Abstract
The invasion of the Ponto–Caspian amphipod Dikerogammarus villosus in European rivers is assumed to reduce macroinvertebrate diversity and to alter ecosystem functions. D. villosus shows an extraordinarily flexible feeding behavior including the ability to use various food sources. On the other hand, its response to predation risk seems to depend on environmental factors. To evaluate the ecological function of D. villosus, we estimated the daily food consumption for different food sources and analyzed potential effects of predator avoidance behavior on feeding. D. villosus consumption of willow leaves or chironomid larvae was quantified in 24-h laboratory experiments with and without kairomones of the European bullhead (Cottus gobio). Consumption rates were estimated based on gut content and gut evacuation rate under semi-natural laboratory conditions enabling the animals to feed over the whole time of the evacuation rate experiment. We observed very high evacuation rates and consequently high consumption rates up to 89% of body weight per day. Consumption rates differed significantly between food sources: D. villosus ingested more leaves than chironomid larvae. In contrast, predator cues did not affect the feeding of D. villosus. This might be explained by its strong refuge affinity and probably benefits its successful invasion. A comparison of the estimated consumption rates with results of an own consumption experiment (and other studies) under more artificial conditions indicated that more natural conditions result in higher consumption rates. Consequently, feeding rates from highly artificial experiments should be used with great caution to assess the ecosystem function of D. villosus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although migration of organisms is a natural process, the number of invasive alien species in aquatic ecosystems has dramatically increased during the last century (Kinzelbach 1995; Krisp and Maier 2005; Strayer and Dudgeon 2010) due to growing importance of anthropogenic vectors, such as canals or ballast water (Gollasch 1996; Tittizer 1997). Because the establishment of invasive species can endanger the native biodiversity and change ecosystem functions (Kinzelbach 1995; Strayer 2010; Dodd et al. 2013), the effects of aquatic invaders on the biotic community and ecosystem function of rivers have become an important issue of limnological research (Strayer 2010). A ‘notorious’ example of an aquatic invasive species is Dikerogammarus villosus (Sowinski 1894). This freshwater amphipod, which is native to the Ponto–Caspian region, colonized the River Rhine from the River Danube via the Main–Danube Canal and is currently spreading into other rivers of Continental Europe and the British Isles (Bij de Vaate and Klink 1995; Tittizer 1997; Devin et al. 2001; Bij de Vaate et al. 2002; Tricarico et al. 2010; MacNeil et al. 2010; Rewicz et al. 2014). D. villosus possesses several traits making it a potentially strong predator or competitor in benthic communities. It displaces other gammarid species from colonized habitats (e.g. Jermacz et al. 2015; Kobak et al. 2016; Beggel et al. 2016), has a high reproduction rate (Devin et al. 2004; Pöckl 2007) and differs from indigenous gammarid species with respect to its large size (Dick and Platvoet 2000). D. villosus is expected to have high predation rates and a wide food spectrum (Maazouzi et al. 2007; Bollache et al. 2008; Platvoet et al. 2009b; Dodd et al. 2013; Hellmann et al. 2015). Under laboratory conditions it shows an aggressive and predacious behavior (Dick et al. 2002; MacNeil and Platvoet 2005), whereas in the field, stable isotopes and gut content analyses of D. villosus indicate a highly opportunistic and omnivorous feeding behavior (Maazouzi et al. 2009; Hellmann et al. 2015; Koester et al. 2016). A diverse as well as temporally and spatially variable diet composition reflects the feeding strategy (sit-and-wait) (Pellan et al. 2016) and the ‘domicolous’ behavior of D. villosus (Platvoet et al. 2006). The amphipod is also highly tolerant against salinity and temperature changes (Bruijs et al. 2001; Wijnhoven et al. 2003) and prefers rip-rap habitats (Van Riel et al. 2006a), which probably contributes to the species’ tolerance against anthropogenic habitat degradation (Grabowski et al. 2007; MacNeil and Platvoet 2013).
