mellifera) were obtained from a local nursery and seeds for three common invasive species in these vegetation communities (Brassica nigra, Nicotiana glauca, Ricinus communis) were germinated. It is widely thought that rooting depth explains inter-specific patterns of water use across functional groups (Noy-Meir 1973; Weltzin and McPherson 1997, Gebauer and Ehleringer 2000; Schwinning and Ehleringer 2001, Jenerette et al. 2008); thus, we selected co-occurring native and invasive species with similar rooting depths. All native species are perennial subshrubs with fibrous root systems that penetrate less than 2 m into the soil (Hellmers et al. 1955). Nicotiana and Ricinus are perennial shrubs. In its native range, Ricinus has course major roots growing downwards but not exceeding 1.5 m in depth (Comar et al. 2004). Brassica is an annual herb with a fibrous root system not exceeding 1 m in depth. All species employ the C3 photosynthetic pathway and all species co-occur throughout disturbed coastal sage scrub habitat in southern California.
Plants were grown in a 4:1 mixture of potting soil (cactus mix, E.B. Stone Organics) and perlite. For the first two months, all plants were grown in the Chapman University greenhouse where light levels averaged 300 μmol photon m−2 and average daily maximum temperature ranged from 21–38°C. Plants received monthly doses of 7 g of 8-7-6 (N–P–K plus micronutrients, percent by volume) fertilizer (Miracle Gro, The Scotts Company, Marysville, OH). A foliar systemic insecticide was applied monthly (Orthenex, The Ortho Group, Marysville, OH). In April 2008, all plants were moved outside of the greenhouse and exposed to full sunlight (2,000 μmol photon m−2 s−1) and ambient temperature conditions. Plants were allowed to acclimate to these environmental conditions for one month prior to water and light manipulation studies.
Water manipulation
In May, five individuals each of Encelia, Salvia, Nicotiana and Ricinus were randomly placed into one of three treatments: control (watered every day, soil volumetric water content (VWC) 25–30%), stress (VWC < 10%), and stress-recovery (VWC < 10% before watering resumed). Each treatment included five plants per species. Because of the large number of measurements required with three treatments, we only used four of the six species for this experiment. Encelia and Salvia were selected as the two native species because their planar leaves permit easy gas exchange measures. Nicotiana and Ricinus were chosen as the two invasive species because they are most similar to Encelia and Salvia in life form (perennial shrub) and rooting depth. VWC was measured daily with a soil moisture meter (Delta-T HH2 with ML2x probe). For the stress and stress-recovery treatments, water was withheld from plants until VWC was less than 10% (approximately one week). When VWC fell below 10%, initial physiological measures (Day 0) were conducted (see below). Following physiology measurements, irrigation was resumed in the stress-recovery treatment and VWC was maintained at 25–30% for the remainder of the experiment. No natural precipitation occurred during the study period.
One recently mature leaf on each plant was selected for physiology measurements, tagged and repeatedly measured over the course of the experiment. Physiological traits were measured on Day 0 (before and after resuming irrigation for the stress-recovery treatment), and on Days 1, 2 and 7. Photosynthetic capacity (A max, μmol m−2 s−1), transpiration rates (E, mol m−2 s−1), stomatal conductance (g s , mol m−2 s−1) and chlorophyll fluorescence were measured with a LI-6400 portable photosynthesis system with a fluorescence chamber (LI-COR, Lincoln, NE). All measures were conducted at saturating light levels (1,900 μmol photon m−2 s−1), at 400 μL L−1 CO2, at 40–60% relative humidity, and under ambient temperature (26–30°C). The effective quantum yield of PSII (ΦPSII) was calculated as (F m′−F s)/F m′, where F s is the fluorescence yield of a light-adapted leaf and F m’ is the maximal fluorescence during a saturating light flash. Leaf chlorophyll content (Chl, μmol m−2) was determined with a SPAD-502 meter (Spectrum Technologies, IL, USA).
After physiological measures on Day 7, leaves were collected, scanned to determine leaf area, dried at 65°C for 3 days, and weighed to determine leaf mass per area (LMA, g m−2). All plants were then harvested and separated into above- and below-ground biomass. To minimize fine root loss, roots were carefully separated from soil and washed. All material was dried and weighed to determine root to shoot ratio (R:S) and total biomass.
