Abstract
The reproductive systems of invasive species play a key role in determining their geographical distributions. Oxalis pes-caprae is a clonal, polyploid, heterostylous, plant native to South Africa, but now invasive in all major Mediterranean climatic regions. Here, we extend earlier surveys in the Western Mediterranean basin of floral morph ratios, reproductive traits and ploidy level to include populations from South Africa and introduced regions of Australia, California and Chile. We sampled a total of 104 populations, 33 in South Africa, 29 in Australia, 27 in California and 15 in Chile and collected data on floral morph representation (trimorphic, dimorphic, monomorphic), flower size, fruit set, and ploidy level using flow cytometry. There were significant differences among regions in floral morph structure of populations, reproductive traits and ploidy level. Populations in South Africa were exclusively tristylous and largely tetraploid (4x); Australian populations were mostly pentaploid (5x, 65.5%), comprised exclusively of the short-styled morph, with the remaining populations either dimorphic or trimorphic. Populations in California and Chile were comprised exclusively of the 5x short-styled morph. Fruit set varied dramatically among populations with no fruit produced in 5x populations. Our study demonstrates striking geographical variation among regions in reproductive systems ranging from a mixture of sexual and clonal reproduction in the native range to exclusively clonal propagation in some introduced regions. This variation is likely to have important consequences for local adaptation and should be considered in future management decisions of invasive populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive and genetic systems of species are important for determining their capacity for invasive spread and local adaptation. Flowering plants are of special importance in this context because of their extraordinary variation in sexual and asexual strategies, ploidy levels and mating systems (Lewontin 1965; Harper 1977; Barrett 2002). Moreover, many angiosperm species maintain considerable intraspecific diversity in reproductive and genetic systems with important ecological and evolutionary consequences. In invasive species, this variation is often non-randomly distributed across their geographical range (Barrett 1977; Amsellem et al. 2001; Kliber and Eckert 2005; Kubátová et al. 2008). Comparisons of patterns of geographical variation in native and introduced populations can provide valuable insights into colonizing strategies and the potential for local adaptation.
Changes in reproductive systems associated with colonization are particularly evident in invasive plant species that possess the outcrossing mechanism heterostyly. Population of heterostylous species are usually comprised of two (distyly) or three (tristyly) mating groups (floral morphs) that differ reciprocally in stigma and anther height (Barrett 2019). The degree of sex-organ reciprocity among floral morphs, i.e. the physical match in height of stigmas and anthers among morphs, promotes (intermorph) disassortative pollen transfer (Sánchez et al. 2013). The floral polymorphism is most often associated with a heteromorphic incompatibility system that prevents self and intramorph mating. In equilibrium populations, the floral morphs should occur at equal frequencies (isoplethy) and are maintained by negative frequency-dependent selection as a result of disassortative mating (Fisher 1941; Barrett 1993). However, founder events associated with colonization often lead to morph loss and breakdown in the functioning of heterostylous systems. When this occurs, it can result in evolutionary transitions to alternative reproductive systems based on uniparental reproduction, including high selfing rates (Barrett and Husband 1990) or extensive clonal propagation (Castro et al. 2007).
Plant genome size may play a role during the naturalization process in invasive plants (Kubešová et al. 2010) and polyploidy, i.e. the process of whole genome duplication through autopolypoidy (intraspecific genome duplication) or allopolyploidy (interspecific hybridization and subsequent genome duplication) has frequently been invoked as an important correlate of invasiveness in plants (te Beest et al. 2012). Polyploidy is often associated with changes in ecological breadth leading to range expansion (Levin 1983; Soltis and Soltis 2000). In some cases, multiple cytotypes (ploidy levels) occur in the native range of a species, but only polyploids are represented in the introduced range (e.g. Lafuma et al. 2003; Kubátová et al. 2008; Schlaepfer et al. 2008; Treier et al. 2009). Changes in ploidy level and reproductive system may not be independent of one another and joint investigations of both features are important for disentangling the role of these plant traits in invasiveness. One of the main objectives of this study was to investigate the reproductive systems and cytotype diversity in native and introduced populations of an invasive heterostylous species with prolific clonal reproduction.
Oxalis pes-caprae (Bermuda buttercup) is an invasive weed of arable land and disturbed sites, native to the Western and Northern Cape Provinces of South Africa (Salter 1944) but introduced to all Mediterranean climatic regions of the world. In South Africa, populations are predominantly tristylous with three ploidy levels (diploids 2x, tetraploids 4x, pentaploids 5x) and reproduction occurs sexually by seeds and asexually via bulbils (Ornduff 1986, 1987; Michael 1964; Richardson and Pyšek 2012; Ferrero et al. 2015). Thus, in its native range O. pes-caprae displays considerable geographical variation in reproductive and cytotype diversity, but the few available reports from non-native areas suggest that a series of inter-continental migration events have dramatically reduced patterns of variation.
Historical records provide some insight into the early introduction of O. pes-caprae to its alien range. Although many authors (Rappa 1911; Sommier and Caruana Gatto 1915; Pignatti 1982) speculated that European introductions were derived from the Botanical garden of La Valletta in Malta (Henslow 1891), recent surveys of exsiccata and the historical literature clearly demonstrate that O. pes-caprae arrived several times independently in different places in the Euro-Mediterranean region (Papini et al. 2017), and by the second half of the nineteenth century the species was locally abundant in some areas. In Australia, one of the earliest references to the introduction of O. pes-caprae is from the “Catalogue of plants cultivated in, and for sale at the Hackney Nursery, Adelaide, 1845” (Michael 1964). In California and Chile, historical records are scarce and the earliest recorded date of occurrence of O. pes-caprae in Chile appears to be from 1916 (Matthei 1995).