With regard to all these specific traits, the occurrence of D. villosus has not only been associated with the decrease of native gammarids and other macroinvertebrate species (e.g. Dick and Platvoet 2000; Devin et al. 2001; Bij de Vaate et al. 2002; Devin et al. 2004; Boets et al. 2010) but also been suspected to change functions of ecosystems (MacNeil and Platvoet 2005; Van Riel et al. 2006b; Bollache et al. 2008; MacNeil et al. 2010, 2011). However, some studies detected neither a high predation intensity by D. villosus nor major structural changes in the resident macroinvertebrate community caused by this amphipod (Koester and Gergs 2014; Hellmann et al. 2015; Koester et al. 2016; Hellmann et al. 2017).
Due to its very wide food spectrum, the ecological function of D. villosus is hard to define. Recent studies even showed that it might be river-specific, because this species seems to be omnivorous in the middle River Rhine but almost exclusively a primary consumer in the upper River Elbe (Hellmann et al. 2015, 2017). Consequently, if D. villosus would indeed replace native species, it is not clear whether the invader might then be able to provide the same ecological functions as the native species. For example, the leaf consumption rate of D. villosus has been observed to be lower than that of native species (Piscart et al. 2011; Boeker and Geist 2015; Jourdan et al. 2016). Therefore, a large-scale species turnover in benthic communities could affect the important ecosystem function leaf decomposition (MacNeil et al. 2011, 2011; Piscart et al. 2011; Jourdan et al. 2016).
In any case, the potential impact of an invader on a community and on ecosystem functions depends largely on the quantity of food it consumes, whether of other (prey) species or of shared resources. There are two principal methods to quantify the consumption rate of an animal directly (in contrast to bioenergetics models quantifying food consumption indirectly). One of them is the ‘subtraction method’ which is based on the presentation of pre-defined food sources and the counting or weighing of the remains after a defined time in a laboratory consumption experiment (e.g. Willoughby and Earnshaw 1982; Normant and Lamprecht 2006; Gergs and Rothhaupt 2008). This method is often used to investigate the food consumption as a physiological response and therefore the experimental conditions are often designed in a standard and thus artificial manner in order to minimize the effects of additional environmental factors (Agatz and Brown 2014). In contrast, the alternative method is based on the determination of the stomach or gut content over 24 h and the gut evacuation rate (e.g. Bajkov 1935; Elliott and Persson 1978; Jobling 1981) and is mostly used under field conditions (e.g. Lockwood 1980; Weisberg et al. 1981; Worischka and Mehner 1998; Amundsen et al. 1999). Therefore, the second method can be expected to give more realistic results with respect to the feeding behavior of a species in a natural community.
Because D. villosus is also a prey organism (like most of the benthic invertebrates), quantifying food consumption in a natural community, one should also consider predation risk. Predation risk is in general one of the factors influencing feeding behavior (e.g. Åbjörnsson et al. 2000; Szokoli et al. 2015) by inducing spatial or temporal avoidance behavior. The presence of predators can therefore affect the daily food consumption (e.g. Werner et al. 1983; Viherluoto and Viitasalo 2001). This can be mediated by a trade-off between feeding and hiding (e.g. Pettersson and Brönmark 1993; Lehtiniemi 2005; Szokoli et al. 2015) or the development of a feeding periodicity (e.g. Lampert 1989; Metcalfe et al. 1999). For instance, Gammarus spp. shows a reduced activity and an increased hiding behavior when it is exposed to chemical cues of potential predators (kairomones) (Andersson et al. 1986; Wudkevich et al. 1997; Baumgärtner et al. 2003; Schäffer et al. 2013), which might reduce the daily food consumption. Generally, chemical cues seem to be a very important stimulus for gammarids in order to detect potential predators (Wudkevich et al. 1997; Åbjörnsson et al. 2000; Jermacz et al. 2017), D. villosus being no exception although its responses depend on additional circumstances (Jermacz et al. 2017; Jermacz and Kobak 2017).