Light manipulation
In May, after plant species had acclimated to 2,000 μmol photon m−2 s−1 conditions for one month, five individuals each of Encelia, Artemesia, Salvia, Brassica, Nicotiana, and Ricinus were moved to the greenhouse (300 μmol photon m−2 s−1) for one week. At the end of the week (Day 0), measures of A max, E, ΦPSII, and Chl were conducted as described above. In addition, pre-dawn fluorescence measurements were taken to assess the fraction of absorbed photons that are used for photochemistry (F v /F m ) which was calculated as (F m−F o/F m), where F o is the fluorescence in total darkness and F m is fluorescence of a dark-adapted leaf during a saturating light flash. F v /F m is around 0.8 for healthy plants, with smaller values indicating photoinhibition (Cavender-Bares and Bazzaz 2004). Photoinhibition is caused when more energy is generated by light harvesting than can be used by the Calvin cycle. Severe photoinhibition over a long time period can lead to the production of highly reactive free oxygen radicals, which degrade photosynthetic components.
Because of the various patterns of branching and leaf initiation employed by the six species, stem diameter at the base of the plant was used as a measure of plant productivity. While we did not conduct allometric measures for these species, stem diameter is highly correlated with total plant biomass in many species (Coyle and Coleman 2005; Monclus et al. 2006; Yamashita et al. 2000). After Day 0 measures, plants were returned to a high-light environment (2,000 μmol photon m−2 s−1) and the physiological and growth traits were measured again on Day 1, 2 and 7.
Statistical analysis
The influence of light stress, water stress, and irrigation following water stress on physiological and morphological variables was determined by planned (a priori) orthogonal contrasts among treatments within species at Day 0 and Day 7 and among species within a treatment. Data that violated the ANOVA assumptions of normality were Box Cox transformed. Following Quinn and Keough (2002); Moran (2003); Gotelli and Ellison (2004), sequential Bonferroni corrections for multiple statistical tests were not conducted. Because we lacked enough degrees of freedom to contrast native and invasive species, we discuss how individual species responded to light and water treatments. All analyses were performed in JMP 8.0 (SAS Institute Inc., Cary, NC).
Results
Water manipulation
Volumetric water content for plants in the control treatment was maintained between 25–30% for all species (Fig. 1). VWC for plants in the stress and stress-recovery treatments fell below 10% at the beginning of the experiment (Day 0) and VWC quickly returned to control levels after irrigation was resumed in the stress-recovery treatment. VWC for plants in the stress treatment was maintained at 10% for the duration of the experiment.
Soil volumetric water content (VWC), leaf photosynthetic rate, leaf transpiration rate, and the effective quantum yield of PSII in control (closed circles), stress-recovery (open circles), and stress (closed triangles) treatments for two native (Encelia californica, Salvia mellifera) and two invasive species (Nicotiana glauca, Ricinus communis) over seven days (n = 5 individuals per species). For the stress-recovery treatment, plants were initially water stressed (VWC < 10%) and irrigation was resumed on Day 0. VWC was maintained at 10% in the stress treatment for the duration of the experiment. Data are means and standard errors. Significant differences (P < 0.05) between the control and stress treatment (S) and the control and stress-recovery treatment (R) are shown for each species
Physiological and morphological traits differed among the four species examined and across the three treatments (Figs. 1, 2) with the exception of Chl content, which did not vary across treatments (data not shown). Encelia displayed the highest A max and ΦPSII, followed by Salvia and Ricinus. Nicotiana had the lowest A max and ΦPSII (Fig. 1). However, while Encelia displayed the highest A max under control conditions, A max fell to less than 18% of control values when water stressed (Fig. 1).
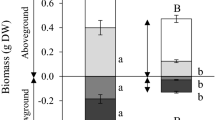
Leaf morphological and plant-level growth data for two native and two invasive species in control, water stress (stress), and stress-recovery treatments after 7 days. See text for description of treatments. Data are means and standard errors. Asterisks denote statistically significant differences between stress treatments and the control treatment (P < 0.05)
All traits except Chl content were lower in stressed plants relative to control plants at Day 0 (Fig. 1). However, traits in some species recovered to control values by Day 7 despite the maintenance of VWC < 10%. In Encelia, Salvia and Ricinus, ΦPSII recovered over the 7-day experiment. Similarly, A max recovered to control values in Salvia and Ricinus, although g s and E remained low in Ricinus (high water use efficiency). Only Ricinus showed changes in morphological traits following prolonged water stress. Stressed Ricinus plants had lower biomass and higher LMA relative to control plants following the 7-day measurement period (Fig. 2).