Based largely on anecdotal observations, it is widely believed that a 5x short-styled morph dominates throughout much of the introduced range of O. pes-caprae (e.g. Australia, the Mediterranean basin, California and Chile; Ornduff 1986, 1987). However, a small number of populations containing tetraploids are reported from Australia (Symon 1961), and Iberia (Castro et al. 2007, 2013). Pentaploid populations appear to be largely sterile and hence clonal propagation by bulbils may be the exclusive mode of reproduction in most of the introduced range. Interestingly, Baker (1965) used the widespread pentaploid form of O. pes-caprae to illustrate his concept of the ‘general-purpose-genotype strategy’: a genotype with broad ecological tolerance owing to well-developed phenotypic plasticity. However, detailed surveys of morph representation and ploidy level in O. pes-caprae in Mediterranean regions are needed to establish the global patterns of reproductive and cytotype diversity, and as a context for understanding future invasive spread. This is the primary goal of our study.
Here, we report results of surveys of morph representation, reproductive traits and ploidy level in populations of O. pes-caprae in South Africa, Australia, California and Chile. Along with previously published work (Castro et al. 2007, 2013, 2016; Ferrero et al. 2015), this data provides a comprehensive picture of geographical patterns of variation in these traits on a global scale. Our study had two specific goals: (1) to determine the representation and frequency of floral morphs and ploidy level of native and introduced populations; (2) to examine the extent to which populations in different regions differ in floral traits and their capacities for fruit production. Our results indicate that generalizations about the reproductive and cytotype diversity of widespread invasive plants, such as O. pes-caprae, based on restricted sampling of only parts of their geographical range, may lead to misleading inferences on future opportunities for local adaptation.
Methods
Study system
Oxalis pes-caprae (Oxalidaceae) is a perennial winter-growing geophyte with a true bulb that is borne at the apex of a vertical rhizome (Young 1968; Oberlander et al. 2009). Every year the bulb produces a contractile root that pulls it deeper into the soil. Bulbils, the organs of clonal regeneration, are produced in the axillary buds of the vertical rhizome (Salter 1944; Pütz 1994). Flowers of O. pes-caprae are yellow, showy, actinomorphic and native populations are tristylous, with two whorls of five stamens and one whorl of five stigmas, arranged at three heights according to the floral morph of the plant: long-, mid- and short-styled (hereafter L- M- or S-morph; see Castro et al. 2007). Oxalis pes-caprae flowers in late winter to early spring, i.e., January–March in the introduced regions of California and the Mediterranean basin, and July–September in South Africa, Australia and Chile. The species is reported to have a relatively low competitive ability (Sala et al. 2007; Tavares et al. 2019) and pollinators of O. pes-caprae in the Western Mediterranean basin are mostly bees including Anthophora spp., Apis mellifera, Bombus terrestris, Bombus pascuorum and Psythirus spp. and also long-tongued Diptera such as Bombylius sp. (Ferrero et al. 2013; Albrecht et al. 2016; Costa et al. 2016). To our knowledge detailed observations of pollinators visiting O. pes-caprae in other regions have not been conducted.
Sampling of populations, floral morph representation and reproductive traits
We conducted extensive sampling of O. pes-caprae populations in South Africa, Australia, California and Chile in 2012–2015 during the flowering season in each region. All required permits for plant collection were obtained. We sampled a total of 104 populations; 33 in South Africa (2011), 29 in Australia (2013), 27 in California (2015), 15 in Chile (2014) and 39 in the Western Mediterranean (2010). We made an effort to sample populations distributed over as much of the known distribution of the species in each range. However, for logistical reasons this was not possible in Chile and the Mediterranean region because of the very large geographical areas over which the species is distributed. Data on ploidy level and floral morph ratios of 10 of the 33 populations sampled in South Africa were previously reported in Ferrero et al. (2015) as part of a study of microsatellite variation. Populations from the Western Mediterranean were previously examined by Castro et al. (2013) for morph representation, ploidy level and fruit set, but not for flower size and sex-organ length, traits we measure here (see below). In South African and Australian populations, we determined floral morph frequencies by sampling plants randomly distributed throughout populations each separated by at least 5 km. We followed sampling protocols described in Castro et al. (2007, 2013) and Ferrero et al. (2015). Briefly, this consisted of examining flowers of plants at 5 m intervals along transects to reduce the likelihood of the repeated inclusion of the same clone. Different numbers of plants were observed depending on population size. In South Africa and Australia, the average sample size for sampling of morph ratios was 64 individuals (range 23–96 individuals). In California and Chile, where floral morph uniformity was detected, 50 individuals were randomly examined in all populations.
In each population, we collected several bulbils from 10 individuals of each morph and these were stored in paper bags. For these individuals we also collected one flower to measure corolla size (hereafter flower size) and stigma and anther heights to confirm the identity of the floral morph and to estimate the degree of sex-organ reciprocity. We used digital photography and Image J software for floral measurements (see Schneider et al. 2012). Fruit set (number of fruits/number of flowers per inflorescence) was estimated by sampling infructescences from 10 plants (where possible) of all morphs detected in populations. These plants were not always the same individuals for which floral traits were measured due to the absence of mature infructescences. In pentaploid populations there was no fruit production. Data on fruit set from Western Mediterranean populations is presented in Castro et al. (2013).