Our aim was therefore to assess the ecological potential of the invader D. villosus with regard to leaf decomposition and predation and the potential effect of a predator presence on that ecological potential. We estimated the consumption rate in a laboratory set-up under conditions as close to nature as possible (e.g. refuge availability, keeping in groups, feeding during the whole experimental time, ‘semi-natural’). We compared the daily food consumption of D. villosus of the two food sources chironomid larvae and willow leaves and analyzed the effect of fish kairomones as predator cues on the daily food consumption. To estimate the effect of the experimental set-up on the results, we compared the results gained under these semi-natural conditions with the standard subtraction method under highly controlled artificial conditions, hypothesizing that more natural conditions would increase the consumption rate.
Methods
Collection and maintenance of study organisms
Individuals of D. villosus used in the semi-natural consumption experiment were collected between June and August 2014 and in July 2015 from River Elbe near Dresden, Germany (51°05′46.3″N, 13°38′36.8″E), whereas the individuals for the experiment under artificial conditions were collected in August 2014 from River Moselle near Koblenz, Germany (50°21′17.6″N, 7°33′21.8″E). After collection by gentle kick-sampling, the animals were quickly and carefully transported from the collection sites to the laboratories in aerated containers equipped with pebbles as refuges, and kept in the laboratories as described in the respective sections below, allowing them to acclimatize for 7 days.
The predator avoidance behavior of D. villosus was investigated using kairomones (chemical cues) of the European bullhead (Cottus gobio) which is known to be an effective predator of amphipods (Mills and Mann 1983; Kaldonski et al. 2008). In order to produce kairomones, three bullheads were captured in May 2013 at the River Elbe in substrate baskets (50 × 20 × 20 cm) which were exposed on the riverbed (Hellmann et al. 2017). The bullheads were kept in an aquarium filled with 150 L aerated and filtered tap water at 13 ± 1 °C and fed with chironomid larvae six times per week. Light conditions were the same as for the consumption experiments of D. villosus and stones were provided as refuges. Once a week, the aquarium was cleaned and one-third of the water was exchanged.
To collect fresh fish kairomones for respective treatments in the experiments, the aeration/filtration unit was removed 24 h before each experiment. During these 24 h, aeration was provided by an air stone only, allowing the accumulation of kairomones in the water. The amount of kairomone water taken out and used in the fish treatments of the predator avoidance experiments equaled a simulated fish density of 1.82 individuals m−3. The bullheads were fed once at the beginning of the 24 h period for kairomone collection when the filtration unit was removed.
Estimation of daily food consumption under semi-natural conditions
To estimate daily food consumption as realistically as possibly while still ensuring comparable experimental conditions, we designed an experiment with so called semi-natural conditions, representing the counterpart of standard experiments with highly controlled laboratory conditions. These semi-natural conditions included group-keeping, the hideouts and no starvation during the estimation of gut evacuation.
After the field-collection, all animals destined for the semi-natural consumption experiment were kept in an indoor flow channel in streamed cages (diameter 18 cm, mesh size 0.8 mm). The cages were equipped with stones and ceramic tiles for hiding and leaf litter or chironomid larvae as food for 1 week prior to the experiments to allow acclimatization of the amphipods. To keep the animals at a natural diurnal rhythm during this period, the flow channel was exposed to natural daylight. We used artificial medium (Borgmann 1996, S1) during the acclimatization phase and the experiments because it mirrors the ion composition of natural river water reasonably well while providing equal conditions for all four experiments in contrast to water from the collection site in the urban-catchment influenced River Elbe.