Photosynthetic function (A max, ΦPSII) recovered in three of the four species when irrigation was resumed (Fig. 1). Ricinus and Salvia recovered to levels of the control treatment by Day 1 while Encelia recovered by Day 7 (Fig. 1). Photosynthetic function in Nicotiana never recovered in the stress-recovery treatment. After resuming irrigation at Day 0, g s and E recovered to control-level values in all species by Day 7 (Fig. 1). Species in the stress-recovery treatment showed no morphological changes relative to control plants (Fig. 2).
Light manipulation
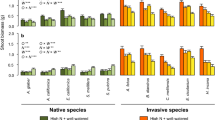
Many species decreased A max and ΦPSII (except Artemisia) and experienced photoinhibition (low F v /F m , except Encelia and Brassica) when transitioning from shade to sun (Fig. 3). Leaf Chl content decreased in the three invasive species (Ricinus, Nicotiana, Brassica) and remained unaltered in two of the native species (Encelia, Salvia; Fig. 3). Artemesia leaves were too small to be measured for Chl content. Stomatal response to high-light availability was extremely variable across species (Fig. 3). Stomatal conductance decreased in two natives and one invasive (Encelia, Salvia, Ricinus), increased in one native (Artemesia) and remained unaltered in two invasive species (Nicotiana, Brassica). Species increased growth over the 7-day period to varying degrees (Fig. 4). Two of the three species that displayed the highest growth (34% increase in stem diameter) were invasive (Ricinus, Nicotiana). Growth (change in stem diameter) was negatively correlated with three measures of leaf function (A max, ΦPSII, g s), but these relationships were not statistically significant (P > 0.10).
Leaf-level physiological data for three native and three invasive species before (Day 0, 300 μmol m−2 s−1) and after (Day 1, 2, and 7, 2,000 μmol m−2 s−1) transferring plants to a high-light environment (n = 5 individuals per species). Data are means and standard errors. Asterisks denote statistically significant differences between high-light (Day 1, 2, 7) and low-light (Day 0) conditions (P < 0.05)
Above-ground growth data for three native and three invasive species before (Day 0, 300 μmol m−2 s−1) and after (Day 7, 2,000 μmol m−2 s−1) transferring plants to a high-light environment (n = 5 individuals per species). Data are means and standard errors. Asterisks denote statistically significant differences between high-light (Day 7) and low-light (Day 0) conditions (P < 0.05)
Discussion
The ability of invasive species to tolerate stress (Funk and Vitousek 2007) and respond to resource pulses when available (Davis et al. 2000) will strongly influence invasion dynamics in low-resource systems. Here, stress tolerance was examined by subjecting plants to water and high-light stress, which are commonly experienced by species growing in arid habitats. In response to water and high-light stress, we found that species differentially responded to stress; however, there were no consistent differences between invasive and native species. Thus, our data neither support nor refute our hypothesis. Instead, our data suggest that species in arid systems display a range of physiological responses to stress.
Stress response
In the water manipulation experiment, one native (Salvia) and one invasive (Ricinus) species showed partial photosynthetic recovery during severe water stress (VWC < 10%) while one native (Encelia) and one invasive (Nicotiana) species did not. Unexpectedly, these species patterns were reversed for high-light stress. Upon transfer from low light (300 μmol m−2 s−1) to high-light (2,000 μmol m−2 s−1), Encelia and Nicotiana showed smaller reductions in photosynthetic function (A max, ΦPSII) than Salvia and Ricinus. In response to high-light stress, photoinhibition (as measured by F v /F m ) was lower in native species relative to invasive species, although one invader (Brassica) showed no photoinhibition and low reductions in A max. Collectively, the data from our stress treatments demonstrate that some invasive species have the ability to retain physiological function while living in water stressed conditions (Ricinus) or when exposed to high-light stress following shade acclimation (Brassica).