Ploidy level estimation
We used flow cytometry to estimate the ploidy of 10 individuals per morph (maximum of 30 individuals for tristylous populations) in each population of O. pes-caprae. We used fresh leaves from plants grown in the glasshouse from collected bulbils. We isolated nuclei following the procedure of Galbraith et al. (1983) by chopping simultaneously with a razor blade, 1 cm2 of leaf tissue of O. pes-caprae and 1 cm2 of leaf tissue of Solanum lycopersicum ‘Stupické’ [internal reference standard with 2C = 1.96 pg, Doležel et al. (1992)] in 1 mL of WPB buffer [0.2 M Tris–HCl, 4 mM MgCl2·6H2O, 1% Triton X-100, 2 mM EDTA Na22H2O, 86 mM NaCl, 10 mM metabisulfite, 1% PVP-10, pH adjusted to 7.5 and stored at 4 °C; see Loureiro et al. (2007)]. After filtration with a 50 µm nylon filter, we added 50 µg/mL of propidium iodide and 50 µg/mL of RNase to the nuclear suspension to stain the DNA and remove double-stranded RNA, respectively.
We analysed samples in a Partec CyFlow Space flow cytometer (Partec GmbH, Görlitz, Germany) equipped with a green solid-state laser (Cobolt Samba 532 nm, operating at 30 mW; Cobolt, Stockholm, Sweden). We analysed more than 3000 nuclei per sample and used a pooled sampling strategy, in which leaflets from five individuals were analysed simultaneously (Castro et al. 2013, Ferrero et al. 2015). When several peaks were obtained in a pooled preparation, we prepared individual samples and ploidy levels were assigned to each plant. To obtain the results we used Partec Flomax software (v. 2.5). As a quality standard, we only accepted histograms with a coefficient of variation (CV) lower than 5% for both sample and standard G1 peaks. Ploidy level was determined by calculating the DNA index, i.e. the ratio between the mean fluorescence of O. pes-caprae G0/G1 peak with that of S. lycopersicum. We identified each plant as either diploid, for DNA indices of 0.35 ± 0.01 (mean ± SD), tetraploid for DNA indices of 0.70 ± 0.03, and pentaploid for DNA indices of 0.86 ± 0.02.
Statistical analyses
The rare 2x individuals occurring at very low frequencies in mixed populations of tetraploids and diploids in South Africa and the single 4x plant from California were not included in statistical analyses. For each population polymorphic for style morph, we used G-tests (Sokal and Rohlf 1995) to compare the frequencies of floral morphs to isoplethic (1:1:1) expectations, and sequential Bonferroni adjustment to control the overall Type 1 error rate. Additionally, for the polymorphic populations we calculated sex-organ reciprocity among morphs using the index proposed by Sánchez et al. (2013). Pearson’s product moment correlation coefficient (r) was calculated between fruit set and the reciprocity index for South Africa, Australia and the Western Mediterranean region, where polymorphic populations occurred. Then, we grouped the data collected from population sampling described above according to: (a) region: including the native range South Africa (SA), and the introduced ranges of Australia (AU), California (CA), Chile (CH) and the Western Mediterranean basin (IB); (b) floral morph: L-, M-, S-morph and, (c) cytotype: diploid, tetraploid, and pentaploid. We compared flower size and fruit set among groups using linear mixed-effect models (LMMs). We considered population as a random effect and removed this term from models whenever its variance was lower than the variance of the residuals (Bolker et al. 2009). As a second dependent variable, we transformed fruit set with arcsine (square root) before analyses and followed the same approach. For both analyses we tested differences between least-square means pairwise by multiple comparisons. First, we tested for differences among morphs in each region and for each cytotype. When morphs did not differ within a particular region and cytotype, we excluded this factor from the analysis and used a fixed factor combining cytotype and region to avoid the incomplete factorial design. We performed all statistical analyses in R version 3.1.1 (R Core Team 2016) using the packages “lme4” for the LMMs, “car” for Type-III analysis of variance, “multcomp” for multiple comparisons after Type-III analysis of variance, “lsmeans” to obtain least-squares means and “ggplot2” for graphical representation (R Core Team 2016).
Results
Representation of floral morphs and sex-organ reciprocity among regions and populations
The number and types of populations sampled in all Mediterranean climatic regions, including results previously reported by Castro et al. (2013) from the Western Mediterranean, are summarized in Table 1. The representation of floral morphs differed among regions, particularly between the native and introduced range (Fig. 1). Detailed data on floral morph frequencies for all populations are presented in Supplementary Table 1.
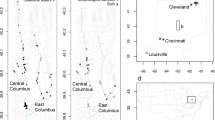
The geographical distribution of Oxalis pes-caprae populations sampled (grey areas) for floral morph (L-morph, M-morph, S-morph) and cytotype (diploid 2x; tetraploid 4x, pentaploid 5x) composition. A South Africa, the native range; B California, C Chile and D Australia, the introduced range. Pie diagrams for polymorphic populations illustrate floral morph frequencies and the solid grey small circles are monomorphic populations comprised of the 5x S-morph only. A bar divides diploid populations in South Africa from tetraploids. The four 5x S-morph individuals in South Africa are not indicated in the figure. Population localities and morph frequency data are listed in Appendix S1. A map with the distribution of populations sampled in the Western Mediterranean region is presented in Castro et al. (2013)
In South Africa, all sampled populations were tristylous and floral-morph ratios departed from isoplethy (1L:1M:1S) in 11 populations (33.3%). Total and pooled G values indicated significant deviation from equal morph frequencies (Gtotal = 528.74, df = 32, P < 0.001; Gpooled = 15.13, df = 2, P = 0.01) and morph ratios were significantly heterogeneous (Ghet = 519.90, df = 30, P < 0.001). In the 11 anisoplethic populations, the M-morph dominated in seven and the L- and S-morphs were each the most common morph in two. The remaining 22 populations were isoplethic, with average morph frequencies of: L-morph = 0.34; M-morph = 0.32; S-morph = 0.34. In Australia, 19 (65.5%) populations were monomorphic for the S-morph, six were dimorphic and were composed of the S- and M-morph (20.7%), three of which were isoplethic. One population was dimorphic for the L- and S-morph and also isoplethic. Three populations (10.3%) were tristylous and all were anisoplethic with the S-morph dominant in each population. Total, pooled and heterogeneity G values calculated for polymorphic Australian populations were all statistically significant (Gtotal = 532.55, df = 9; Gpooled = − 542.61, df = 2, Ghet = 1075.16, df = 7, in all P < 0.001). Sampling in California and Chile revealed that all populations were monomorphic for the S-morph.