During the consumption and gut evacuation experiments, the animals were kept in groups of five adult individuals (both sexes were randomly used) in small aerated aquaria (14.5 × 10 × 10 cm). In the chironomid food evacuation experiments, only three animals per aquarium were used because of limited availability. The aquaria were filled with 500 mL artificial medium and about six stones were arranged in form of a foraging arena with the food placed in the center. This ensured that each amphipod had equal foraging facilities (S2). All experiments were conducted at 14 ± 1 °C water temperature and in an artificially lighted room. The day-night rhythm was adapted to season-specific field conditions (16:8 h) with two 1-h periods of low light intensity representing dawn and dusk. While the low light intensity was achieved by using commercial neon tubes only, daylight was simulated by additional high pressure mercury lamps (HQL 80 W, Osram, Munich, Germany). The experiments were performed with animal prey (commercially available live Chironomus spp. larvae) and with plant food (dry willow leaves, Salix spp., collected during autumn at the riverside and conditioned for 1 week prior to the experiments in water from the collection site).
Gut evacuation experiment
The gut evacuation rate of D. villosus was not estimated during the consumption experiments but in separate experiments as recommended for continuously feeding species (Héroux and Magnan 1996) and for each food source separately. In order to provide realistic conditions comparable to the field, we used a method which allowed the animals to feed throughout the experiment. Prior to the experiments, the animals were starved for 12 h (chironomid food experiment) or 24 h (leaves food experiment). To avoid cannibalism caused by starvation, the individuals were removed from the indoor flow channel and separated in small glass jars (diameter 6 cm, aerated 300 mL artificial medium, with a stone for orientation inside). Two hours before the evacuation experiment started, each individual was fed with the respective experimental food (one willow leaf or four chironomid larvae). After feeding, the amphipods were transferred in groups of five individuals (leaves food experiment) or three individuals (chironomid food experiment) into 42 of the above described experimental aquaria and fed with a different, well-distinguishable food (post-experimental food, i.e. willow leaves in the chironomid evacuation experiment, customary red color coding dots made of glued paper in the leaves evacuation experiment). Samples were taken over 24 h, at seven time points (0, 1, 3, 5, 9, 16 and 24 h). At each time point, all animals from three aquaria of each treatment were sampled, blotted and flash-frozen in liquid nitrogen for conservation. Every 4 h, three of the six experimental aquaria were treated with 50 mL fresh kairomone water (collected as described above) and three were treated with 50 mL aerated tap water containing no kairomones (control).
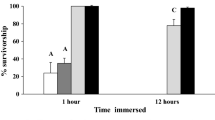
To exclude any effects of the color coding dots (CCD) on the gut evacuation of D. villosus, a separate 48-h experiment was conducted to investigate the gut evacuation of CCD. After 24 h starvation in isolation and 2 h feeding time with CCD, three groups of five individuals each were transferred to experimental aquaria (control) and fed with conditioned willow leaves as post-experimental food. Samples of 3 individuals each were taken at five time points (0, 6, 12, 24 and 48 h) as described above. During experiments, red CCD (diameter 8 mm, AVERY Zweckform®, Holzkirchen, Germany) were used because preliminary tests indicated a preference by D. villosus over other colors. Long-term damage due to the feeding on CCD can be excluded because in another preliminary experiment, animals fed with CCD had a normal mortality rate (only one of twelve animals died within 7 days after feeding with CCD for 24 h).
Consumption experiment
Similar to the evacuation experiments, the daily food consumption of D. villosus was investigated in 24-h experiments as described by Elliott and Persson (1978) for both experimental food types and for the two predator treatments (with and without kairomones). Three hours before the experiment started, groups of five individuals were transferred from the indoor flow channel into each of 42 experimental aquaria for acclimatization. Animals were fed with the experimental food during the acclimatization and the experimental time. To quantify the gut content of D. villosus, samples were taken in 4-h intervals over 24 h. As described for the evacuation experiment, at each time, all amphipods from three experimental aquaria per treatment were sampled, blotted and flash-frozen in liquid nitrogen. All samples were stored at − 20 °C until further sample processing.
