Abstract
Polyploidy resulting from whole genome duplication is common in plants and is increasingly being recognised as a critical factor in conservation actions, particularly when within-species variation in ploidy exists. Pomaderris (Rhamnaceae), a genus of 70 species endemic to Australia and New Zealand, has many species listed as threatened and that are subject to conservation management but of unknown ploidy. To provide a better understanding of polyploidy in the genus we sampled 36 of 70 species of Pomaderris and used flow cytometry to establish genome sizes and infer ploidy. Additionally, to examine within-species variation, we screened 104 individuals of eight rare species subject to conservation management. We did not find evidence for infraspecific variation in ploidy, suggesting that from a cytological perspective, conspecific individuals from the screened populations do not need to be kept geographically separated in conservation management. There is, however, considerable variation among species, with genome sizes suggesting the occurrence of diploidy, triploidy, tetraploidy and hexaploidy. Finding several species to be triploid but capable of seed production, we then explored reproductive biology using the flow cytometric seed screen. Results suggested that triploid species produce seeds asexually, as previously reported for two New Zealand congeners. While asexual reproduction through apomixis is generally a means of odd-ploid taxa overcoming sterility, we found that more than half of examined diploids and tetraploids also produced seeds asexually. Asexual reproduction means genetic diversity is potentially low, and these results should therefore be considered in future conservation actions and seed sampling designs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetics and conservation management
Successful conservation management of rare species depends on the availability of information on their biological traits (Morris and Doak 2002; Nevill et al. 2016) but, because of their rarity, this information is missing particularly often in the case of threatened species (Jain et al. 2013). Knowledge of the genetic characteristics and the reproductive biology of species are key elements of successful conservation, regardless of the conservation method (Kramer and Havens 2009; Hoban and Strand 2015; Nevill et al. 2016). Genetic characteristics of the target species are important for a variety of reasons (Frankham et al. 2010). Maintenance of genetic diversity is crucial to enable a species to respond to changing conditions (Reed and Frankham 2003). Inbreeding depression threatens the viability of small and isolated populations, leading to the concept of genetic rescue as a management action (Pickup and Young 2007). Genetic variants of a species can, however, be adapted to climatic and edaphic conditions, in which case translocation or revegetation efforts into an unsuitable area would fail, and genetic rescue may reduce population viability (Waller 2015). In vascular plants, polyploidy (or whole genome duplication) is increasingly recognised as another aspect of genetics to be considered in management actions (Severns and Liston 2008; Wallace et al. 2017). Polyploidy can cause breeding barriers within and among populations and therefore has implications for both in situ and ex situ conservation.
Polyploidy
Most plant and animal species are diploid, i.e. their cells carry two chromosome sets or genome copies, one inherited from each parent. Polyploidy, the state of having more than two genome copies, is the result of whole genome duplication (Thompson and Lumaret 1992; Leitch and Bennett 1997).
Two main forms of polyploidy are recognised. Autopolyploids have multiple copies of the same genome, whereas allopolyploids arise from genome duplication after the hybridisation of two different species. Depending on the degree of differentiation between genomes and the resultant behaviour of chromosomes during meiosis, allopolyploids are characterised as genomic or segmental, with the latter still sufficiently homologous to form multivalents (Jenkins and Rees 1991). While polyploidy is rare in animals, it has been well documented and is widespread in plants (Muller 1925; Masterson 1994; Soltis et al. 2009) with the frequency and maximum ploidy varying among and within taxa (Husband et al. 2013).
Genome duplication can have a variety of consequences. It can have an impact on the ecology of a species, which then influences diversification and speciation (Leitch and Bennett 1997; Comai 2005). Polyploidy may lead to instantaneous speciation, either through creating a barrier to gene flow within one species or by producing a fertile, allopolyploid hybridogenic species in cases where diploid hybrids are sterile. Polyploidy has been hypothesised to facilitate the shift from self-incompatibility to selfing (Miller and Venable 2000) and apomixis (Carman 1997). Globally, across thousands of species, ploidy has been associated with rarity (endangered plants are disproportionately diploid) and invasiveness (invasive plants are disproportionately polyploids) (Pandit et al. 2011; te Beest et al. 2012). Polyploid plants are particularly frequent in previously glaciated areas of the northern hemisphere (Brochmann et al. 2004). It has been suggested that polyploidy is advantageous for the evolutionary success of species, particularly in harsh environments (Comai 2005; Madlung 2013), but this has rarely been tested empirically (Godfree et al. 2017).
From the perspective of conservation management, within-species polyploid complexes are of particular concern. Where within-species variation in ploidy exists in fragmented landscapes diploid populations are more prone to local extinction than polyploids (Plue et al. 2018). In species where populations may have different ploidy, crosses between such populations result in odd-ploid offspring, e.g. the cross between a diploid (2n) and a tetraploid (4n) is triploid (3n). Odd-ploid plants are often partially or fully sterile, and this is generally attributed to the failure of chromosome pairing in meiosis (Köhler et al. 2010). In conservation management, the creation of sterile odd-ploids is undesirable, as it reduces population viability unless the plants can reproduce asexually (Severns and Liston 2008; Wallace et al. 2017). Ultimately, the less common ploidial level in a mixed population is likely to become locally extinct through minority cytotype exclusion (Levin 1975). Therefore, it is important to know if species subject to management actions, such as seed production (Nevill et al. 2016), translocation or revegetation, constitute polyploid complexes, as well as about their reproductive biology. If populations with different ploidy exist within the same species, the relevant plants and seeds should be separated both in natural populations and in seed orchards (Schmidt-Lebuhn et al. 2018).