Detailed measurements for sexual-organ heights and reciprocity values for polymorphic populations are summarised in Appendix S1. Within each of the regions sampled values for reciprocity and fruit set were not correlated (South Africa: r = − 0.085, P30 = 0.643; Australia: r = − 0.267, P8 = 0.454; Western Mediterranean: r = 0.412, P14 = 0.113).
Ploidy level determination
Flow cytometric determination of ploidy levels indicated that in South Africa the majority of populations were 4x, but four populations were composed mostly of 2x individuals; these were largely distributed at the northern part of the range (see Fig. 1). Some 2x individuals occurred sporadically in other 4x populations but at very low frequency (see Supplementary Table 1). Additionally, a small number of 5x individuals were found, three in a population at Cape Point and one individual at Botrivier, Western Cape. In Australia, most populations were monomorphic for the 5x S-morph (65.5%). The remaining 10 populations contained a mixture of 4x and 5x cytotypes. No purely 4x populations or 2x cytotypes were identified in Australia. In both California and Chile, all individuals sampled from populations were 5x. Baker (1965) reported a 4x M-morph individual in a garden at Berkeley, California and we found a single tetraploid plant at a similar garden location at Berkeley, which may or may not correspond to the same clone observed by Baker.
Reproductive traits
There was no significant difference in flower size among 4x floral morphs within South Africa (P2,832 = 0.070) and Australia (P2,127 = 0.093). Therefore, floral morph was removed from the analysis and the following groups were compared: SA—2x, 4x; AU—4x, 5x; CA—5x; CH—5x; IB—4x, 5x. There were significant differences among these groups (χ 27 ,2225 = 2627.1, P < 0.001, Fig. 2A). Overall, flower size was significantly larger in 5x plants in all regions in comparison with 2x and 4x plants from South Africa, which had similarly smaller flowers (Fig. 2A). Flower size of 4x cytotypes in Australia and the Western Mediterranean region was very similar but larger than tetraploids in South Africa. Similarly, flowers of 5x plants from the Western Mediterranean basin were larger in size than pentaploids from all other regions. Detailed measurements of flower size and sexual organs are summarized for regions and cytotypes in Supplementary Table 2 and for individual populations in Supplementary Table 3.
Geographical variation in: A mean flower size of floral morphs and cytotypes and B mean fruit set per cytotype and region from the native and introduced ranges of Oxalis pes-caprae. For flower size, the floral morphs within cytotypes are indicated and values for fruit set are the arcsine of the proportion of flowers developing into fruit. Values given are model-adjusted back-transformed least-squared means and 95% confident intervals. Significant differences among factors are indicated with different letters (P < 0.05). Fruit set in California and Chile was zero, and information for the Western Mediterranean region is presented in Castro et al. (2013). Floral morphs: L-morph, M-morph, S-morph; cytotypes: 4x tetraploid; 5x pentaploid
There was significant variation among regions, morphs and ploidy levels in fruit set (Fig. 2B). The following groups were therefore considered for comparison: SA—2x L-, M-, S-morph, 4x L-, M-, S-morph; AU—4x L-, M-, S-morph, 5x S-morph; CA—5x S-morph; CH—5x S-morph (Fig. 2B). Because in South Africa plants used for ploidy estimates were not the same plants used in the analyses of other traits, only populations comprised of exclusively 2x or 4x individuals were included in our analysis. Fruit set differed significantly among the groups (χ 211,1648 = 2275.6, P < 0.001). In general, fruit set was significantly higher in the native range than in the introduced ranges, being zero in pentaploid populations in Australia, California and Chile. In the 4x cytotype in South Africa, fruit set of the L-morph was significantly higher than the M- and S-morphs but there was no significant difference in fruit set among floral morphs of the 2x cytotype. In Australia, the overall level of fruit set in the 4x cytotype was significantly lower than the corresponding cytotype in the native range, but there were no significant differences among floral morphs.
Discussion
Our global sampling of Oxalis pes-caprae revealed striking differences among Mediterranean climatic regions in floral morph and cytotype composition, particularly between the native and introduced range. Although the sterile pentaploid S-morph dominated throughout the introduced range, occasional tetraploids populations occur in Australia and the Iberian Peninsula allowing some degree of sexual reproduction. We now discuss in detail these findings within the context of the introduction history of the species and the implications for the reproductive biology of populations in the native and introduced range.
Geographical patterns of floral morph and cytotype occurrence
Historical contingency involving the particular floral morphs that initiate heterostylous populations during migration can cause biased morph ratios or extensive areas of stylar monomorphism. For example, the limited representation of the M-morph in northern populations of Decodon verticillatus in eastern North America (Eckert and Barrett 1995), and the absence of the S-morph from the introduced range of Eichhornia crassipes (Barrett 1977) provide examples of the influence of founder events on geographical patterns of morph-frequency variation. A similar process is apparent in O. pes-caprae, where founder events involving the sterile pentaploid S-morph have probably resulted in the predominance of this form throughout much of the introduced Mediterranean climatic regions of the world. Indeed, based on our surveys this appears to be the only morph that is represented in invasive populations in California and Chile.