Does the ability to maintain photosynthetic function during water stress preclude a plant from protecting itself from photoinhibition? Leaf adaptations for water stress should be similar to those for high-light stress. For example, species in arid systems typically have trichomes or increased pigment content (i.e., xanthophyll pigments) to deflect or dissipate excess radiation (Demmig-Adams and Adams 1992; Ehleringer and Mooney 1978), which lowers the amount of radiation absorbed (i.e., decreases photoinhibition) and lowers leaf temperature (i.e., lowers evaporative loss and maintains an optimal temperature range for photosynthesis). While morphological traits such as trichome density and carotenoid content were not measured, the gas exchange results are inconsistent with the idea that species should have integrated stress responses (Chapin 1991) and respond similarly to water and high-light stress. As stated above, A max recovered in Ricinus and Salvia following water stress while Brassica and Encelia were most robust to photoinhibition.
Recovery from stress
Few studies have examined plant responses on short time scales to determine how plants respond to rapidly changing environmental conditions or to precipitation pulses (Barker et al. 2006; BassiriRad et al. 1999; Gebauer and Ehleringer 2000; Ignace et al. 2007; James and Richards 2005; Loik 2007; Nobel and Sanderson 1984; Peek and Forseth 2009; Resco et al. 2008). We found that photosynthetic function in three of four species (all but Nicotiana) fully recovered from water stress after irrigation was resumed. Recovery was fastest in Salvia and Ricinus, occurring after just one day, while Encelia recovered after seven days. Although there have been many studies examining photosynthetic recovery following drought stress (e.g., BassiriRad et al. 1999; Gebauer and Ehleringer 2000), few generalizations can be made regarding the mechanistic basis of recovery. Ennahli and Earl (2005) found that partial recovery of photosynthesis following severe water stress resulted from chloroplast limitations of photosynthesis (e.g., reduced Rubisco activity, increased photoinhibition) rather than stomatal limitations (e.g., low intercellular CO2 concentration). It seems unlikely that photoinhibition inhibited photosynthetic recovery during water stress in Encelia and Nicotiana given their resilience to photoinhibition in the high-light study. Thus, it is plausible that stomatal limitations to photosynthesis resulted in slow or incomplete recovery after seven days in these two species.
Root to shoot ratios strongly influence stomatal limitation of photosynthesis by controlling plant water status. For example, high R:S of a non-native C4 bunchgrass (Eragrostis lehmanniana) in southeastern Arizona is believed to contribute to the ability of this species to increase g s and, consequently, to rapidly increase A max following a precipitation pulse (Ignace et al. 2007). Plants may also increase R:S immediately following a precipitation event. Nobel and Sanderson (1984) found that root growth in cactus (Agave deserti) can be induced within six hours of soil wetting, highlighting the potential importance of new root growth in response to precipitation pulses. Root to shoot ratios remained unchanged in our stress-recovery treatment for all four species relative to control plants, suggesting that root growth was not rapidly initiated and that new root growth is unlikely to explain the rapid photosynthetic recovery that we observed in Ricinus and Salvia. Across treatments, R:S was higher in Ricinus (1.24 ± 0.11) and Nicotiana (1.05 ± 0.22) than in either native species (Encelia, 0.61 ± 0.08; Salvia, 0.68 ± 0.13), which may partially explain faster photosynthetic recovery in Ricinus, but not Salvia. However, low E observed for Ricinus and Encelia suggests that rapid photosynthetic recovery of these species resulted from increased water use efficiency (WUE) rather than increased g s mediated by root dynamics.
While Chl content was unaffected by water stress in all species, all three invasive species displayed lower Chl content in response to high-light stress, suggesting that invaders were adjusting to the new light environment by reallocating N away from light-harvesting pigments to carboxylation components of photosynthesis (Yamashita et al. 2000) or possibly initiating senescence of existing leaves. A recent study found that an invasive species (Ageratina adenophora) allocates more nitrogen to photosynthetic protein at the expense of structural protein [(e.g., cell walls; Feng et al. (2009)], but the timescale on which plants can reallocate nitrogen to various protein fractions, and the potential for nitrogen biochemistry to explain how species will respond to rapid changes in environmental factors, is unknown. Because there was no significant difference between leaf function (A max, ΦPSII, g s ) and stem diameter, we cannot conclude that invasive species decreased the function of existing leaves while diverting resources to new structures (e.g., Ackerly and Bazzaz 1995). However, future resource-pulse studies should monitor the potential trade-off between the function of existing leaves and new leaf growth over longer time periods.