Asexual (apomictic) reproduction is often associated with polyploidy (Carman 1997). While ploidy is increasingly addressed in conservation management (Severns and Liston 2008; Wallace et al. 2017), the associated apomixis that may be present has rarely been investigated or considered in conservation protocols.
Study group
Pomaderris (Rhamnaceae: Pomaderreae; Fig. 1) is a genus of shrubs comprising 70 species occurring in Australia and New Zealand (Walsh and Coates 1997). Sixty-five species occur in Australia, and 61 of these are endemic. A number of species are listed as threatened in Commonwealth and State legislation (Australian Government 1999; New South Wales Government 2016), and the whole group is therefore of conservation interest.
Ex situ conservation is being undertaken in an effort to halt or reverse the decline of the numerous threatened Pomaderris species. These conservation activities have revealed that for many species it is often difficult to obtain seeds (Roberts and Trueman 2016; McAuliffe et al. 2016). If seeds are obtained physical dormancy is likely and needs to be alleviated with scarification, heat or acid treatment to enable germination (Turner et al. 2005; Haines et al. 2007; Patykowski et al. 2016), and if seed cannot be obtained then clonal production via cuttings or tissue culture can be a viable option (Roberts and Trueman 2016; McAuliffe et al. 2016). Currently, these conservation and propagation activities are undertaken without information on cytology and reproductive biology, both of which are important to ensure adequate sampling (Hoban and Strand 2015), management of collections or seed production areas (Nevill et al. 2016), and translocation design.
Genome size in the genus Pomaderris has not been studied; no values are available in the scientific literature, nor in the Kew Plant DNA C-values Database (http://data.kew.org/cvalues/, Accessed 24 Feb 2019). At the same time, there is no published information on chromosome numbers in Australian Pomaderris except for a few species shared with New Zealand. Diploid, triploid and tetraploid species have been reported among New Zealand Pomaderris (Hair 1963; Harvey and Braggins 1985; Harvey and Rattenbury 1985). This raises the possibility that the closely related Australian Pomaderris species may also show variation in ploidy. Interspecific variation in ploidy, in turn, would suggest within-species variation may also be present, as polyploid complexes generally show frequent and parallel whole genome duplication events as opposed to single, isolated events (Thompson and Lumaret 1992; Soltis and Soltis 1999).
New Zealand triploid species of Pomaderris are agamospermous, producing seeds asexually, and a tetraploid species was suggested to be facultatively apomictic (Harvey and Braggins 1985). Should threatened Australian congeners be apomictic, then this would have practical consequences for conservation management, as it would imply lower genetic diversity within populations (Lo et al. 2009) which would in turn need to inform sampling designs for ex situ conservation (Hoban and Strand 2015).
Aims of the study
Ongoing conservation management work on eight Australian species, Pomaderris adnata N.G.Walsh & F.Coates, P. bodalla N.G.Walsh & F.Coates, P. brunnea N.A.Wakef., P. cotoneaster N.A.Wakef., P. delicata N.G.Walsh & F.Coates, P. pallida N.A.Wakef., P. reperta N.G.Walsh & F.Coates, and P. walshii J.C.Millott & K.L.McDougall, required testing for intraspecific polyploidy to avoid the inadvertent creation of odd-ploids during seed production. This study aimed to advance ex situ conservation practices by providing cytological and reproductive biology information. Specifically, the aims of the present study were to:
-
1.
Determine the genome size of Australian Pomaderris, as far as available, to test for the first time if there is polyploidy in the Australian species of the genus.
-
2.
Conduct chromosome counts of selected individuals to establish the relationship between genome sizes and ploidy.
-
3.
Test seeds of available species to determine reproductive biology.
-
4.
Determine if polyploid complexes exist within rare or near-rare species subject to conservation management.
-
5.
Provide management recommendations based on these results, to inform seed collection, plant and seed production, and the creation of augmented, translocated or additional populations.
Materials and methods
Study species and sampling
Sampling of 46 species across the genus was possible due to availability of fresh leaf samples and/or seeds at various botanic gardens (Online Appendix 1). Leaf samples were obtained from potted or tubestock plants of 36 species. These were recently added accessions in the living collection of the Australian National Botanic Gardens (ANBG) and Wollongong Botanic Garden (WBG), except for a sample of P. parrisiae kindly provided by Francis Nge of the State Herbarium of South Australia. Seeds of 25 species were sampled from the National Seed Bank of the ANBG, PlantBank at the Australian Botanic Garden Mount Annan, and the research collection of Dr Mark Ooi at the University of New South Wales.