Two questions remain unresolved with regard to the colonizing history of the 5x cytotype of O. pes-caprae. First, it is unclear where the introduction(s) come from, especially given the extreme rarity of the 5x cytotype in the native range. Ornduff (1987) proposed the Cape region of South Africa as the source region since this is the native range of the species. However, various alternative hypotheses have been suggested, including the possibility that the 5x form originated from hybridization between cytotypes in the introduced range and subsequent introduction to the Cape region occurred during contemporary times through nurseries and/or botanical gardens (Michael 1964; Krejčíková et al. 2013; Ferrero et al. 2015). Compared to the diploid and tetraploid forms, the pentaploid has larger showy flowers (Fig. 2A), and the ease with which it can be propagated clonally together with its sterile nature may have made it a desirable choice for ornamental use.
Second, what is the colonization history of O. pes-caprae within the introduced range? Various scenarios can be envisioned depending on where the 5x cytotype originated and whether or not it has had multiple independent origins through hybridization among cytotypes. What seems evident, based on an extensive survey of microsatellite variation in populations from South Africa and the Western Mediterranean region, is that there were multiple introductions of O. pes-caprae from the native to the introduced range (Ferrero et al. 2015) and this is supported by historical evidence (D’Austria 1884; Gimeno et al. 2006; Papini et al. 2017). However, given the highly restricted distribution of the 5x cytotype in South Africa this may be unlikely for this particular cytotype. Future studies of the molecular phylogeography of populations throughout the range of O. pes-caprae would be valuable in determining the complex migratory history of the species.
Our surveys of morph ratios in 33 populations in the native range indicated that they were all tristylous and mostly exhibited 1:1:1 morph ratios, which is consistent with Fisherian predictions based on negative frequency-dependent selection (Fisher 1941; Barrett 1993). The maintenance of trimorphism indicates that sexual reproduction must occur regularly in these populations in addition to regeneration by clonal propagation. Recurrent gene flow by seed and/or clonal propagules in the Cape region is probably sufficiently frequent to counter the stochastic loss of the S-morph from populations that occurs commonly in other tristylous species with prolific colonizing ability (Husband and Barrett 1992; Balogh and Barrett 2016). There was also no evidence in South African populations of a reduced frequency or loss of the M-morph from populations, as occurs in Oxalis alpina in association with modifications to trimorphic incompatibility (Weller et al. 2016). The occurrence of trimorphic incompatibility in O. pes-caprae (see Costa et al. 2017) and its promotion of disassortative mating clearly serve to maintain trimorphism in South African populations. Anisoplethic morph structure in approximately one-third of the populations we surveyed most probably results from rapid clonal propagation after the founding of populations by particular morphs. These populations are likely in a non-equilibrium state with genetic drift also playing some role in smaller populations. Indeed, biased morph ratios have been reported elsewhere in other tristylous populations of Oxalis species (Ornduff 1972; Barrett and Arroyo 2012; Weller et al. 2016).
South Africa is the only region we sampled in which 2x, 4x and 5x populations occur. Diploid populations were less abundant than 4x populations and were primarily distributed in the succulent Karoo vegetation at the northern range limit, with rare sporadic individuals occurring in some 4x populations (and see Krejčíková et al. 2013). Our field observations indicated that 2x populations appeared to be less weedy than 4x populations and were less commonly observed in highly disturbed sites. This is in accord with previous observations by Krejčíková and co-authors (2013) who describe the 4x cytotype as having a higher weedy potential than the diploids. Their observations indicate that tetraploids appear to have a greater potential for growing in disturbed and anthropogenically-modified habitats and forming larger stands than the diploids, which in contrast occur largely in habitats with natural vegetation. These differences in ecology may explain the absence of the 2x cytotype from the invasive range, although its narrower distribution in the native range (Krejčíková et al. 2013) may have also reduced opportunities for introduction to other Mediterranean climatic regions.
In Australia, 19 of the 29 (65.5%) populations sampled were monomorphic for the 5x S-morph, six were dimorphic for the S- and M-morph and one was dimorphic for the L- and S-morph. A similar diversity of morph and cytotypes to those in Australia has recently been revealed in the Western Mediterranean basin (Castro et al. 2007, 2013; Supplementary Table 1). The occurrence of all three floral morphs and both 4x and 5x cytotypes in this region is consistent with multiple introductions (Ferrero et al. 2015). The main difference between invasive populations in these two regions appears to be that in the Iberian Peninsula the 5x cytotype is not completely sterile and is capable of producing limited amounts of viable seed (Castro et al. 2013; Costa et al. 2014). In contrast, all populations of the 5x S-morph that we surveyed in Australia, California and Chile were sterile and produced no seed. The opportunities for sexual reproduction in populations of the 5x cytotype in the Mediterranean (Castro et al. 2013; Costa et al. 2017), although limited, may explain the occurrence of multiple genotypes in this region based on studies of genetic markers (Rottenberg and Parker 2004; Ferrero et al. 2015), although somatic mutations may be another potential source of genetic variation in these largely clonal populations (reviewed in Barrett 2015). Regardless of the source of the observed variation, if sexual offspring produced by the 5x cytotype are capable of growth and reproduction under field conditions in the Mediterranean, opportunities for the evolution of local adaptation would be enhanced, a process that appears to be constrained in California and Chile. Comparisons of genetic diversity among regions, particularly for ecologically relevant traits, would be useful to determine the scope for future adaptive changes associated with range expansion.