Sample processing
In order to determine the gut content of the experimental animals, the guts of three animals per sample were extracted after measuring the body length (mm) and identifying the sex. If females were breeding, the offspring was removed before weighing. If individuals were infected with Acanthocephala (Pomphorhynchus sp., see Emde et al. 2012), the parasite was removed. Although parasite infection can influence the feeding of gammarids (Dick et al. 2010; Agatz and Brown 2014), the animals (n = 6, total of all experiments) were not excluded because the estimate of the consumption rates of our study were intended to reflect to the feeding behavior of individuals in a natural community. Therefore, we decided to include a representative sample of adult individuals (male/female, parasitized/healthy) in the analysis. Nevertheless, data of infected individuals did not affect the results of the data analysis of the whole dataset (S3). The gut extraction was conducted analogous to Bentley and Hurd (1995). For each sample, the gut contents and animals together with the empty guts were placed separately on one previously heated (30 min at 500 °C in muffle furnace) and weighed piece of glass-microfibre discs (grade MGF, diameter 50 mm, Sartorius, Goettingen, Germany) and were stored at − 20 °C. After overnight freeze-drying (Alpha 1–2, Christ, Osterode, Germany), samples were stored in a desiccator and weighed with a microbalance (M3P, Sartorius, Goettingen, Germany).
Estimation of leaf consumption under artificial conditions
In contrast to the consumption experiments under semi-natural conditions, within the artificial consumption experiment the feeding rates were determined in individually kept and pre-starved specimens using a ‘subtraction method’-type approach basically following Naylor et al. (1989). Experimental data are available for conditioned willow leaves (Salix spp.) only because these consumption rates were originally intended as response variable in a physiological experiment. This is also why the experiment was designed as standardized as possible, which is the case in many consumption experiments and can therefore serve as an example for such experiments.
The experiment was conducted in an artificially lighted room using fluorescent daylight tubes (Biolux 36 W/965, Osram, Munich, Germany) simulating a 16:8 h (day:night) photoperiod. All animals were kept in continuously aerated water or media as specified below using membrane pumps. Temperature was controlled by placing the respective animal-containing containers in a water bath that could be regulated by an external thermostat (± 0.1 °C, Lauda RE 304 Ecoline, Lauda-Koenigshofen, Germany). After field-collection, only the male specimens of D. villosus were kept for 1 week in groups of 10–15 individuals in small plastic boxes containing 750 mL of the experimental medium (1:1 mixture, water from the collection site and tap water, both aerated), small pebbles as well as preconditioned willow leaves (S2).
During the actual consumption experiment, 15 of the specimens were placed individually in experimental chambers consisting of 125 mL plastic cups and containing 110 mL of the experimental medium at 15 °C, and starved for 24 h. Each chamber was fitted with a double-bottom consisting of a gauze inlet (1.5 mm mesh size, approx. 20 mm above the original bottom) to separate excreted faecal pellets from the animals by falling through the gauze and consequently prevent coprophagy during the experiments. Each experimental chamber contained one small pebble to provide a shelter.
Subsequent to the starvation period, each individual was transferred into a new experimental chamber containing temperate fresh medium and allowed to feed on one pre-weighed, conditioned leaf disc (approx. 13 mm diameter) for 24 h. The remaining parts of the leaf disc as well as smaller leaf particles, which could be easily discriminated from cylindrical faeces (Gergs and Rothhaupt 2008), were then transferred onto filter paper to remove adhering water and weighed using a microbalance (Mettler Toledo, 205 DR, Greifensee, Switzerland) to the nearest 0.01 mg.
This experimental procedure was repeated using the same individuals for another 24-h period by transferring the animals in new experimental chambers containing fresh medium and a new leaf disc. At the end of the second experimental trial, the fresh weight as well as the dry weight of each individual was determined after blotting it dry with soft tissue paper and, for the latter, subsequent drying at 55 °C for 24 h. To account for a potential change in the weight of the leaf discs during the experiment, 10 control treatments (without animals) were included in each trial. Moreover, to establish a specific weight conversion factor for willow leaves the fresh and dry weight of 32 leaf discs was determined within this experiment as well.
