Abstract
Because Upper Midwest temperate forests lack native earthworms, the invasions of European and Asian earthworms can significantly alter soils and understory vegetation. Earthworms’ ability to increase leaf litter decay, alter nutrient cycling by mixing the organic layer with mineral soil, and decrease plant species richness leads to concern about the Asian ‘jumping earthworm’ (Amynthas agrestis and A. tokioensis) species that were recorded in the University of Wisconsin—Madison Arboretum in 2013. In 2015, we found A. agrestis and A. tokioensis in a distinct 8-ha region of a 23-ha hardwood forest surveyed in the Arboretum; by 2016 A. agrestis and A. tokioensis had spread over an additional 7 ha. Plots also contained the European earthworm species Lumbricus terrestris, L. rubellus, and Apporectodea spp., whose distributions decreased from 2015 to 2016. While leaf litter, plant species richness, and tree and shrub seedling abundance were generally reduced in areas with European earthworms, they were typically slightly increased in areas with A. agrestis and A. tokioensis versus those without. Although our results do not show substantial impacts of A. agrestis and A. tokioensis on vegetation in the initial years of invasion, the rapid replacement of European earthworms by A. agrestis and A. tokioensis suggests continued monitoring of these new invasive species is important to better understand their potential to change the Upper Midwest’s forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessing the impacts of invasive species soon after establishment is vital for understanding species’ roles in a new ecosystem and can help anticipate changes that will accompany their spread. Because the northern temperate region of the United States lacks native earthworms due to widespread glaciation in the last ice age (Bohlen and Hendrix 2002; Bohlen et al. 2004, Fig. 1a), the Upper Midwest is an ideal location to study the ecosystem effects of earthworms. Although European earthworms have been present in the Upper Midwest since European settlement (Hale et al. 2005), the ecological impacts of the Asian genus Amynthas have not been well studied, representing an important gap in knowledge of invasive earthworm biology (Richardson et al. 2009). The recent arrival of Amynthas agrestis and Amynthas tokioensis earthworms to the region has allowed for observation of these species’ initial effects in Wisconsin forests. Our research observed the understory vegetation and forest floor immediately following the introduction of Amynthas earthworms.
a Location of study area within the Midwest, with bold line representing the southern extent of hypothesized earthworm absence due to glaciation, adapted from Bohlen and Hendrix (2002). b Madison, Wisconsin, with the University of Wisconsin—Arboretum in light grey, and the study area in dark grey
European earthworms have been well studied for their effects on the naturally earthworm-lacking hardwood forests of the Upper Midwest (Hale et al. 2006; Holdsworth et al. 2007). Known impacts of earthworms on forest floor structure include increases in leaf litter decay, soil pH, rates of nitrogen transformation and carbon flux, and litter-soil mixing (Burtelow et al. 1998; Bohlen et al. 2004; Drouin et al. 2016). The influence of an earthworm species can depend on its functional group; epigeic species live in and alter the litter layer and upper surface of the soil, endogeic species alter both mineral and organic soil, and anecic species form deep vertical burrows, altering the forest floor and mineral horizons (Bouché 1971; Bohlen and Hendrix 2002; Asshoff et al. 2010). Soil impacts of exotic earthworms include a disruption of native soil biota, including microbes and fungi. For example, earthworms have been found to increase soil microbial biomass (Burtelow et al. 1998; Drouin et al. 2016), and to decrease mycorrhizal associations in forests (Lawrence et al. 2003). Through these abiotic and biotic mechanisms, European earthworms have been found to negatively affect vegetation in invaded regions lacking native earthworms. Native plant species richness is typically negatively correlated with abundance of the European earthworms in the genus Lumbricus (Hale et al. 2006; Holdsworth et al. 2007; Gibson et al. 2013), while the presence of multiple non-native earthworm species can lead to an increase in plant mortality, particularly of Acer seedlings (Hale et al. 2008).
Unlike European species of the Lumbricidae family, the impacts of invasive Amynthas earthworms are not yet thoroughly understood. Native to east-central Asia, the Amynthas genus, family Megascolecidae, has been present in North America since the late 1800s (Snyder et al. 2011) and populations of A. agrestis have established in areas of New England (Burtelow et al. 1998; Gorres and Melnichuk 2012; Gorres et al. 2014) and the southern Appalachian mountains (Callaham et al. 2003; Snyder et al. 2011). A. agrestis earthworms typically occur in high densities, even in areas with native North American earthworms (Callaham et al. 2003), and their invasions result in a long-lasting granular soil surface structure, a decrease of the organic horizon, and an increase in soil pH and soil microbial biomass (Burtelow et al. 1998; Greiner et al. 2012). Optimum conditions for A. agrestis are around 25 °C with high soil moisture (Richardson et al. 2009) and their high potential for invasion has been partially credited to the ability to shift their diet among leaf litter, soil bacteria, and other soil fauna depending on availability (Zhang et al. 2010). Although A. tokioensis has not been studied as much as A. agrestis, the two are believed to share traits with each other and with others in the genus: they are typically epigeic or epi-endogeic, reproduce parthenogenetically, have faster growth rates than invasive European earthworms, and undergo an annual life cycle, experiencing mortality of adults in the fall and emergence from cocoons in spring (Burtelow et al. 1998; Greiner et al. 2012).
In 2013, the first documentation of Amynthas spp. in Wisconsin was recorded at the University of Wisconsin-Madison Arboretum, located in the south-central part of the state. Recent developments in Amynthas taxonomy and genetic testing of samples from the Arboretum have confirmed the presence of both A. agrestis and A. tokioensis (Chang et al. 2016a; Damhnait McHugh, Colgate University, unpublished data and pers. comm. 2017). Both species are colloquially known as ‘jumping worms’ for the defensive thrashing behavior displayed when handled, and they are thought to have similar ecological impacts. Because the field research in this study occurred before confirmation of the Amynthas species present, the two were not differentiated and will be referred to collectively. A recent mesocosm study done at the UW Arboretum found that these two species of Amynthas significantly decreased leaf litter and increased the percentage of carbon, nitrogen, and phosphorous in the soil (Qiu and Turner 2016). Because of known vegetation impacts from European species, researchers and managers are concerned with potential negative effects of A. agrestis and A. tokioensis on temperate deciduous forests. In an experiment conducted in Michigan, no changes to vegetation biomass were found in treatments with or without Amynthas (now Metaphire) hilgendorfi (Greiner et al. 2012), but observational studies of the potential impacts of A. agrestis and A. tokioensis on vegetation in the Great Lakes region, in addition to the potential legacy effects of European earthworms, are needed.