The differences in floral morph and cytotype diversity among regions in the introduced Mediterranean climatic range of O. pes-caprae are likely to have resulted from variation in propagule pressure associated with the frequency of trading exchange and tourism. The Western Mediterranean basin and Australia have been important trade routes with South Africa since colonial times and more recently via tourism (Crosby 1986; Kruger et al. 1989). In contrast, multiple introductions to California and Chile from South Africa seem less likely (Thuiller et al. 2005). Indeed, it is quite plausible that Chilean populations of O. pes-caprae were derived from California, although introduction from Western Europe cannot be ruled out as this region has contributed significantly to the invasive Mediterranean flora of Chile (Figueroa et al. 2004).
Flower size and fruit set
We detected considerable variation in flower size among O. pes-caprae cytotypes and regions. As mentioned earlier, the 5x cytotype had by far the largest flowers with no significant difference among population in Australia, California and Chile (Fig. 2A). However, flowers of the 5x cytotype in the Western Mediterranean basin were 25% larger than from other Mediterranean climatic regions and this difference was statistically significant (mean 24.82 mm ± 4.04, n = 362 flowers sampled from 39 populations in the Western Mediterranean basin (V. Ferrero, unpublished data) versus mean 19.04 mm ± 3.52, n = 1003 pentaploid flowers sampled in 72 populations in the rest of the areas, F4,998 = 49.87, P < 0.001). In South Africa there were no significant difference in flower size among morphs, nor between 2x and 4x cytotypes; however, the flower size of 4x cytotypes in Australia was significantly larger than that of 4x cytotypes in the native range (Fig. 2A). Interestingly, the flower size of 4x cytotypes in Australia and the Western Mediterranean Region were very similar. It is important to note that our data on flower size variation are phenotypic measures and are therefore influenced by local environmental conditions. Common garden studies of samples from the global range of the species would be required to establish whether the flower size differences we report have a heritable basis.
Our measurements of fruit set in O. pes-caprae populations capable of sexual reproduction revealed differences among morphs and regions (Fig. 2B). There was no significant difference among morphs for 2x populations in South Africa, although sample sizes were relatively low. Among 4x populations from this region the L-morph set more fruit than the M- and S-morphs, perhaps because stigmas of long-styles are more accessible to pollinators and capture more pollen (Nishihiro et al. 2000), although in tristylous Lythrum salicaria the L-morph produced fewer seeds per fruit than the M- and S-morphs (Ågren and Ericson 1996).
Experimental cross-pollinations of 4x plants from the native range did not reveal differences in maternal seed fertility among the morphs (Costa et al. 2017). The overall level of fruit set (35% ± 38) of the 4x cytotype in Australia was substantially lower than the 4x cytotype in the native range (72% ± 30, data for each population in Appendix S1), despite their larger flowers. Lower fruit set in comparison with the native range in the 4x cytotype was also evident in the Western Mediterranean region (43% ± 34; Castro et al. 2013). This pattern of reduced maternal fertility is not unexpected for a heterostylous species in its invasive range and probably results from pollen limitation of fruit set owing to insufficient pollinator visitation or mate limitation (see Barrett 1980 for an example).
In conclusion, our study of geographical variation in reproductive and cytotype diversity of O. pes-caprae revealed striking regional variation in the style morph composition of populations and in their ploidy levels. This variation should result in different opportunities for sexual reproduction and genetic recombination, with implications for the magnitude and tempo of local adaptation. It has been suggested that climatic niche expansion in the introduced range of O. pes-caprae most likely reflects the well-developed capacity for phenotypic plasticity in clonal populations rather than rapid evolution (González-Moreno et al. 2015; and see Baker 1965). However, although clonal propagation is indeed the predominant mechanism of reproduction in all invasive populations, the occurrence of sexual tetraploids in Australia and the Western Mediterranean may enable populations to become adapted to local environmental conditions. In contrast, this process is likely to be much slower in California and Chile because of the apparent absence of genetic recombination. Future, comparative studies of the ecology and genetics of population across the global range of O. pes-caprae would be worthwhile to test these predictions.