In conclusion, we found no universal differences between native and invasive species in response to high-light stress, water stress and irrigation following water stress. Instead, responses were species specific. Climate change projections for the southwestern United States call for intensified intra-annual precipitation resulting in larger precipitation events with longer intervening dry periods (Diffenbaugh et al. 2005; Knapp et al. 2008). Thus, species will need to both tolerate stress and respond to large precipitation pulses. Understanding how species use water and light during pulse events may suggest strategies for the restoration of invaded systems by identifying native species with similar resource use patterns (thereby increasing community resilience to invasion) or through manipulation of pulsed resources (Funk et al. 2008).
References
Ackerly DD, Bazzaz FA (1995) Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101:289–298
Barker DH, Vanier C, Naumburg E, Charlet TN, Nielsen KM, Newingham BA, Smith SD (2006) Enhanced monsoon precipitation and nitrogen deposition affect leaf traits and photosynthesis differently in spring and summer in the desert shrub Larrea tridentata. New Phytol 169:799–808
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121:183–192
BassiriRad H, Tremmel DC, Virginia RA, Reynolds JF, de Soyza AG, Brunell MH (1999) Short-term patterns in water and nitrogen acquisition by two desert shrubs following a simulated summer rain. Plant Ecol 145:27–36
Burns JH, Winn AA (2006) A comparison of plastic responses to competition by invasive and non-invasive congeners in the Commelinaceae. Biol Invasions 8:797–807
Cavender-Bares J, Bazzaz FA (2004) From leaves to ecosystems: using chlorophyll fluorescence to assess photosynthesis and plant function in ecological studies. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, The Netherlands, pp 737–755
Chapin FS III (1991) Integrated responses of plants to stress. Bioscience 41:29–36
Chesson P, Gebauer RLE, Schwinning S, Huntly N, Wiegand K, Ernest MSK, Sher A, Novoplansky A, Weltzin JF (2004) Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141:236–253
Comar V, Tilley D, Felix E, Turdera M, Chagas Neto M (2004) Comparative energy evaluation of Castor bean (Ricinus communis) production systems in Brazil and the U.S. In: Ortega E, Ulgiati S (eds) Proceedings of IV biennial international workshop “Advances in energy studies”, pp 227–237, Unicamp, Campinas, SP, Brazil
Coyle DR, Coleman MD (2005) Forest production responses to irrigation and fertilization are not explained by shifts in allocation. For Ecol Manag 208:137–152
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 73:599–626
Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F (2005) Fine-scale processes regulate the response of extreme events to global climate change. Proceedings of the national academy of science 102: 15774–15778
Durand LZ, Goldstein G (2001) Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126:345–354
Ehleringer JR, Mooney HA (1978) Effects on physiological activity and adaptive value to a desert shrub. Oecologia 37:183–200
Ennahli S, Earl HJ (2005) Physiological limitations to photosynthetic carbon assimilation in cotton under water stress. Crop Sci 45:2374–2382
Feng Y, Lei Y-B, Wang Y-P, Callaway RM, Valiente-Banuet A, Inderjit, Li Y-P, Zheng Y-L (2009) Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc Natl Acad Sci 106:1853–1856
Funk JL (2008) Differences in plasticity between invasive and native plants from a low resource environment. J Ecol 96:1162–1174
Funk JL, Vitousek PM (2007) Resource use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Funk JL, Cleland EE, Suding KN, Zavaleta ES (2008) Restoration through re-assembly: plant traits and invasion resistance. Trends Ecol Evol 23:695–703
Gebauer RLE, Ehleringer JR (2000) Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81:1415–1424
Gleason SM, Ares A (2004) Photosynthesis, carbohydrate storage and survival of a native and an introduced species in relation to light and defoliation. Tree Physiol 24:1087–1097
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates Inc., Sunderland, MA
Grotkopp E, Rejmanek M, Rost TL (2002) Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159:396–419
Hamilton MA, Murray BR, Cadotte MW, Hose GC, Baker AC, Harris CJ, Licari D (2005) Life-history correlates of plant invasiveness at regional and continental scales. Ecol Lett 8:1066–1074