Given the opportunity to document a novel invasion at the UW Arboretum, we assessed the changes in forest understory and earthworm communities in areas of A. agrestis and A. tokioensis’ spread within the first 3 years of their confirmed presence. Based on the changes seen in forests after European earthworm introduction, we expected to see decreases in plant species health such as reduced abundance and diversity. We hypothesized that leaf litter would be diminished in areas with high densities of A. agrestis and A. tokioensis based on the recent work in the Arboretum (Qiu and Turner 2016), and we expected to find European earthworm species presumed to be present pre-Amynthas invasion. The goal of this study was to better understand what changes land managers can expect in the initial years of A. agrestis and A. tokioensis invasion in forests of the Upper Midwest so that priorities for forest management can be set. We hope that these initial surveys will mprove the understanding of invasions by earthworm species into historically earthworm-free areas.
Methods
Study location
Field studies were conducted in Wingra and Gallistel Woods at the University of Wisconsin-Madison Arboretum in Madison, Wisconsin (43.045477°N, − 89.423853°W, Fig. 1b). The area surveyed is a 23-ha sugar maple and oak dominated mesic forest with predominantly Military loam and Dodge silt loam soils at around 260 meters elevation. Gallistel and Wingra Woods are remnant forests on land that was partially cleared but never plowed, and both areas now contain trees that were planted between 1941 and 1964 (UW Arboretum 1981). The two parcels of woods contain hiking trails open to the public and are divided by a two-lane paved road. White-tailed deer are not excluded from the Arboretum, but populations are culled annually. The hypothesized introduction point of A. agrestis and A. tokioensis to the Arboretum, a mulch depository, is located just southwest of the survey area, and mulch is frequently spread in horticultural gardens that are located uphill and to the west of the study area.
Selection of plots
71 points were randomly generated using ArcMap for initial placement of surveys in 2015. Each point was located with a Trimble GeoXH unit and represented the southwest corner of a 1-m2 plot oriented north–south. In 2016, 15 additional plots were added at random locations in the central portion of Wingra and Gallistel Woods to cover an underrepresented area.
Vegetation and forest floor surveys
Each 1-m2 plot was surveyed for understory vegetation in August of 2015 and August of 2016. Abundances of herbaceous species were recorded in 6 cover classes, adopted from the Daubenmire scale (Mueller-Dombois and Ellenberg 1974): 1 (0–5%), 2 (6–25%), 3 (26–50%), 4 (51–75%), 5 (76–95%), 6 (96–100%). The same cover classes were used to estimate cover of bare soil, coarse woody debris, and total vegetation of each plot. Stem counts and percent cover of woody seedlings and saplings were recorded. Canopy cover was estimated at chest height with a densiometer in 2015, and average leaf litter depth was calculated by measuring depth at the center and two corners of each plot. In addition, leaf litter mass was measured in August 2016 by collecting all leaf litter in adjacent 0.36-m2 earthworm survey plots and weighing after drying at 60 °C for at least 48 h. In 2016, pH and moisture content of plot soils were measured using a Hannah Instruments 99,121 pH meter and Extech MO750 soil moisture meter. Averages were calculated based on three measurements taken in each vegetation plot for pH in the SW corner, center, and NE corner, and moisture in the SE corner, center, and NW corner.
Earthworm surveys
In August 2015 and 2016, earthworm surveys were conducted at each plot using liquid mustard extraction (Gunn 1992; Hale et al. 2005). Each earthworm plot was 0.36-m2 and shared one border with a vegetation plot, with the adjacent border selected randomly from one of the four cardinal directions. Earthworm surveys involved removal of leaf litter and debris from the plot area followed by saturating the soil with a solution of 10 L water:100 g (1/3 cup) ground mustard, which agitates earthworms to the surface. After pouring half of the solution, we waited 5 min for epigeic and endogeic earthworms to emerge, and then poured the second half and waited 5 min for anecic earthworms. Earthworms were counted and identified to the genus or species level in the field. Area of earthworm spread was calculated with minimum bounding geometry in ArcGIS using convex hull polygons.
Statistical analysis
Nonmetric multidimensional scaling ordinations built with the Bray–Curtis distance metric were used to visualize the plant and earthworm community. The plant community was represented by midpoints of cover class percentage ranges and included only plants that occurred in at least five plots and only those plots that had at least two plant species. Values were square root transformed and underwent Wisconsin double standardization. Additional ordination axes were retained if reduction in stress was > 5 until final stress values were < 20 (McCune and Grace 2002). Vectors for earthworm abundance were added with the vegan function ‘envfit’ in R using 1000 permutations (R Development Core Team 2009).
T tests were conducted to compare areas with and without each earthworm taxon for vegetation and forest floor variables. Linear regressions were conducted to compare earthworm abundance to vegetation and forest floor variables, using data only from plots where each earthworm group being examined was present (i.e. data points with zero A. agrestis and A. tokioensis were excluded for regressions on A. agrestis and A. tokioensis). Cover classes were converted to midpoints of percentage ranges for use in analyses, and residual plots were assessed for assumptions of normality. Fischer’s Exact tests were conducted using presence/absence of individual herbaceous species and presence/absence of earthworm taxa to determine if any herbs were more likely to be present when an earthworm taxon was present. Herbaceous species were also categorized based on traits including: early blooming (before June), late blooming (June and after), high-moisture preference, low-moisture preference, and mycorrhizal or non-mycorrhizal, and analyzed using Fischer’s Exact tests to determine if any traits were more likely to be present when an earthworm taxon was present.