Collaboration partners collected plants from field sites in New South Wales and the Australian Capital Territory from September 2015 to January 2018 (Fig. 2). Sampling aimed to collect from as many sites per species as possible and from multiple individuals per site. Pomaderris adnata, P. reperta, and P. walshii are known only from a single locality each. Pomaderris delicata is known from two localities, both of which were sampled. Pomaderris brunnea and P. cotoneaster were sampled across their ranges with the exception of their two respectively southernmost populations which were inaccessible. A previously recorded northern outlier population of P. brunnea was not found during field work, suggesting that it may be extinct (J. McAuliffe, pers. comm.). Pomaderris bodalla has a small continuous, coastal distribution of which the northern and southern ends were sampled. Pomaderris pallida was sampled only across the northern half of its range.
Cuttings, seeds and herbarium vouchers were collected, and cuttings were propagated in nurseries in south-eastern Australia. Living plants for analysis were held as tubestock or in pots in the ANBG and WBG nurseries.
Flow cytometry
Flow cytometry is a rapid and reliable method to analyse properties of microscopic particles in liquid suspension. The method has a wide range of applications in the plant sciences including the estimation of nuclear DNA content, often expressed as the 2C value of DNA in picograms (pg) (Doležel et al. 2007; Vrána et al. 2014). The relationship between ploidy and nuclear DNA content generally allows the recognition of polyploid complexes among close relatives, e.g. if genome sizes of different individuals show ratios of c. 1:2 (potentially diploid and tetraploid, or tetraploid and octoploid) or 1:1.5 (potentially diploid and triploid, or tetraploid and hexaploid). Although living tissue is required, only a small amount is needed.
For flow cytometry, fresh young leaves were collected from Pomaderris specimens and kept on ice or refrigerated for up to 2 days. Glycine max ‘Polanka’ (2C = 2.50 pg) (Doležel et al. 1994) was used as an internal standard. CyStain PI Absolute P (Sysmex Partex GmbH, Görlitz, Germany) was used for sample preparation following the manufacturer’s instructions. A leaf area of approximately 1 cm2 from the Pomaderris plant and the standard was placed in 500 μL of nuclei extraction buffer and chopped manually with a double edge razor in a Petri dish lid for approximately 30 s. The leaf material was swirled gently in the buffer to release intact nuclei. The liquid was filtered through a 40 μm cell strainer, and the filtered sample was transferred to a sample tube. The sample was mixed with 2000 μL staining solution (consisting of 2 mL staining buffer, 12 μL of 1 mg/mL propidium iodide and 6 μL of 1 mg/mL RNAse) and loaded into a flow cytometer with a 488 nm laser (BD Accuri C6 Plus equipped with a BD CSampler Plus, BD Biosciences, San Jose, CA, USA) and run at a flow rate of 14 μm/min per min and core size of 10 μm. Histogram data were collected using the FL2 detector while eliminating events with a value of less than 5000 on FL2-H. Analysis was performed with the BD Accuri C6 Software version 1.0.23.1.
For screening, flow cytometry was run until at least 5000 events were counted in total. For reporting representative 2C values for each species, a plant was selected from each species and measured in triplicate, with standard errors calculated for each individual. In this case, runs were terminated after counting 2000 events for the sample peak.
For species where seeds were available, seeds were screened with flow cytometry to measure the genome size ratios between embryo and endosperm, which can provide information on the reproductive biology of a species (Matzk et al. 2001; Dobeš et al. 2013). A ratio of 1.5, for example, is typical of normal sexual reproduction with diploid embryo and triploid endosperm resulting from double fertilisation, whereas a ratio of 2.5 may indicate a diploid embryo and pentaploid endosperm. As the latter is most parsimoniously explained as the result of unreduced (diploid) polar nuclei fusing with a reduced (haploid) sperm nucleus, this situation would imply that the embryo was produced asexually.
Depending on availability, we used between one and ten seeds per measurement and collected varying numbers of events, as small numbers of seeds do not always release thousands of usable nuclei. Glycine max ‘Polanka’ and Zea mays ‘CE-777’ (2C = 5.43 pg) (Doležel et al. 2007) were used as internal standards. In cases where the internal standard peak was too close to the endosperm peak we repeated flow cytometry without a standard to determine embryo: endosperm ratios and infer reproductive biology.
Chromosome counts
Our methodology was adapted from that used by Castro and Rosselló (2007). Several root tips were collected from a representative plant of selected species approximately 1–2 h after watering and immediately pre-treated with 0.002 M 8-hydroxyquinoline solution for c. 4 h at 4 °C, washed with distilled water, fixed in fresh Carnoy I (95% alcohol and acetic acid in a 3:1 ratio) overnight and preserved in 70% ethanol at − 20 °C. For examination, root tips were hydrolysed for 10 min in 1 M HCl at 60 °C and stained in aceto-orcein for approximately 4 h. The first c. 1 mm of two root tips were spread out on a glass slide by tapping with a brass rod for 30 s in a drop of 45% acetic acid. A coverslip was added and then pressed down firmly under tissue paper.
Chromosome counts were made from well-spread metaphases on digital photographs taken with Leica Application Suite (Version 4.8.0) software from a Leica DM 2500 LED compound microscope at the 100 × oil immersion level.