References
Ågren J, Ericson L (1996) Population structure and morph-specific fitness differences in tristylous Lythrum salicaria. Evolution 50:126–136
Albrecht M, Ramis MR, Traveset A (2016) Pollinator-mediated impacts of alien invasive plants on the pollination of native plants: the role of spatial scale and distinct behaviour among pollinator guilds. Biol Inv 18:1801–1812
Amsellem L, Chevallier MH, Hossaert-McKey M (2001) Ploidy level of the invasive weed Rubus alceifolius (Rosaceae) in its native range and in areas of introduction. Plant Syst Evol 228:171–179
Baker HG (1965) Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, New York, pp 147–172
Balogh CM, Barrett SCH (2016) Stochastic processes during invasion: the influence of population size on style-morph frequency variation in Lythrum salicaria (purple loosestrife). Int J Plant Sci 177:409–418
Barrett SCH (1977) Tristyly in Eichhornia crassipes (Water Hyacinth). Biotropica 9:230–238
Barrett SCH (1980) Sexual reproduction in Eichhornia crassipes (Water Hyacinth). II. Seed production in natural populations. J Appl Ecol 17:113–124
Barrett SCH (1993) The evolutionary biology of tristyly. In: Futuyma D, Antonovics J (eds) Oxford surveys in evolutionary biology. Oxford University Press, Oxford, pp 283–326
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev Gen 3:274–284
Barrett SCH (2015) The influences of clonality on plant sexual reproduction. Proc Natl Acad Sci USA 112:8859–8866
Barrett SCH (2019) ‘A most complex marriage arrangement’: recent advances on heterostyly and unresolved questions. New Phytol 224:1051–1067
Barrett SCH, Arroyo MTK (2012) Variation in floral morph ratios in tristylous Oxalis squamata (Oxalidaeae). Botany 90:1180–1185
Barrett SCH, Husband BC (1990) Variation in outcrossing rates in Eichhornia paniculata: the role of demographic and reproductive factors. Plant Species Biol 5:41–56
Bolker BM, Brooks ME, ClarkCJ Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trend Ecol Evol 24:127–135
Castro S, Loureiro J, Santos C, Ater M, Ayensa G, Navarro L (2007) Distribution of flower morphs, ploidy level and sexual reproduction of the invasive weed Oxalis pes-caprae in the western area of the Mediterranean region. Ann Bot 99:507–517
Castro S, Ferrero V, Costa J, Sousa AJ, Navarro L, Loureiro J (2013) Reproductive strategy of the invasive Oxalis pes-caprae: distribution patterns of flower morphs, ploidy levels and sexual reproduction. Biol Inv 15:1863–1875
Castro S, Castro M, Ferrero V, Costa J, Tavares D, Navarro L, Loureiro J (2016) Invasion fosters change: independent evolutionary shifts in reproductive traits after Oxalis pes-caprae L. introduction. Front Plant Sci 7:874
Costa J, Ferrero V, Loureiro J, Castro M, Navarro L, Castro S (2014) Sexual reproduction in the invasive pentaploid short-styled Oxalis pes-caprae allows the production of viable offspring. Plant Biol 16:208–214
Costa J, Ferrero V, Castro M, Jorge A, Afonso A, Loureiro J, Castro S (2016) Pollen flow between flowers of the same morph in invasive populations of Oxalis pes-caprae L. in the Western Mediterranean region. Plant Biosyst 150:923–932
Costa J, Ferrero V, Castro M, Loureiro J, Navarro L, Castro S (2017) Variation in the incompatibility reactions in tristylous Oxalis pes-caprae: large-scale screening in South African native and Mediterranean basin invasive populations. Perspect Plant Ecol Evol Syst 24:25–36
Crosby AW (1986) Ecological imperialism: the biological expansion of Europe, 900–1900. Cambridge University Press, Cambridge
D’Austria S (1884) Die Balearen-Menorca, vol II (Translated to Spanish in 1982). Ed. Sa Nostra, Palma de Mallorca
Doležel J, Sgorbati S, Lucretti S (1992) Comparison of 3 DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol Plant 85:625–631
Eckert CG, Barrett SCH (1995) Style morph ratios in tristylous Decodon verticillatus (Lythraceae): selection vs. historical contingency. Ecology 76:1051–1066
Ferrero V, Castro S, Costa J, Acuña P, Navarro L, Loureiro J (2013) Effect of invader removal: pollinators stay but some native plants miss their new friend. Biol Inv 15:2347–2358
Ferrero V, Barrett SCH, Castro S, Caldeirinha P, Navarro L, Loureiro J, Rodríguez-Echeverría S (2015) Invasion genetics of the Bermuda buttercup (Oxalis pes-caprae): complex intercontinental patterns of genetic diversity, polyploidy and heterostyly characterize both native and introduced populations. Mol Ecol 24:2143–2155
Figueroa JA, Castro SA, Marquet PA, Jaksic FM (2004) Exotic plant invasions to the Mediterranean region of Chile: causes, history and impacts. Rev Chil Hist Nat 77:465–483
Fisher RA (1941) The theoretical consequences of polyploid inheritance for the mid style form of Lythrum salicaria. Ann Eugen 11:31–38
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell-cycle in intact plant tissues. Science 220:1049–1051
Gimeno I, Vilà M, Hulme PE (2006) Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae L. in the Western Mediterranean. J Biogeogr 33:1559–1565
González-Moreno P, Diez JM, Richardson DM, Vilà M (2015) Niche shifts in invasive species. Glob Ecol Biogeogr 24:360–370
Harper JL (1977) Population biology of plants. Academic Press, London
Henslow G (1891) On the northern distribution of Oxalis cernua Thunb. In: Proceedings of the Royal Society of London (Session 1890-1891), pp 31–36
Husband BC, Barrett SCH (1992) Genetic drift and the maintenance of the style length polymorphism in tristylous populations of Eichhornia paniculata (Pontederiaceae). Heredity 69:440–449
Kliber A, Eckert CG (2005) Interaction between founder effect and selection during biological invasion in an aquatic plant. Evolution 59:1900–1913
Krejčíková J, Sudová R, Oberlander KC, Dreyer LL, Suda J (2013) Cytogeography of Oxalis pes-caprae in its native range: where are the pentaploids? Biol Inv 15:1189–1194
Kruger FJ, Breytenbach GJ, Macdonald IAW, Richardson DM (1989) The characteristics of invaded mediterranean-climate regions. In: Drake J, Mooney HA, Di Castri F, Groves RH, Kruger FJ, Rejmanek M, Williamson M (eds) Biological invasions: a global synthesis. Wiley, New York, pp 181–213