Data analysis
For the experiments under semi-natural conditions, the relative gut content G was calculated as the ratio of gut dry weight W gut (mg dw) to body dry weight W body (mg dw). On the basis of G, the gut evacuation rate R (mg dw mg−1 animal dw h−1) as well as the daily food consumption C (mg dw mg−1 animal dw d−1) were calculated according to Elliott and Persson (1978). In this model, R is an exponential rate and C equals the sum of food consumptions in i intervals of 24/i h duration. We used 6 intervals of 4-h duration each. In contrast, the daily food consumption under artificial conditions was calculated as the mean fresh weight loss of the provided leaf discs within the two consecutive 24 h periods, corrected by the mean trial-specific weight change of the control discs and was then converted to (mg dw mg−1 animal dw d−1) using specific conversion factors for D. villosus and willow leaves as specified below.
The data analysis was performed using R 3.2.0 (R Core Team 2015). The assumptions of parametric tests were checked graphically from boxplots and quantile–quantile plots. Confidence intervals, standard error, coefficient of variation, and quantiles (0.025, 0.5 and 0.975) of C were estimated by nonparametric bootstrapping (Efron and Tibshirani 1993; number of bootstrap samples B = 1000). A permutation test (cf. Efron and Tibshirani 1993; stratified resampling test, 7 strata with 2 × 3 measurements per stratum, number of permutation samples P = 1000) was employed in order to evaluate differences of R respectively C between treatments (with and without kairomones) or food sources (chironomids and leaves). Significance level was set to α = 0.05. In order to avoid a two-factorial permutation test, data of different treatments were combined when no treatment effect was observed.
In order to compare the results of the semi-natural and the artificial consumption experiments of our study with the consumption rates in other studies, the leaf consumption/breakdown rates and shredding efficiency values of D. villosus found in the literature were converted to mg dw consumed food mg−1 animal dw per day, if necessary, or directly provided by the authors in the case of Gergs and Rothhaupt (2008). For all conversions we used a mean dry weight of 16.8 ± 0.89 mg for male D. villosus (mean ± SE, n = 120) with a mean body length of 11.6 ± 0.20 mm (mean ± SE, n = 206), a standardization of leaf pieces [alder leaves, 4.62 cm2, 0.035 ± 0.007 g dry weight, Boeker and Geist (2015)] and the ratios of fresh weight to dry weight of 4.09 for D. villosus (n = 15); dry weight to fresh weight of 0.163 for alder leaves (Jourdan et al. 2016) and 0.297 for willow leaves (n = 32). In the following text, all evacuation and consumption rates are stated in (mg dw food mg−1 animal dw).
Results
D. villosus showed very high daily consumption rates in the semi-natural experimental setting (54–89% of its body weight, Table 1). The daily consumption rate for willow leaves was significantly higher than that for chironomid larvae but this was not the case for the gut evacuation rate (Table 2). On the other hand, neither gut evacuation nor consumption differed significantly between the fish kairomone and control treatments (Table 2). Consequently, the comparison between food sources is based on a combined data set of both predator treatments. All individuals had evacuated the experimental food within max. 24 h (Fig. 1a, c). In case of the willow leaf treatment, the relative gut content was lower at t 0 than after 1 h (Fig. 1c). Therefore the t 0 samples of this trial were excluded from the calculation of the evacuation rate. In the 24-h consumption experiments under semi-natural conditions, no pronounced diel rhythm was observed, although the relative gut content showed a maximum at 5 p.m. for chironomids as food (Fig. 1b).
Under artificial conditions, D. villosus showed a lower mean daily consumption of willow leaves (0.24 ± 0.07, n = 12) than under semi-natural conditions.
The analysis of the gut evacuation of color coding dots (CCD) revealed that amphipods are able to normally evacuate CCD, although the gut evacuation rate of CCD (R CCD = 0.08 ± 0.02 h−1, mean ± SE) was lower than R of willow leaves or chironomid larvae (compare Table 1). The mortality in the experiments under semi-natural conditions (gut evacuation, consumption and CCD gut evacuation) averaged 4.9%. The mortality in the CCD experiments was also comparably low (2 out of 75 animals died during 48 h). Although no mortality was observed under artificial conditions during the experimental period, 3 individuals were excluded from calculating the consumption rate because of their molting during the experiments.