Results
Genome size and chromosome counts across the genus
Measurement of genome size across 36 species (37 taxa including subspecies) of Pomaderris revealed a group of putative diploids with genome sizes of 0.783 ± 0.009 to 0.998 ± 0.003 pg (mean ± standard error), a gradient of species with genome sizes ranging from 1.319 ± 0.004 to 2.046 ± 0.009 pg, and a single taxon, P. paniculosa subsp. paralia, with a genome size of 2.908 ± 0.004 pg (Fig. 3; Online Appendix 1).
Mean genome size ± S.E. from triplicate measurements of Pomaderris species. Bar colours indicate sections of the genus in the classification of Walsh and Coates (1997). Plain numbers above bars indicate chromosome counts, with those marked with asterisks representing counts published before the present study (Hair 1963; Harvey and Rattenbury 1985). Numbers with multiplier signs indicate endosperm: embryo genome size ratios
Our chromosome counts from root tip squashes were 2n = 24 for P. bodalla (genome size 2C = 0.966 ± 0.004), 36 for P. pallida (2C = 1.394 ± 0.006) and P. elliptica var. elliptica (2C = 1.667 ± 0.005), and at least 43 for P. walshii (2C = 1.993 ± 0.002).
Flow cytometric seed screen
Flow cytometric seed screen showed ratios in genome size between embryo and endosperm of c. 1.5 for Pomaderris forrestiana, P. lanigera, P. ledifolia, P. notata, P. obcordata, and P. vellea, c. 2.5 for Pomaderris bodalla, P. costata, P. brunnea, P. cotoneaster, P. delicata, P. discolor, P. mediora, P. parrisiae, and P. velutina, and c. 3.0 for P. andromedifolia, P. argyrophylla, P. eriocephala, P. intermedia, P. pallida, P. prunifolia var. prunifolia, P. queenslandica, and P. reperta (Online Appendix 1). In the case of P. cocoparrana, measurement of two seeds together produced three peaks, with the second c. 1.5 and the third c. 2.5 times the size of the first. A repeat measurement of a single seed showed only a ratio of c. 1.5, but unfortunately no more seeds were available for further sampling.
Only two taxa showed inconsistent genome sizes between leaf samples and seed accessions. A seed sample of P. parrisiae showed nearly exactly twice the genome size of an independently collected leaf sample, suggesting the existence of diploids and tetraploids in the same species. Although measurements of leaves and seeds of P. prunifolia var. prunifolia similarly suggest tetraploidy and triploidy, respectively, the first of these two specimens is morphologically aberrant, raising the possibility that there is an unresolved taxonomic issue.
Population screening
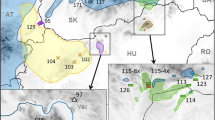
A total of 104 Pomaderris plants from twenty populations of eight species subject to conservation management were screened (Fig. 4; Online Appendix 2). No variation large enough to indicate ploidy differences was detected within any of the eight species. The greatest difference in measurements within a species was 10.5%, in P. pallida, and the difference was only between 2.7% and 6.5% in the other species.
Genome size measurements for individuals from screened populations of eight rare species of Pomaderris (2C values). Within each species results are grouped by populations. No species shows variation of a degree that would indicate intraspecific polyploidy. Pomaderris adnata, P. bodalla, P. brunnea, and P. delicata have genome sizes suggesting diploidy, P. cotoneaster and P. walshii have sizes suggesting tetraploidy, and P. pallida and P. reperta have intermediate sizes suggesting triploidy
Discussion
In the present study we aimed to determine cytology and reproductive biology of Australian Pomaderris to inform conservation management, particularly ex situ seed conservation and production protocols. We sampled a large portion (over half) of the genus and revealed that polyploidy and apomixis occur frequently across the genus, in both rare and common species. The conservation and evolutionary implications of the prevalence of polyploidy and apomixis in Australian Pomaderris are discussed.
Polyploidy in Pomaderris
Our data from three different lines of evidence—genome sizes of mature plants, genome size ratios in seeds, and chromosome counts—demonstrate widespread ploidy differences in Australian Pomaderris. A group of species with the smallest genome sizes of c. 0.8–1.0 pg was clearly distinct. A gradient of species showed genome sizes of c. 1.3–2.0 pg, and Pomaderris paniculosa subsp. paralia stood out with a larger size of c. 2.9 pg. We produced chromosome counts of selected species and measured genome sizes for two taxa for which counts had been previously published—P. aspera (2n = 24, 2C = 0.945 pg) and P. phylicifolia (2n = 36, 2C = 1.34 pg) (Hair 1963; Harvey and Rattenbury 1985). Considered together, these data support the assumption that samples in the small genome size group are diploid, those at the lower end of the intermediate gradient are triploid, and those at the upper end of the gradient are tetraploid. Based on our count of 2n = 36 for P. elliptica var. elliptica, it appears most likely that it is the triploid species with the largest genome size in our study, and that the next four larger taxa are tetraploid. Although P. paniculosa subsp. paralia has not been counted, its genome size is consistent with the assumption of hexaploidy.