Shannon Diversity Index was calculated for each plot using the midpoint of each species cover class to represent proportional abundance. Shannon Diversity Index was compared with earthworm presence/absence using t tests and with earthworm abundance using linear regression. Herbaceous species were ranked by their Coefficient of Conservatism (CC) as described by the Wisconsin State Herbarium (2015), a scale from 0 to 10 that gives plants with a higher tolerance for site disturbance a lower value and those that have specific habitat requirements a higher value. Average CC per plot was compared with earthworm presence/absence and abundance using t tests and regressions. Paired t tests comparing differences in plot variables between 2015 and 2016 were conducted, in addition to ANOVAs comparing differences in plot variables based on the spread of A. agrestis and tokioensis between the 2 years. Statistics were performed using R Studio software (RStudio Team 2015).
Results
Earthworm surveys
In addition to A. agrestis and A. tokioensis, the European earthworms Lumbricus terrestris, Lumbricus rubellus, and Apporectodea spp. were found through mustard pour sampling (Table 1). Lumbricus terrestris is a large, pigmented, anecic species, L. rubellus is a moderate sized pigmented epi-endogeic earthworm, and the species of Apporectodea surveyed, most likely A. caliginosa or A. rosea, are smaller than Lumbricus, unpigmented, and endogeic (Hale et al. 2006; Bernadette Williams, Wisconsin DNR, personal communication).
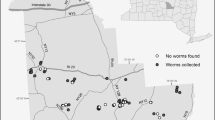
In 2015, A. agrestis and A. tokioensis were found in the western half of Wingra and Gallistel Woods, in an area of 8.24 ha (excluding one eastern outlier plot; Table 1, Fig. 2) while L. terrestris, L. rubellus, and Apporectodea spp. were concentrated in the eastern half but widespread throughout the survey area, covering an average area of 14.2 ha (Table 1, Fig. 2). In 2016, A. agrestis and A. tokioensis were much more widespread throughout the eastern half of the forest, covering an area of 15.58 ha (Table 1, Fig. 2), while the distribution of European earthworm species was reduced to an average area of 1.96 (Table 1, Fig. 2).
Non-metric multidimensional scaling ordinations
A stress value of 12.4 resulted from 20 iterations of a three-dimensional non-metric multidimensional scaling. No significant relationships were found between plant community and earthworm taxa, with all earthworms’ r2 < 0.05 (Table 2), but the A. agrestis and A. tokioensis vector was opposite of the vectors of L. terrestris, L. rubellus, and Apporectodea spp. in vegetation space (Fig. 3). Distributions of plots by sampling year had strong overlap, showing no clear shifts in vegetation between 2015 and 2016.
Nonmetric multidimensional scaling ordination of plots based on plant species percent cover data. Points represent plots analyzed (n = 65), outlines in a and b enclose sampling years, text represents plant species included (n = 15): RHCA = Rhamnus cathartica, ACSA = Acer saccharum, PAQU = Parthenocissus quinquefolia, VIAC = Viburnum acerifolium, CILU = Circaea lutetiana, IMCA = Impatiens capensis, MARA = Maianthemum racemosum, GECA = Geum canadense, CATH = Caulophyllum thalictroides, ARTR = Arisaema triphyllum, GEMA = Geranium maculatum, PEPE = Persicaria pensylvanica, ASCA = Asarum canadense, CEOC = Celtis occidentalis, FRPE = Fraxinus pennsylvanica. Amynthas spp. refers to A. agrestis and A. tokioensis. Ordinations c and d include overlay of earthworm vectors and symbols based on Amynthas spp. status of plots: None = plots with no Amynthas spp., Gained = plots that did not have Amynthas spp. in 2015, but gained Amynthas spp. in 2016, Both = plots that had Amynthas spp. in 2015 and 2016, New = plots that were added in 2016, all contained Amynthas spp.
Forest floor characteristics
T tests showed no differences in August leaf litter depth between plots with presence of absence of any earthworm grouping (A. agrestis and A. tokioensis, L. terrestris, L. rubellus, Apporectodea spp., European earthworms combined and total earthworms combined) in 2015, but in 2016 we found significantly higher August leaf litter depth in plots with A. agrestis and A. tokioensis present than with A. agrestis and A. tokioensis absent (p = 0.013) and significantly lower depth in plots with L. terrestris present (p = 0.006), Apporectodea spp. present (p = 0.037), or European earthworms present (p = 0.045; Table 3). Leaf litter mass reflected these trends in 2016; t tests showed significantly lower leaf litter mass in plots with L. terrestris present (p < 0.001), L. rubellus present (p < 0.001), or Apporectodea spp. present (p < 0.001), but no significant difference was detected between plots with and without A. agrestis and A. tokioensis (Table 3, Fig. 4). Similarly, linear regressions showed weak evidence for a negative relationship between the abundance of L. terrestris and leaf litter mass (p = 0.084, R2 = 0.16, slope = − 5.3), and strong evidence for a negative relationship between the abundance of total earthworms and leaf litter mass (p = 0.005, R2 = 0.08, slope = − 1.11) and between the abundance of European earthworms and leaf litter mass (p = 0.030, R2 = 0.21, slope = − 0.89; Online Resource Table S1). Canopy cover was significantly lower in plots containing L. rubellus, in plots with any European earthworms compared to plots without, and in plots with any earthworm compared to plots absent of all earthworms (Online Resource Table S2). Average cover of coarse woody debris in vegetation plots did not differ based on earthworm presence or abundance (Online Resource Table S1 and S2). The average cover of bare soil in plots was significantly higher in areas with each earthworm taxon present compared to areas with the respective earthworms absent, except for A. agrestis and A. tokioensis, which had a lower cover of bare soil when present (Table 3).