Discussion
The aim of this study was to analyze the ecological potential of D. villosus to perform different ecological functions (as a predator or shredder) in river ecosystems. We therefore estimated daily food consumption of D. villosus under semi-natural laboratory conditions and tested whether it would differ between chironomid larvae and leaf-litter or whether it would decrease in presence of fish kairomones. Mainly as a result of the high gut evacuation rates (0.14–0.26 h−1), the daily food consumption of D. villosus amounted to 54–89% of its own body weight. By comparison with consumption/leaf litter breakdown rates of former studies (Gergs and Rothhaupt 2008; MacNeil et al. 2011; Piscart et al. 2011; Truhlar et al. 2014; Boeker and Geist 2015; Jourdan et al. 2016) and with our results from the artificial experimental setting (Fig. 2), this enormous food consumption potential of D. villosus was unexpected. The high values were even more remarkable as most of these studies observed lower leaf consumptions for the invader than for native gammarid species (MacNeil et al. 2011; Boeker and Geist 2015; Jourdan et al. 2016) with the exception of Truhlar et al. (2014). The very high consumption rates and the apparent large variability of these rates underline the high potential of D. villosus to affect ecosystem functioning, especially with regard to the very high biomasses and the dominance in the macroinvertebrate community observed for instance at the River Elbe or the River Rhine (Hellmann et al. 2015). In consequence, D. villosus can be assumed to be a potentially strong competitor of other shredding macroinvertebrate species and may act as a key species in benthic food webs.
Comparison of the leaf consumption of D. villosus per day between different studies with respect to the applied estimation methods and experimental conditions (estimation method, grouping). Left bars represent animals kept in groups while right bars represent individually kept animals. Grey bars symbolize the consumption estimation using relative gut contents, black bars indicate the use of the subtraction method. Upper/lower margin of bars: Max/min consumption rates. If only the mean consumption rate was published, max/min were estimated by addition/subtraction of SD
The large variation between estimated feeding rates in the different laboratory experiments might result from differences in the environmental factors like food source, food quality or temperature (e.g. Agatz and Brown 2014; Pellan et al. 2016). Because of its high flexibility and ability to cope with changing environmental conditions or different food sources, the food consumption of D. villosus may be perceived as a reaction to environmental factors—illustrating the variability of D. villosus ecological functions. We tested two of these potential environmental factors under semi-natural conditions: food source and potential predation.
While predation risk did not affect consumption rate, the type of food significantly affected the daily food consumption of D. villosus with higher values for leaves than for chironomids. Higher consumption rates for plant material are also known for other amphipod species (Cruz-Rivera and Hay 2000; Dick et al. 2005) and seem to be related to the lower nutritional quality of the food source (Cruz-Rivera and Hay 2000; Dick et al. 2005; Gergs and Rothhaupt 2008; Hellmann et al. 2015): chironomid larvae are expected to be higher-quality food than leaves and during food choice experiments, amphipods consume more of the preferred high-quality food than of plant material (Cruz-Rivera and Hay 2000; Dick et al. 2005; Pellan et al. 2016). But when amphipods are confined to only one food source, consumption of low-quality food is much higher, possibly because of compensatory feeding (Cruz-Rivera and Hay 2000; Dick et al. 2005). Our observation is in accordance with this compensatory feeding assumption, but contradicts the results of Gergs and Rothhaupt (2008), who observed higher feeding rates on chironomids than on alder leaves. One reason for this could be an effect of the tree species/leaf type. We used willow leaves from site-specific vegetation instead of alder leaves. But, although selection among leaf types is known for invertebrates (e.g. summarized in Graça 2001), Jourdan et al. (2016) couldn’t observe an effect of leaf type on the consumption of D. villosus. Another reason could be the shorter acclimatization time regarding the food source (only 24 h) in the experiments of Gergs and Rothhaupt (2008), i.e. the animals did probably not perceive the potential loss of energy or nutrients by feeding low-quality food and did not show compensatory feeding responses. Moreover, consumption rates for chironomids might have been overestimated when using the subtraction method because D. villosus is known to bite off parts of prey organisms but not to consume the whole item (Dick et al. 2002) and injured chironomid larvae lose body fluids and thereby weight. Another explanation for the higher D. villosus consumption rate for plant material than for chironomids in our experiments could be the more effortless food availability of leaves because of the trade-off between hiding and feeding (see above, Ahlgren et al. 2011; Szokoli et al. 2015) and the resulting avoidance of energy loss for prey handling.