Polyploidy appears to be widespread in Australian Pomaderris, with the diploid group accounting for 20 of the 37 taxa whose genome sizes we measured. Whole genome duplication has been identified as a driver of speciation as it confers instantaneous genetic isolation (Soltis et al. 2007), with the caveat that polyploid species are usually of polyphyletic origin (Soltis and Soltis 1999). It also enables hybridogenic speciation through restoration of fertility (Rieseberg 2001) and can be a key event preceding radiations into harsher environments (Chen et al. 2007; Schmidt-Lebuhn et al. 2010).
To understand the evolutionary history of Pomaderris, it is therefore important to place polyploidization events in a phylogenetic context. At the same time, this may enable us to make predictions about species whose ploidy has not yet been established. A nested member of a clade whose other species are known to be triploid and apomictic could, for example, be assumed to share these traits, which would provide guidance for the development of management plans.
The most complete currently available phylogenies of Pomaderris were sampled broadly across the tribe Pomaderreae and included only a limited number of Pomaderris taxa—thirteen represented by nrITS sequences (Kellermann et al. 2005) and 12 by trnL-trnF sequences (Kellermann and Udovicic 2008), only five of which were included in this study.
The genus has been divided into seven sections based on floral, fruiting and indumentum characters (Walsh and Coates 1997). Existing phylogenies do not have sufficient sampling and resolution to test the monophyly of the sections, but in the absence of such data this classification provides the best available framework in which to examine variation in ploidy. Taxa from four sections have been sampled in this study, and sections Apetalae and Pomaderris contain different ploidy levels. This suggests that polyploidy in the genus may have arisen several times independently. Without a detailed phylogeny, however, it has to remain unclear how often and when polyploidy arose in the genus.
Once our results can be placed in the context of a well resolved phylogeny, we will be able to gain a clearer understanding of the evolution of genome size and ploidy and of the impacts of polyploidization on patterns of diversification in Pomaderris. Such information would also provide useful background for focusing conservation efforts on maintaining evolutionary potential and genetic diversity.
Reproductive biology
Our results indicate that numerous species of Pomaderris reproduce asexually through pseudogamous apomixis (Online Appendix 1, Table 1). Understanding the factors influencing seed set and fitness is crucial to assure the long-term security of rare plant species. Management of rare triploid species, confirmed (P. pallida) and putative (P. reperta), is of particular concern, as triploid species are generally expected to require asexual means of reproduction. Although the results demonstrate that asexual reproduction does occur widely across the genus, it may succeed only infrequently in some species where seed set is commonly observed to be very low.
Obtaining seed has so far proven difficult for P. pallida with 196 seeds being retrieved from 18 plants in one population and no seed from another population; on the other hand, P. reperta has produced 35,497 seeds from 30 bagged plants. Pomaderris delicata, a diploid apomict, produced very little seed in the wild but much larger numbers in cultivation (all data T. North, pers. comm.). Potential explanations are low mate availability, pollinator limitation or stress-induced seed abortion in situ, but experimental data are needed to resolve the question. In particular in triploid individuals, failure of meiosis would be an additional possibility but immediately raises the question of how a species would have historically persisted and dispersed without producing appreciable numbers of seeds.
The flow cytometric seed screen of Pomaderris pallida, P. reperta and six other species showed an endosperm:embryo ratio of c. 3 and embryonic genome sizes of c. 1.14–1.51 pg. We suggest that the most parsimonious explanation of these values is triploidy (Fig. 3) and pseudogamous apomixis with apomeiotic origin of the pollen: both embryo sac and pollen are produced without meiosis, the embryo is a clone of the mother, and the endosperm obtains six genome copies from the mother and three from a pollen nucleus. Production of unreduced pollen grains has been observed in triploid Pomaderris in New Zealand (Harvey and Rattenbury 1985). The same process could occur in diploids and tetraploids (Ramsey and Schemske 1998, 2002), likewise leading to a genome size ratio of c. 3 in the seed, but we consider this less likely given that all species for which an even level of ploidy can be assumed with some confidence appear to produce pollen meiotically (see below).
More surprisingly, our results suggest that many even-ploid Australian species of Pomaderris may likewise regularly produce seeds asexually. Seven species showed a ratio of c. 2.5 and embryonic genome sizes of c. 0.84–0.92 pg. We consider it most likely that this is the result of diploidy and pseudogamous apomixis with meiotic origin of the pollen. In this scenario the embryo sac would be produced without meiosis, the embryo is accordingly a clone of the mother, and the endosperm obtains four genome copies from the mother and one from a haploid pollen nucleus (Dobeš et al. 2013). The same applies to P. cotoneaster and P. parrisiae but at the level of tetraploidy with a decaploid endosperm derived from eight maternal and two paternal genome copies.
The implication is in all cases pseudogamous agamospermy, meaning that offspring are genetically uniform but reproductive success is still dependent on pollination (or at least self-pollination) (Noirot et al. 1997). As we aimed to screen across many species we did not systematically screen multiple seed samples per species, and it remains possible that apomixis is facultative in the even-ploid species, as we observed within the same seed lot in P. cocoparrana. The large number of apomictically produced seed in our analyses indicates, however, that apomixis is at least very common in Australian Pomaderris.