Soil characteristics
The average soil pH across all plots was 5.913. T tests based on presence and absence of earthworm groups found higher pH in areas with A. agrestis and A. tokioensis (p = 0.054), but significantly lower pH in areas with L. terrestris (p = 0.007), L. rubellus (p = 0.007), and combined European earthworms (p = 0.044; Table 3, Fig. 5a). Linear regressions showed significantly positive relationships between soil pH and the abundance of A. agrestis and A. tokioensis (p = 0.035, R2 = 0.05, slope = 0.01) and the abundance of L. terrestris (p = 0.0004, R2 = 0.62, slope = 0.14; Fig. 5b). There was weak evidence for a positive relationship between soil pH and L. rubellus abundance (p = 0.083, R2 = 0.16, slope = 0.03). No relationships were detected between soil pH and Apporectodea spp., total earthworm abundance, or European earthworm abundance (Online Resource Table S1). Moisture was significantly negatively related to the abundance of Apporectodea spp. (p = 0.023, R2 = 0.25, slope = − 1.6), combined European earthworms (p = 0.033, R2 = 0.206, slope = − 2.5) and total earthworms (p = 0.025, R2 = 0.05, slope = − 1.02), and was lower on average in areas containing each of L. terrestris (p = 0.029) and L. rubellus (p = 0.019). Moisture was significantly higher in areas containing A. agrestis and A. tokioensis (p = 0.009; Table 3).
a Average soil pH from plots with and without each earthworm grouping in 2016. T tests on A. agrestis and A. tokioensis (p = 0.054), L. terrestris (p = 0.007), L. rubellus (p = 0.007) showed significant differences, Apporectodea spp. (p = 0.063) showed weak evidence for significant difference. b Linear regressions between soil pH and number of A. agrestis and A. tokioensis (p = 0.035, R2 = 0.05, slope = 0.01), L. terrestris (p = 0.0004, R2 = 0.62, slope = 0.14), and L. rubellus (p = 0.083, R2 = 0.16, slope = 0.03) in 2016. Each taxon regression analysis excluded plots with zero earthworms
Vegetation characteristics
Fifteen herbaceous species were found in the vegetation surveys in 2015, and seventeen herbaceous species were found in 2016, all of which were native to southern Wisconsin. The average number of herbaceous species per plot was 1.4 in 2015 (SD 1.59, SE 0.18) and 1.6 in 2016 (SD 1.78, SE 0.19). The most abundant herbaceous species was Parthenocissus quinquefolia, found in 60% of plots in 2015 and 2016, followed by Circaea lutetiana, found in 20% of plots in 2015 and 25% of plots in 2016. The most abundant tree species was Acer saccharum, found in 90% of plots. The only invasive plant species found were honeysuckle (Lonicera morrowii) and buckthorn (Rhamnus cathartica), found in only 4 and 21% of plots in 2015, respectively, and 2 and 15% of plots in 2016, with no clear spatial patterns.
T tests on a number of vegetation characteristics based on presence and absence of earthworm taxa, including average CC and SD Index, mostly found no significant differences (Table 3, Online Resource Table S3), but trends typically showed increased diversity, richness, and abundance in areas with A. agrestis and A. tokioensis and in areas without each European earthworm (Table 3, Fig. 6). Linear regressions between each earthworm group’s abundance and vegetation response variables largely showed no significant relationships in either year, except for significantly negative relationships in 2016 between plant species richness, herbaceous species richness, Acer saccharum abundance and each of L. rubellus, Apporectodea spp., and total European earthworms (Online Resource Table S4, Fig. 7). No herbaceous species or traits were significantly more or less likely to be present based on earthworm presence using Fischer’s Exact tests for 2015 and 2016.
Linear regressions between plant species richness and number of A. agrestis and A. tokioensis (p = 0.977, R2 = − 0.01, slope = − 0.0008), L. rubellus (p = 0.036, R2 = 0.26, slope = − 0.166), Apporectodea spp. (p = 0.015, R2 = 0.27, slope = 0.104), and total European earthworms (p = 0.026, R2 = 0.22, slope = − 0.05) in 2016. Each taxon analysis excluded plots with zero earthworms
Plots from 2016 were categorized as being completely absent of A. agrestis and A. tokioensis, being absent of A. agrestis and A. tokioensis in 2015 but present in 2016, and containing A. agrestis and A. tokioensis in both 2015 and 2016. Using these three categories, ANOVAs were conducted on multiple site characteristics, none of which showed significant differences or had large enough sample sizes to be statistically testable. Paired t tests comparing leaf litter depth, Acer saccharum abundance, Shannon Diversity Index, and plant species richness between 2015 and 2016 showed the same trends for all earthworm taxa, suggesting that earthworm presence did not cause plot-wise temporal changes within the 2 years.
Discussion
Previous research on earthworm invasions has largely found negative impacts to both above- and below-ground communities in forests (Gundale 2002; Hale et al. 2006; Holdsworth et al. 2007; Nuzzo et al. 2009; Craven et al. 2017). This field study provides evidence for a rapid invasion of A. agrestis and A. tokioensis earthworms and their potential replacement of European earthworms, as has been suggested by previous researchers (Greiner et al. 2012). While much of the understory vegetation did not show detectable changes in relation to the spread of A. agrestis and A. tokioensis, some forest floor variables such as soil pH and leaf litter mass reveal differences in areas where A. agrestis and A. tokioensis were found to be present or absent. Although we were unable to assess their invasion into a completely earthworm-free forest, the changes seen in A. agrestis and A. tokioensis distribution represent the first observational evidence to track the early invasion of these earthworms in the Upper Midwest, and there are many potential implications of the trends seen in this 2-year study.