Kubátová B, Trávníček P, Bastlová D, Čurn V, Jarolímová V, Suda J (2008) DNA ploidy-level variation in native and invasive populations of Lythrum salicaria at a large geographical scale. J Biogeogr 35:167–176
Kubešová M, Moravcová L, Suda J, Jarošík V, Pyšek P (2010) Naturalized plants have smaller genomes than their non-invading relatives: a flow cytometric analysis of the Czech alien flora. Preslia 82:81–96
Lafuma L, Balkwill K, Imbert E, Verlaque R, Maurice S (2003) Ploidy level and origin of the European invasive weed Senecio inaequidens (Asteraceae). Plant Syst Evol 243:59–72
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Lewontin RC (1965) Selection for colonizing ability. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, London, pp 77–91
Loureiro J, Rodriguez E, Doležel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Matthei O (1995) Manual de las malezas que crecen en Chile. Alfabeta Impresores, Santiago de Chile
Michael P (1964) The identity and origin of varieties of Oxalis pes-caprae L. naturalized in Australia. Trans R Soc S Aust 88:167–173
Nishihiro J, Washitani I, Thomson JD, Thomson BA (2000) Patterns and consequences of stigma height variation in a natural population of a distylous plant, Primula sieboldii. Funct Ecol 14:502–512
Oberlander KC, Emshwiller E, Bellstedt DU, Dreyer LL (2009) A model of bulb evolution in the eudicot genus Oxalis L. Mol Phylogen Evol 51:54–63
Ornduff R (1972) The breakdown of trimorphic incompatibility in Oxalis section Corniculatae. Evolution 26:52–65
Ornduff R (1986) The origin of weediness in Oxalis pes-caprae. Am J Bot 73:779–780
Ornduff R (1987) Reproductive systems and chromosome races of Oxalis pes-caprae L. and their bearing on the genesis of a noxious weed. Ann Miss Bot Gard 74:79–84
Papini A, Signorini MA, Foggi B, Della Giovampaola E, Ongaro L, Vivona L, Santosuosso U, Tani C, Bruschi P (2017) History vs. legend: retracing invasion and spread of Oxalis pes-caprae L. in Europe and the Mediterranean area. PLoS ONE 12:e0190237
Pignatti S (1982) Oxalis pes-caprae L. In: Pignatti S (ed) Flora d’Italia, vol II. Edagricole, Bologna, p 3
Pütz N (1994) Vegetative spreading of Oxalis pes-caprae (Oxalidaceae). Plant Syst Evol 191:57–67
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. R version 3.3.2. http://www.R-project.org/
Rappa F (1911) Osservazioni sull’Oxalis cernua. Bollettino reale orto botanico e giardino coloniale di Palermo 10:142–185
Richardson DM, Pyšek P (2012) Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytol 196:383–396
Rottenberg A, Parker JS (2004) Asexual populations of the invasive weed Oxalis pes-caprae are genetically variable. Proc R Soc B 271:S206–S208
Sala A, Verdaguer D, Vilà M (2007) Sensitivity of the invasive geophyte Oxalis pes-caprae to nutrient availability and competition. Ann Bot 99:637–645
Salter TM (1944) The genus Oxalis in South Africa: a taxonomic revision. J S Afr Bot Suppl 1:1–355
Sánchez JM, Ferrero V, Navarro L (2013) Quantifying reciprocity in distylous and tristylous plant populations. Plant Biol 15:616–620
Schlaepfer DR, Edwards PJ, Semple JC, Billeter R (2008) Cytogeography of Solidago gigantea (Asteraceae) and its invasive ploidy level. J Biogeogr 35:2119–2127
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman and Company, New York, p 887
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci USA 97:7051–7057
Sommier S, Caruana Gatto A (1915) Flora melitensis nova. Stabilimento Pellas, Firenze
Symon DE (1961) The species of Oxalis established in South Australia. Trans R Soc S Aust 84:71–77
Tavares D, Loureiro J, Martins A, Castro M, Roiloa Castro S (2019) Genetically based phenotypic differentiation between native and introduced tetraploids of Oxalis pes-caprae. Biol Inv 21:229–243
te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubesová M, Pysek P (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109:19–45
Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC (2005) Climate change threats to plant diversity in Europe. Proc Natl Acad Sci USA 102:8245–8250
Treier UA, Broennimann O, Normand S, Guisan A, Schaffner U, Steinger T, Müller-Schärer H (2009) Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90:1366–1377
Weller SG, Sakai AK, Gray T, Weber JJ, Tsyuslo OV, Domínguez CA, Fornoni J, Molina-Freaner FE (2016) Variation in heterostylous breeding systems in neighbouring populations of Oxalis alpina (Oxalidaceae). Plant Biol 18:104–110
Young D (1968) Oxalis L. In: Tutin T, Heywood V, Burges N et al (eds) Flora Europaea, vol 2. Cambridge University Press, Cambridge, p 193
Acknowledgements
The authors thank the Western Cape Nature Conservation Board and Department of Environment and Nature Conservation, Northern Cape, and State Governments in Australia for providing authorizations to collect and undertake scientific research. The authors also thank Joana Costa for help with sampling. This research was supported by FEDER funds through the COMPETE Program and by Portuguese Foundation for Science and Technology (FCT) funds in the ambit of the project PTDC/BIA-BIC/110824/2009, CRUP Acções Integradas Luso-Espanholas 2010 with the project E10/10, MCI-Programa de Internacionalización de la ICD (PT2009-0068) of the Spanish DGICYT (CGL2009-10466 and CGL2013-45941), the Xunta de Galicia (INCITE09-3103009PR, CITACA and R2014/036), the Percy Sladen Memorial Fund (2014 and 2015 calls) and the British Ecological Society (BES Small Research Grant 5355/6399). FCT also supported the work of SC (BPD/41200/2007, Starting Grant IF/01267/2013) and VF (SFRH/BPD/108707/2015). SCHB was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferrero, V., Navarro, L., Castro, S. et al. Global patterns of reproductive and cytotype diversity in an invasive clonal plant. Biol Invasions 22, 1691–1703 (2020). https://doi.org/10.1007/s10530-020-02213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02213-9