Previous research showed that Pomaderris kumeraho, which is endemic to the North Island of New Zealand, is a diploid (2n = 24) that is self-fertile and reproduces sexually (Harvey and Braggins 1985; Harvey and Rattenbury 1985). However, two triploid taxa, P. hamiltonii and P. phylicifolia var. ericifolia, reproduce by agamospermy. In the tetraploid P. phylicifolia reproduction is mainly sexual, but there is some indication that plants may be facultative apomicts.
A predominance of asexual reproduction would have implications for the conservation management of a species, because many or all individuals of a given population may be genetically identical. For species with low genetic diversity (e.g. due to high selfing) it is recommended that sampling for seed conservation focus on increasing the number of mothers sampled rather than number of seeds per plant (Hoban and Strand 2015). This recommendation could also be applied to apomictic species. Some allelic diversity may be produced if apomixis is facultative (Berthaud 2001) and again in this case sampling many mothers would increase the likelihood of capturing unique alleles.
If apomixis is obligate for some species, seed production areas may not need to control paternity in those cases (as for pseudogamous apomixis the pollen does not contribute genes to the offspring) and effort may be best directed at including as many maternal lines as possible. The evolutionary ecology of apomixis is not widely studied and as such has not necessarily been applied to translocation planning. While low genetic diversity is often considered problematic in conservation (Hoban and Strand 2015), in the case of apomictic species it can be associated with specialisation or adaptation to the environment and proven site of the mother plant (van Dijk 2003). In species where both sexual and apomictic plants occur the apomicts often have wider geographic distribution than the sexuals. This may, however, only be beneficial in the short-term, as in the longer term evolutionary success may be limited by lack of adaptive potential (van Dijk 2003). These patterns may also affect translocation success.
Within-species ploidy variation
No variation in ploidy was detected within the eight species subject to conservation management (Pomaderris adnata, P. bodalla, P. brunnea, P. cotoneaster, P. delicata, P. pallida, P. reperta and P. walshii). Although the existence of such variation cannot be excluded entirely unless every individual of each species is measured, our sample was representative for many of the extant populations used to source seeds and cuttings in ongoing conservation work. Our results accordingly suggest that during conservation management of the aforementioned eight selected rare species, screened populations of the same species do not need to be kept geographically separated from a cytological perspective.
The only significant gaps are single southern outlier populations of P. brunnea and P. cotoneaster. Should these populations be used as sources for seed production and revegetation in the future, it would be recommended to test the ploidy of representative individuals.
It was beyond the scope of this study to examine whether different species should be kept geographically separated where they are not already co-occurring to avoid hybridisation between species. This information would be valuable for conservation management and could be obtained through crossing trials.
Data availability
Flow cytometric data are available in the Supplementary Information.
References
Australian Government (1999) Environment Protection and Biodiversity Conservation Act 1999
Berthaud J (2001) Apomixis and the management of genetic diversity. In: Savidan Y, Carman JG, Dresselhaus T (eds) The flowering of apomixis: from mechanisms to genetic engineering. CIMMYT, IRD, and EU RTD FAIR, Houston, pp 8–23
Brochmann C, Brysting AK, Alsos IG et al (2004) Polyploidy in arctic plants. Biol J Linn Soc 82:521–536. https://doi.org/10.1111/j.1095-8312.2004.00337.x
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc 61:51–94. https://doi.org/10.1111/j.1095-8312.1997.tb01778.x
Castro M, Rosselló JA (2007) Karyological observations on plant taxa endemic to the Balearic Islands. Bot J Linn Soc 153:463–476. https://doi.org/10.1111/j.1095-8339.2007.00617.x
Chen G, Sun W-B, Sun H (2007) Ploidy variation in Buddleja L. (Buddlejaceae) in the Sino-Himalayan region and its biogeographical implications. Bot J Linn Soc 154:305–312. https://doi.org/10.1111/j.1095-8339.2007.00650.x
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846. https://doi.org/10.1038/nrg1711
Dobeš C, Lückl A, Hülber K, Paule J (2013) Prospects and limits of the flow cytometric seed screen—insights from Potentilla sensu lato (Potentilleae, Rosaceae). New Phytol 198:605–616. https://doi.org/10.1111/nph.12149
Doležel J, Doleželová M, Novák FJ (1994) Flow cytometric estimation of nuclear DNA amount in diploid bananas (Musa acuminata and M. balbisiana). Biol Plant 36:351. https://doi.org/10.1007/bf02920930
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244. https://doi.org/10.1038/nprot.2007.310