Earthworm community changes
The clearest finding of this research was the rapid spread of A. agrestis and A. tokioensis from 2015 to 2016, gaining approximately 7 ha of the 23-ha study area. This trend was in opposition to that of the three European earthworm taxa found, all of which saw a reduction in distribution. Although the initial distribution of A. agrestis and A. tokioensis in 2015 was largely in the western half of the study area, many more plots in the eastern half were surveyed with A. agrestis and A. tokioensis in 2016, and the overlap between Amynthas species and European earthworms was reduced (Online Resource Figs. S1 and 2). The high population densities and epi-endogeic niche of A. agrestis and A. tokioensis, leading to many earthworms and likely cocoons on the upper soil surface, may have led to increased dispersal via human disturbance and precipitation runoff compared to other earthworm groupings. The total precipitation for the spring and summer of 2015 (March through August) was 55 cm, while the same period in 2016 received 68 cm (U.S. Climate Data 2017). This increase in rainfall could explain the increase of plots containing A. agrestis and A. tokioensis in areas of slightly lower topography in the eastern part of the study area. In the Great Smoky Mountains National Park, Snyder et al. (2011) found that the distribution of an A. agrestis population varied monthly with weather conditions, reflected in a receding boundary into lower, moist areas during drought conditions. Our findings suggest A. agrestis and A. tokioensis may be similarly affected by weather conditions in this study site, since such a dramatic spread was seen after one rainy season and because soil moisture was significantly higher in areas where A. agrestis and A. tokioensis were found than in areas without them (Table 3). However, this trend is reflected in only two annual August surveys; multiple monthly surveys, with additional sampling after rain events, would more clearly assess whether the changes in earthworm distribution correlate with increased moisture or heavy rain events as well as if patterns are short-lived, as seen by Gorres et al. (2014). Trends through the early fall should be considered in order to understand how the spread of A. agrestis and A. tokioensis varies with distribution and survival of cocoons. In addition, long-term trends may reveal important information about how to manage the new invasive species, as earthworm invasion fronts do not always show predictable changes in space and time (Stoscheck et al. 2012).
The opposing patterns in A. agrestis and A. tokioensis distribution and European earthworm distributions may represent competition between these groups. The high dietary flexibility exhibited by A. agrestis in microcosm research by Zhang et al. (2010) included a shift to leaf litter when soil biota was in low abundance, leading to indirect competition with the less flexible litter feeder L. rubellus. However, those implications may be dependent on leaf litter quality and plant composition of the study area (Chang et al. 2016c). Earthworm competition for food resources was suggested in a lab mesocosm study by Chang et al. (2016c), which found reduced leaf litter consumption in L. rubellus and the European earthworm Octolasion lactium when Metaphire hilgendorfi was present. The authors also noted reduced L. rubellus densities in forests where M. hilgendorfi had invaded, but emphasized the importance of differences in species-specific traits such as seasonal versus multi-year life spans as factors that may lead to reduced competition and potential coexistence in the field (Chang et al. 2016c). That research, as well as research focused on changes in carbon and nitrogen associated with earthworm species (Chang et al. 2016b), highlighted the need for considering earthworm species identity rather than functional group to correctly identify their impacts. Our study was limited by not differentiating between A. agrestis and A. tokioensis, but species identity should be considered for future research on Amynthas species in the Upper Midwest.
Changes to the forest floor
Species of Asian earthworms have been shown to have direct effects on soils, with M. hilgendorfi increasing NH4 + and labile C through granular casts with high initial microbial activity (Chang et al. 2016b), and A. agrestis and A. tokioensis increasing mineralization and soil inorganic nitrogen and dissolved organic carbon (Qiu and Turner 2016). Szlavecz et al. (2006) found increased N-mineralization and nitrification in areas containing European earthworms, as well as correlations between those soil characteristics and higher soil pH. Our 2016 sampling found soil pH to be significantly positively related to the abundance of A. agrestis and A. tokioensis, L. terrestris, and L. rubellus in areas where each taxon was present (Fig. 5b), but areas without L. terrestris and without L. rubellus showed significantly higher soil pH on average (Fig. 5a). Increased soil pH in relation to European earthworms has been hypothesized to be due to increased secretion of calcium carbonate by some earthworms (Drouin et al. 2016), or the incorporation of leaf litter into the soil and loss of organic matter causing reduction of organic acids (Burtelow et al. 1998). It is also hypothesized that soil pH may influence earthworm range expansion, based on the tolerance of earthworm species to soil acidity (Stoscheck et al. 2012). It is possible that increases in soil pH could facilitate the invasion of non-native plants, which tend to be less tolerant of acidic soils (Kourtev et al. 1999; Beauséjour et al. 2015), but no changes in exotic plant distribution or abundance were detected to be associated with earthworm distribution in our surveys. However, the differences in the results of the t tests and regressions suggest that the overall variability within these plots is too high to conclude that earthworm distribution is determined by soil pH or that earthworm presence and abundance directly influence pH.
One of the most common changes seen in Midwest forests invaded by European earthworms is the reduction of leaf litter on the forest floor through earthworm feeding and soil mixing (Kourtev et al. 1999; Hale et al. 2008; Nuzzo et al. 2009). While our August surveys found significantly less leaf litter mass in areas containing European earthworms compared to areas without European earthworms, litter mass was slightly higher in areas with A. agrestis and A. tokioensis compared to areas without (Fig. 4). This trend was also reflected in the results of ground cover surveys that showed increased bare soil in plots without A. agrestis and A. tokioensis and in plots with European earthworms (Table 3). Although A. agrestis and A. tokioensis create a granular soil signature, we found individuals predominantly in the upper soil surface under a layer of thick leaf litter, while areas of high European earthworm densities were characterized by exposed roots, middens, and low leaf litter. A preference for soil biota by the Amynthas species could explain this initial lack of reduction in leaf litter, as seen by Zhang et al. (2010) with A. agrestis. In addition, our sampling timing may have not adequately captured the full pattern of leaf litter depletion; Qiu and Turner (2016) found areas with A. agrestis and A. tokioensis in the UW Arboretum had higher leaf litter mass than areas without A. agrestis and A. tokioensis in August, but by September the opposite was true. Different European earthworm species may preferentially feed on older or fresher leaf litter (Hale et al. 2008), suggesting that the timing of sampling may drastically change the observed relationship between earthworm community composition and depth of the forest floor. The full impacts of earthworm invasion, including data on the rate of leaf litter change rather than a static measurement, would be best captured through seasonal or year-round sampling. Furthermore, recent mesocosm work at the Arboretum by Ziter and Turner (unpublished data) suggests that A. tokioensis doesn’t thrive well in low leaf litter conditions. Leaf litter dynamics represent the complex nature of field studies on earthworm invasion and the uncertainty of the directionality of changes. In this case, it is unclear whether A. agrestis and A. tokioensis have a smaller impact on leaf litter than other earthworms, or if they preferentially spread to areas with high leaf litter. As with most of the vegetation and forest floor variables in this observational study, both possibilities exist: for earthworm distribution to be dependent on environmental factors or for environmental factors to be dependent on earthworms.