Hellmers H, Horton JS, Juhren G, O’Keefe J (1955) Root systems of some chaparral plants in southern California. Ecology 36:667–678
Huxman TE, Cable JM, Ignace DD, Eilts JA, English NB, Weltzin J, Williams DG (2004) Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: the role of native versus non-native grasses and soil texture. Oecologia 141:295–305
Ignace DD, Huxman TE, Weltzin JF, Williams DG (2007) Leaf gas exchange and water status responses of a native and non-native grass to precipitation across contrasting soil surfaces in the Sonoran Desert. Oecologia 152:401–413
James JJ, Richards JH (2005) Plant nitrogen capture from pulses: effects of pulse size, growth rate, and other soil resources. Oecologia 145:113–122
Jenerette GD, Scott RL, Huxman TE (2008) Whole ecosystem metabolic pulses following precipitation events. Funct Ecol 22:924–930
Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, Smith MD, Smith SE, Bell JE, Fay PA, Heisler JL, Leavitt SW, Sherry R, Smith B, Weng E (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58:811–821
Krueger-Mangold J, Sheley R, Engel R (2006) Can R*s predict invasion in semi-arid grasslands? Biol Invasions 8:1343–1354
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot 103:561–579
Leishman MR, Thomson VP (2005) Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol 93:38–49
Loik ME (2007) Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentata. Plant Ecol 191:95–108
Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit J, Barbaroux C, Le Thiec D, Brechet C, Brignolas F (2006) Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol 169:765–777
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Muth NZ, Pigliucci M (2007) Implementation of a novel framework for assessing species plasticity in biological invasions: responses of Centaurea and Crepis to phosphorus and water availability. J Ecol 95:1001–1013
Niinemets U, Valladares F, Ceulemans R (2003) Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell Environ 26:941–956
Nobel PS, Sanderson J (1984) Rectifier-like activities of roots of two desert succulents. J Exp Bot 35:727–737
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Padgett PE, Allen EB (1999) Differential responses to nitrogen fertilization in native shrubs and exotic annuals common to mediterranean coastal sage scrub of California. Plant Ecol 144:93–101
Peek MS, Forseth IN (2009) Positive effects of soil nitrogen pulses on individuals can have negative consequences for population growth during drought in a herbaceous desert perennial. J Ecol 97:440–449
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Reed HE, Seasteadt TR, Blair JM (2005) Ecological consequences of C4 grass invasion of a C4 grassland: a dilemma for management. Ecol Appl 15:1560–1569
Rejmanek M, Richardson DM, Pysek P (2005) Plant invasions and invasibility of plant communities. In: van der Maarel E (ed) Vegetation ecology. Blackwell Publishing, Oxford, pp 332–355
Resco V, Ignace DD, Sun W, Huxman TE, Weltzin JF, Williams DG (2008) Chlorophyll fluorescence, predawn water potential and photosynthesis in precipitation pulse-driven ecosystems: implications for ecological studies. Funct Ecol 22:479–483
Rundel PW, Gustafson R (2005) Introduction to the Plant Life of Southern California: Coast to Foothills. University of California Press, Berkeley, CA
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480
Weltzin JF, McPherson GR (1997) Spatial and temporal soil moisture resource partitioning by trees and grasses in a temperate savanna, Arizona, USA. Oecologia 112:156–164
Williams DG, Black RA (1994) Drought response of a native and introduced Hawaiian grass. Oecologia 97:512–519
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419
Yang LH, Bastow JL, Spence KO, Wright AN (2008) What can we learn from resource pulses? Ecology 89:621–634
Acknowledgments
We thank Erick Reisinger and Lori Glenwinkel for their assistance in the lab and field. Molly Cavaleri and Christine Creese provided valuable comments that greatly improved the manuscript. This work was supported in part by a grant from the Office of Sponsored Research, Office of the Chancellor, Chapman University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funk, J.L., Zachary, V.A. Physiological responses to short-term water and light stress in native and invasive plant species in southern California. Biol Invasions 12, 1685–1694 (2010). https://doi.org/10.1007/s10530-009-9581-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9581-6