Frankham R, Ballou JD, Briscoe DA (2010) Introduction to conservation genetics. Cambridge University Press, Cambridge
Godfree RC, Marshall DJ, Young AG et al (2017) Empirical evidence of fixed and homeostatic patterns of polyploid advantage in a keystone grass exposed to drought and heat stress. Open Sci 4:170934. https://doi.org/10.1098/rsos.170934
Haines L, Ennis IL, Blanchon DJ, Triggs CM (2007) Propagating the pale-flowered kumarahou (Pomaderris hamiltonii) and kumarahou (Pomaderris kumeraho) from seeds. N Z J Bot 45:91–100. https://doi.org/10.1080/00288250709509706
Hair JB (1963) Contributions to a chromosome atlas of the New Zealand Flora—6. N Z J Bot 1:243–257. https://doi.org/10.1080/0028825X.1963.10428997
Harvey CF, Braggins JE (1985) Reproduction in the New Zealand taxa of Pomaderris Labill. (Rhamnaceae). N Z J Bot 23:151–156. https://doi.org/10.1080/0028825X.1985.10425316
Harvey CF, Rattenbury JA (1985) Microsporogenesis and pollen viability in New Zealand taxa of Pomaderris Labill. (Rhamnaceae). N Z J Bot 23:321–330. https://doi.org/10.1080/0028825X.1985.10425334
Hoban S, Strand A (2015) Ex situ seed collections will benefit from considering spatial sampling design and species’ reproductive biology. Biol Conserv 187:182–191. https://doi.org/10.1016/j.biocon.2015.04.023
Husband BC, Baldwin SJ, Suda J (2013) The incidence of polyploidy in natural plant populations: major patterns and evolutionary processes. Plant genome diversity, vol. 2. Springer, Vienna, pp 255–276
Jain M, Flynn DFB, Prager CM et al (2013) The importance of rare species: a trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol Evol 4:104–112. https://doi.org/10.1002/ece3.915
Jenkins G, Rees H (1991) Strategies of bivalent formation in allopolyploid plants. Proc R Soc Lond B Biol Sci 243:209–214. https://doi.org/10.1098/rspb.1991.0033
Kellermann J, Udovicic F (2008) Large indels obscure phylogeny in analysis of chloroplast DNA (trnL-F) sequence data: Pomaderreae (Rhamnaceae) revisited. Telopea 12:1–22
Kellermann J, Udovicic F, Ladiges PY (2005) Phylogenetic analysis and generic limits of the tribe Pomaderreae (Rhamnaceae) using internal transcribed spacer DNA sequences. Taxon 54:619–631
Köhler C, Mittelsten Scheid O, Erilova A (2010) The impact of the triploid block on the origin and evolution of polyploid plants. Trends Genet 26:142–148. https://doi.org/10.1016/j.tig.2009.12.006
Kramer AT, Havens K (2009) Plant conservation genetics in a changing world. Trends Plant Sci 14:599–607. https://doi.org/10.1016/j.tplants.2009.08.005
Leitch IJ, Bennett MD (1997) Polyploidy in angiosperms. Trends Plant Sci 2:470–476. https://doi.org/10.1016/S1360-1385(97)01154-0
Levin DA (1975) Minority cytotype exclusion in local plant populations. Taxon 24:35–43. https://doi.org/10.2307/1218997
Lo EYY, Stefanović S, Dickinson TA (2009) Population genetic structure of diploid sexual and polyploid apomictic hawthorns (Crataegus; Rosaceae) in the Pacific Northwest. Mol Ecol 18:1145–1160. https://doi.org/10.1111/j.1365-294X.2009.04091.x
Madlung A (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 110:99–104. https://doi.org/10.1038/hdy.2012.79
Masterson J (1994) Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264:421–424. https://doi.org/10.1126/science.264.5157.421
Matzk F, Meister A, Schubert I (2001) An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J 21:97–108. https://doi.org/10.1046/j.1365-313x.2000.00647.x
McAuliffe J, Taylor D, McDougall K (2016) A non-seed based method for enhancement planting of the critically endangered “Pomaderris delicata”. Australas Plant Conserv 25:14
Miller JS, Venable DL (2000) Polyploidy and the evolution of gender dimorphism in plants. Science 289:2335–2338. https://doi.org/10.1126/science.289.5488.2335
Morris WF, Doak DF (2002) Quantitative conservation biology. Sinauer Associates, Massachusetts
Muller HJ (1925) Why polyploidy is rarer in animals than in plants. Am Nat 59:346–353. https://doi.org/10.1086/280047
Nevill PG, Tomlinson S, Elliott CP et al (2016) Seed production areas for the global restoration challenge. Ecol Evol 6:7490–7497. https://doi.org/10.1002/ece3.2455
New South Wales Government (2016) Biodiversity Conservation Act 2016 No 63
Noirot M, Couvet D, Hamon S (1997) Main role of self-pollination rate on reproductive allocations in pseudogamous apomicts. Theor Appl Genet 95:479–483. https://doi.org/10.1007/s001220050586
Pandit MK, Pocock MJO, Kunin WE (2011) Ploidy influences rarity and invasiveness in plants. J Ecol 99:1108–1115. https://doi.org/10.1111/j.1365-2745.2011.01838.x
Patykowski J, Dell M, Gibson M (2016) Germination ecology and seed dispersal of a critically endangered plant: a case study of Pomaderris vacciniifolia (round-leaf Pomaderris). PLoS ONE 11:e0161665. https://doi.org/10.1371/journal.pone.0161665
Pickup M, Young AG (2007) Population size, self-incompatibility and genetic rescue in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Heredity 100:268–274. https://doi.org/10.1038/sj.hdy.6801070