The impact of the study area on changes in vegetation
The trends seen in leaf litter were also seen in analyses comparing vegetation factors such as species richness and Shannon Diversity Index to the presence and absence of A. agrestis and A. tokioensis and European earthworms. While we did see fewer plant species, less diversity, and less vegetative cover in areas with European earthworms as expected, areas with A. agrestis and A. tokioensis tended to have slightly higher plant species richness, diversity, and vegetative cover than areas without A. agrestis and A. tokioensis (Figs. 6, 7), while many vegetation variables such as individual plant species showed no clear differences based on earthworm distributions. One explanation for these findings in comparison to other studies’ data is the overall low diversity of plants in this forest, with the majority of vegetation being Acer saccharum and Parthenocissus quinquefolia. Had the study area been in a forest with higher herbaceous plant diversity or one with a larger number of rare plant species, the patterns seen in the understory vegetation may have been clearer. This low diversity could be attributed to many factors including a legacy effect of European earthworms. Because of the late summer timing of sampling, we also were not able to capture spring ephemeral trends. Our study area was in a smaller patch of forest compared to much of the earthworm research done in national forests of the Upper Midwest, and in a more managed setting. The many trails that run through Wingra and Gallistel Woods as well as the original restoration of this area in the 1940s mean that our results capture a more disturbed setting than the research done in larger and more remote sites. However, because the initial invasion of A. agrestis and A. tokioensis in Wisconsin has been in urban areas, it is vital to understand how these types of locations respond to their presence.
As an observational field study, this research was influenced by the natural variability of the study area, including the pre-existing populations of earthworms, the state of the understory vegetation, and the health of the overall forest. Results from a study of herbaceous vegetation changes in a Michigan forest using field treatments of Metaphire hilgendorfi found similarly inconclusive effects on plants (Greiner et al. 2012). In the present study, as in the work by Greiner et al., the research site was in a forest historically devoid of earthworms, but with European earthworms already present pre-Amynthas spp., as opposed to studies of the Upper Midwest investigating the leading edge of initial earthworm invasion (Hale et al. 2006). Greiner et al. suggest that plants in their study site may “have been resistant to earthworm treatments given that earthworm populations were present prior to the start of the experiment.” The age of the vegetation can also alter the effects of earthworms: mesocosm research on L. terrestris has indicated that established seedlings may not be negatively affected by invasive earthworms in the same way that seeds can be directly hindered through burial or consumption (Dávalos et al. 2013). Because our research was in a mature forest already invaded by earthworms, our results may not represent the changes that would be seen in an earthworm-free forest invaded by A. agrestis and A. tokioensis. Although canopy cover differed in areas invaded by different earthworms (Table 3), it is unlikely that earthworms are having an impact on the canopy or that they differentially invade areas with varying shade, but cohorts of maple seedlings currently affected could result in differential canopy cover in the future (Bal et al. 2017). As noted by Hale and Host (2005), the natural variability of areas free of earthworms is crucial to understand but often difficult to determine when studying areas fully invaded.
Another possible explanation for our findings of increased vegetation in areas with A. agrestis and A. tokioensis is the potential pulse of nutrients that has been documented after some earthworm invasions. Larson et al. (2010) found patterns in tree rings of earthworm-invaded forests that represented a surge in growth due to the increased availability of nutrients caused by earthworm activity, which eventually diminished as nutrients were leached from the soil. More specifically, the rapid mineralization in soils with A. agrestis and A. tokioensis (Qiu and Turner 2016) suggests more nutrients may be available for plant uptake in areas recently invaded. Asshoff et al. (2010) found an initial positive effect of multiple earthworm groups on seedlings, which they associated with increasing nutrient availability for seed germination in earthworm casts. It is possible that the slight increases in plant species richness and diversity in areas where A. agrestis and A. tokioensis were found could be due to the initial boost in nutrient availability that the worms provide in the upper soil layers, but this interpretation depends on a more definite arrival timeline of Amynthas species in the Arboretum. Furthermore, this hypothesis would suggest that within the next few years, as nutrients become leached from the soils, plants in areas with A. agrestis and A. tokioensis will experience negative health impacts.
The year-to-year pattern of earthworm distribution in our study shows a shift from European earthworm-dominated areas to A. agrestis and A. tokioensis-dominated, which raises the possibility that vegetation is being ‘released’ from effects of multiple European species to those of solely A. agrestis and A. tokioensis in the soil system. While the time range of our study was too short to definitively detect any such effects, Hale et al. (2008) found impacts to vegetation and soil nutrients in mesocosms with multiple earthworm species present while little to no changes were seen in some mesocosms with only one earthworm species present, allowing for speculation on the processes that may be at play here. The three European earthworm taxa found in our study area collectively inhabit all three earthworm niches of the soil, whereas A. agrestis and A. tokioensis do not burrow vertically and typically are not found deeper than 5 cm (Richardson et al. 2009; Qiu and Turner 2016). If the European earthworms were well established prior to A. agrestis and A. tokioensis’ arrival, hindering understory plants, then the shift to soils with no European worms but with A. agrestis and A. tokioensis may have allowed seedlings and understory plants to rebound, explaining why vegetation was not seen to be negatively impacted in areas with A. agrestis and A. tokioensis.
The initial invasion dates of both European earthworms and A. agrestis and A. tokioensis at our study site were unknown, although it is safe to assume that European earthworms have been in the Arboretum on a scale of decades, while A. agrestis and A. tokioensis’ presence is on a scale of years. Knowing the initial distribution of European earthworms pre-Amynthas spp. would be immensely helpful in understanding whether patterns seen in this study were pre-existing or new. Our study area differs from the majority of invasive earthworm studies in that the typical invasion front seen starts with epigeic earthworms and over time leads to a complete assemblage of multiple earthworm groups (Hale et al. 2005). Here we have an established community of epi-endogeic and anecic European earthworms with the new addition of the predominantly epigeic A. agrestis and A. tokioensis. This reverse pattern makes it difficult to predict the time lags and impacts related to A. agrestis and A. tokioensis as they become established within the Arboretum and Wisconsin (Beauséjour et al. 2015), but emphasizes the importance of tracking this informative invasion.