Plue J, Kimberley A, Slotte T (2018) Interspecific variation in ploidy as a key plant trait outlining local extinction risks and community patterns in fragmented landscapes. Funct Ecol 32:2095–2106. https://doi.org/10.1111/1365-2435.13127
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Ramsey J, Schemske DW (2002) Neopolyploidy in flowering plants. Annu Rev Ecol Syst 33:589–639. https://doi.org/10.1146/annurev.ecolsys.33.010802.150437
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237. https://doi.org/10.1046/j.1523-1739.2003.01236.x
Rieseberg LH (2001) Polyploid evolution: keeping the peace at genomic reunions. Curr Biol 11:R925–R928. https://doi.org/10.1016/S0960-9822(01)00556-5
Roberts KS, Trueman SJ (2016) Plant propagation for environmental offset planting: a case study of endangered Pomaderris clivicola and near-threatened Bertya pedicellata—SciAlert responsive version. J Environ Sci Technol 9:452–461. https://doi.org/10.3923/jest.2016.452.461
Schmidt-Lebuhn AN, Fuchs J, Hertel D et al (2010) An Andean radiation: polyploidy in the tree genus Polylepis (Rosaceae, Sanguisorbeae). Plant Biol 12:917–926. https://doi.org/10.1111/j.1438-8677.2009.00297.x
Schmidt-Lebuhn AN, Marshall DJ, Dreis B, Young AG (2018) Genetic rescue in a plant polyploid complex: case study on the importance of genetic and trait data for conservation management. Ecol Evol 8:5153–5163. https://doi.org/10.1002/ece3.4039
Severns PM, Liston A (2008) Intraspecific chromosome number variation: a neglected threat to the conservation of rare plants. Conserv Biol 22:1641–1647. https://doi.org/10.1111/j.1523-1739.2008.01058.x
Soltis DE, Soltis PS (1999) Polyploidy: recurrent formation and genome evolution. Trends Ecol Evol 14:348–352. https://doi.org/10.1016/S0169-5347(99)01638-9
Soltis DE, Soltis PS, Schemske DW et al (2007) Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon 56:13–30. https://doi.org/10.2307/25065732
Soltis DE, Albert VA, Leebens-Mack J et al (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348. https://doi.org/10.3732/ajb.0800079
te Beest M, Le Roux L, Richardson DM et al (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109:19–45. https://doi.org/10.1093/aob/mcr277
Thompson JD, Lumaret R (1992) The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends Ecol Evol 7:302–307. https://doi.org/10.1016/0169-5347(92)90228-4
Turner SR, Merritt DJ, Baskin CC et al (2005) Physical dormancy in seeds of six genera of Australian Rhamnaceae. Seed Sci Res 15:51–58. https://doi.org/10.1079/SSR2004197
van Dijk PJ (2003) Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philos Trans R Soc Lond B Biol Sci 358:1113–1121. https://doi.org/10.1098/rstb.2003.1302
Vrána J, Cápal P, Bednářová M, Doležel J (2014) Flow cytometry in plant research: a success story. Applied plant cell biology. Springer, Berlin, pp 395–430
Wallace MJ, Guja LK, Jouault MA et al (2017) DNA ploidy variation and distribution in the Lepidosperma costale complex (Cyperaceae): implications for conservation and restoration in a biodiversity hotspot. Aust J Bot 65:120–127. https://doi.org/10.1071/BT16197
Waller DM (2015) Genetic rescue: a safe or risky bet? Mol Ecol 24:2595–2597. https://doi.org/10.1111/mec.13220
Walsh NG, Coates F (1997) New taxa, new combinations and an infrageneric classification in Pomaderris (Rhamnaceae). Muelleria 10:27–56
Acknowledgements
We thank the many colleagues who collected Pomaderris vouchers, cuttings and seeds across south-eastern Australia, maintained living collections, and made collections available for this research: the Australian National Botanic Gardens nursery and National Seed Bank; the Wollongong Botanic Garden; PlantBank and The Royal Botanic Gardens & Domain Trust; Francis Nge and Mark Ooi. We thank David Marshall and Mark Wallace for assistance and advice in developing flow cytometry protocols for Pomaderris. Dave Albrecht, Jo Palmer and Neville Walsh kindly confirmed identifications of voucher specimens. Constructive comments by three anonymous reviewers helped us to improve a previous version of the manuscript. The work has been assisted by the New South Wales Government through its Environmental Trust and is part of a project (2015 RD 004) lead by David Taylor in collaboration with the Australian National Botanic Gardens, The Australian Botanic Garden Mount Annan, Wollongong Botanic Garden, Eurobodalla Regional Botanic Garden, Booderee National Park, the NSW Office of Environment and Heritage, the ACT Government and the University of New South Wales. The research was also supported by a CSIRO Undergraduate Research Scholarship awarded to S.H.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10592_2019_1184_MOESM2_ESM.csv
Supplementary material 2 Genome size measurements from screening across populations of rare Australian species of Pomaderris, with voucher information (CSV 10 kb)
Rights and permissions
About this article
Cite this article
Chen, S.H., Guja, L.K. & Schmidt-Lebuhn, A.N. Conservation implications of widespread polyploidy and apomixis: a case study in the genus Pomaderris (Rhamnaceae). Conserv Genet 20, 917–926 (2019). https://doi.org/10.1007/s10592-019-01184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-019-01184-2