Future considerations
While A. agrestis and A. tokioensis differ in behavior compared to many of the European species already in the Upper Midwest, they share similarities with the European earthworm Dendrobaena octaedra found elsewhere in the region, which may be helpful in interpreting our findings. D. octaedra has been described as having high cocoon productivity, as do A. agrestis and A. tokioensis, and the ability to withstand a wider range of environmental conditions than other European species, such as drought and acidic leaf litter (Beauséjour et al. 2015). D. octaedra is an epigeic parthenogenetic species that has been found to not reduce the forest floor as other epi-endogeic and anecic species do, but rather thrives in thick leaf litter (Hale et al. 2005; Beauséjour et al. 2015). A study in northern Wisconsin and Minnesota found negative relationships between Lumbricus and the depth of leaf litter and richness of plant species, but positive relationships between those variables and D. octaedra abundance (Holdsworth et al. 2007). This was attributed to the reduction in D. octaedra populations with increasing L. terrestris invasions—a pattern we may be seeing in reverse in our study with decreasing L. terrestris as A. agrestis and A. tokioensis invade. Although these newly invasive members of the Amynthas genus have additional traits not exhibited by D. octaedra, such as dietary flexibility, rapid growth, and the creation of granular soil, the similarities of Amynthas spp. and D. octaedra, suggest that research done on D. octaedra could inform future studies on A. tokioensis and A. agrestis.
In conclusion, our study provides evidence that A. agrestis and A. tokioensis have the ability to spread rapidly in deciduous forests of Wisconsin, where they were recently recorded for the first time. The pattern of A. agrestis and A. tokioensis spread in the UW Arboretum contrasted the shrinking distributions of the three European earthworm groups surveyed, suggesting potential competition or interaction between invasive earthworms. Leaf litter and plant diversity showed increasing trends in areas with A. agrestis and A. tokioensis compared to areas without, while most traits of the vegetation community did not show conclusive findings. Although there were limitations to this study, including the small survey area and the seasonality of only two annual surveys, we were able to capture a key snapshot of the early years of invasion in a site that exemplifies the areas A. agrestis and A. tokioensis have begun to invade in Wisconsin, establishing a benchmark for future observations. We recommend a continuation of long-term surveys in both the UW Arboretum and throughout the state to understand the patterns to the invasion of A. agrestis and A. tokioensis, their interactions with other earthworms, and the effects on soils and plants to fill the remaining knowledge gaps in the implications for forests of the Upper Midwest.
References
Asshoff R, Scheu S, Eisenhauer N (2010) Different earthworm ecological groups interactively impact seedling establishment. Eur J Soil Biol 46:330–334. https://doi.org/10.1016/j.ejsobi.2010.06.005
Bal TL, Storer AJ, Jurgensen MF (2017) Evidence of damage from exotic invasive earthworm activity was highly correlated to sugar maple dieback in the Upper Great Lakes region. Biol Invasions. https://doi.org/10.1007/s10530-017-1523-0
Beauséjour R, Handa IT, Lechowicz MJ et al (2015) Historical anthropogenic disturbances influence patterns of non-native earthworm and plant invasions in a temperate primary forest. Biol Invasions 17:1267–1281. https://doi.org/10.1007/s10530-014-0794-y
Bohlen PJ, Hendrix PF (2002) Exotic earthworm invasions in North America: ecological and policy implications. Bioscience 52:669–682. https://doi.org/10.1641/0006-3568(2002)052
Bohlen PJ, Scheu S, Hale CM et al (2004) Non-native invasive earthworms as agents of change in northern temperate forests. Front Ecol Environ 2:427–435
Bouché MB (1971) Relations entre les structures spatiales et fonctionelles des ecosystems, illustrées par le role pédobiologique des vers de terre. In: Pesson P (ed) La vie dans les sols, aspects nouveaux, études experimentales. Gauthier-Villars, Paris, pp 187–209
Burtelow A, Bohlen P, Groffman P (1998) Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Appl Soil Ecol 9:197–202
Callaham MA, Hendrix PF, Phillips RJ (2003) Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA. Pedobiologia (Jena) 47:466–470
Chang CH, Snyder BA, Szlavecz K (2016a) Asian pheretimoid earthworms in North America north of Mexico: an illustrated key to the genera Amynthas, Metaphire, Pithemera, and Polypheretima (Clitellata: Megascolecidae). Zootaxa 4179:495–529
Chang CH, Szlavecz K, Buyer JS (2016b) Species-specific effects of earthworms on microbial communities and the fate of litter-derived carbon. Soil Biol Biochem 100:129–139. https://doi.org/10.1016/j.soilbio.2016.06.004
Chang CH, Szlavecz K, Filley T et al (2016c) Belowground competition among invading detritivores. Ecology 97:160–170. https://doi.org/10.1890/15-0551.1
Craven D, Thakur MP, Cameron EK et al (2017) The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob Chang Biol 23:1065–1074. https://doi.org/10.1111/gcb.13446
Dávalos A, Nuzzo V, Stark J, Blossey B (2013) Unexpected earthworm effects on forest understory plants. BMC Ecol 13:48. https://doi.org/10.1186/1472-6785-13-48
Drouin M, Bradley R, Lapointe L (2016) Linkage between exotic earthworms, understory vegetation and soil properties in sugar maple forests. For Ecol Manag 364:113–121. https://doi.org/10.1016/j.foreco.2016.01.010
Gibson KD, Quackenbush PM, Emery NC et al (2013) Invasive earthworms and plants in Indiana old- and second-growth Forests. Invasive Plant Sci Manag 6:161–174. https://doi.org/10.1614/IPSM-D-12-00046.1
Gorres JH, Melnichuk RDS (2012) Asian invasive earthworms of the genus Amynthas Kinberg in Vermont. Northeast Nat 19:313–322. https://doi.org/10.1656/045.019.0212
Gorres JH, Bellitürk K, Keller E (2014) Failure of an Amynthas agresitis (Goto & Hatai 1899) (Oligochaeta: Megascolecidae) population to expand its range within a sugar maple (Acer saccharum) stand. Megadrilogica 17:7–13
Greiner HG, Kashian DR, Tiegs SD (2012) Impacts of invasive Asian (Amynthas hilgendorfi) and European (Lumbricus rubellus) earthworms in a North American temperate deciduous forest. Biol Invasions 14:2017–2027. https://doi.org/10.1007/s10530-012-0208-y
Gundale MJ (2002) Influence of exotic earthworms on the soil organic horizon and the rare fern Botrychium mormo. Conserv Biol 16:1555–1561. https://doi.org/10.1046/j.1523-1739.2002.01229.x
Gunn A (1992) The use of mustard to estimate earthworm populations. Pedobiologia (Jena) 36:65–67
Hale CM, Host GE (2005) Assessing the impacts of European earthworm invasions in beech-maple hardwood and aspen-fir boreal forests of the western Great Lakes region. Natl Park Serv Gt Lakes Invent Monit Netw Rep GLKN/2005/:1–35
Hale CM, Frelich LE, Reich PB (2005) Exotic European earthworm invasion dynamics in northern hardwood forests of Minnesota, USA. Ecol Appl 15:848–860. https://doi.org/10.1890/03-5345
Hale CM, Frelich LE, Reich PB (2006) Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology 87:1637–1649
Hale CM, Frelich LE, Reich PB, Pastor J (2008) Exotic earthworm effects on hardwood forest floor, nutrient availability and native plants: a mesocosm study. Oecologia 155:509–518. https://doi.org/10.1007/s00442-007-0925-6
Holdsworth AR, Frelich LE, Reich PB (2007) Effects of earthworm invasion on plant species richness in northern hardwood forests. Conserv Biol 21:997–1008. https://doi.org/10.1111/j.1523-1739.2007.00740.x
Kourtev PS, Huang WZ, Ehrenfeld JG (1999) Differences in earthworm densities and nitrogen dynamics in soils under exotic and native plant species. Biol Invasions 1:237–245. https://doi.org/10.1023/A:1010048909563
Larson ER, Kipfmueller KF, Hale CM et al (2010) Tree rings detect earthworm invasions and their effects in northern hardwood forests. Biol Invasions 12:1053–1066. https://doi.org/10.1007/s10530-009-9523-3
Lawrence B, Fisk MC, Fahey TJ, Suarez ER (2003) Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytol 157:145–153. https://doi.org/10.1046/j.1469-8137.2003.00649.x
McCune B, Grace J (2002) Analysis of ecological communities. MJM Software Design, Oregon. https://doi.org/10.1016/S0022-0981(03)00091-1
Mueller-Dombois D, Ellenberg H (1974) Community sampling: the releve method. In: Aims and methods of vegetation ecology. Wiley, New York
Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conserv Biol 23:966–974. https://doi.org/10.1111/j.1523-1739.2009.01168.x
Qiu J, Turner MG (2016) Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biol Invasions. https://doi.org/10.1007/s10530-016-1264-5
R Development Core Team (2009) R: a language and environment for statistical computing. http://www.r-project.org
Richardson DR, Snyder BA, Hendrix PF (2009) Soil moisture and temperature: tolerances and optima for a non- native earthworm species, Amynthas agrestis (Oligochaeta: Opisthopora: Megascolecidae). Southeast Nat 8:325–334
RStudio Team (2015) RStudio: integrated development for R. RStudio Inc., Boston, MA. http://www.rstudio.com/
Snyder BA, Callaham MA, Hendrix PF (2011) Spatial variability of an invasive earthworm (Amynthas agrestis) population and potential impacts on soil characteristics and millipedes in the Great Smoky Mountains National Park, USA. Biol Invasions 13:349–358. https://doi.org/10.1007/s10530-010-9826-4
Stoscheck LM, Sherman RE, Suarez ER, Fahey TJ (2012) Exotic earthworm distributions did not expand over a decade in a hardwood forest in New York state. Appl Soil Ecol 62:124–130. https://doi.org/10.1016/j.apsoil.2012.07.002
Szlavecz K, Placella SA, Pouyat RV et al (2006) Invasive earthworm species and nitrogen cycling in remnant forest patches. Appl Soil Ecol 32:54–62. https://doi.org/10.1016/j.apsoil.2005.01.006
U.S. Climate Data (2017) http://www.usclimatedata.com
UW Arboretum (1981) The Arboretum: the outdoor laboratory after its first fifty years. Madison, Wisconsin
Wisconsin State Herbarium (2015) Flora of Wisconsin. In: Univ. Wisconsin. http://wisflora.herbarium.wisc.edu/. Accessed 1 Jan 2016
Zhang W, Hendrix PF, Snyder BA et al (2010) Dietary flexibility aids Asian earthworm invasion in North American forests. Ecology 91:2070–2079. https://doi.org/10.1890/09-0979.1
Acknowledgements
We thank the staff of the University of Wisconsin—Madison Arboretum for assistance with this research, and Jiangxiao Qiu and Carly Ziter for assistance with research design and collaboration. We thank Nicholas Keuler for statistical expertise and guidance, Mark Wegener for GIS support and map-making, and Will Vincent for figure drafting. We also thank Bernadette Williams, of the Wisconsin Department of Natural Resources, for sharing her knowledge of earthworm history and research in the area, and two anonymous reviewers for their helpful feedback.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laushman, K.M., Hotchkiss, S.C. & Herrick, B.M. Tracking an invasion: community changes in hardwood forests following the arrival of Amynthas agrestis and Amynthas tokioensis in Wisconsin. Biol Invasions 20, 1671–1685 (2018). https://doi.org/10.1007/s10530-017-1653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1653-4