However, we observed no effect of added kairomones on the feeding activity or consumption rate of D. villosus indicating no sensitive response to predator presence with respect to the food consumption. This observation is supported by the results of other studies also reporting a lack of predator avoidance behavior (e.g. Rossano et al. 2013) and seems to distinguish D. villosus from native Gammarus species, e.g. G. fossarum and G. pulex (Andersson et al. 1986; Szokoli et al. 2015). It is possible that the daily food consumption of D. villosus would decrease in presence of chemical cues of injured conspecifics (alarm cues), because predator avoidance behavior such as hiding or decreased mobility and activity was observed for D. villosus when it was exposed to cues of injured conspecifics (Wudkevich et al. 1997; Wisenden et al. 1999; Sornom et al. 2012). However, because D. villosus is also known to be cannibalistic (Platvoet et al. 2009a), it seems at least questionable whether alarm cues would result in decreased food consumption. In accordance, Jermacz et al. (2017) found D. villosus to be attracted by chemical cues of predators fed with chironomids and conspecifics. Because we fed the bullheads with chironomid larvae, the results of Jermacz et al. (2017) could also explain the absence of avoidance behavior and effects on the food consumption of D. villosus. Another explanation for the absent effect on the feeding rate might be the direct proximity of accessible food to the shelters (in the mid of the aquarium and near the surrounding stones) as shown by Jermacz and Kobak (2017). Thus, in our study, we observed a ‘domicolous’ behavior (i.e. a strong affinity to refuges) of D. villosus, which has previously been reported by Platvoet et al. (2006). The amphipod stays in its preferred refuge between stones (Van Riel et al. 2006a; Kobak et al. 2015) and leaves its hideouts only for short time (also described by Devin et al. 2003; Platvoet et al. 2009a; Rossano et al. 2013). This behavioral pattern can be described as a form of predator avoidance behavior which—in case of sufficient food availability—does not require a trade-off between feeding and hiding (e.g. summarized in Moore and Eastman 2015). Therefore, it seems unlikely that predator cues cause a decrease in feeding rate, even if the amphipods are familiar to the predator from their natural habitat (Åbjörnsson et al. 2004). In addition, amphipods can also habituate to the chemical cues of predators already after a short time. This phenomenon was shown for G. pulex that consumed less leaves in the presence of fish kairomones than in the control treatment at the beginning, but not after 4 weeks (Åbjörnsson et al. 2000). Additionally, D. villosus did not show a conspicuous diurnal feeding periodicity in our study, indicated by the lack of diurnal variation of the relative gut content. This observation could be also related to the affinity to refuges, because diel rhythms are often triggered by predators (Rusak and Zucker 1975; Wagner 1991; Huhta et al. 2000; Pettersson et al. 2001). For D. villosus, Rossano et al. (2013) observed a negative phototaxis, which might be interpreted as predator avoidance but could also be explained by its strong refuge affinity. Therefore, negative phototaxis and the absence of a diel feeding rhythm do not contradict. This applies even more under conditions of consistently high food availability as in our experiments.
For a validation of our observations, we compared the results from our ‘semi-natural experiment’ (others were not available from the literature) with those of our ‘artificial experiment’ as well as other ‘artificial’ consumption estimation studies for D. villosus with leaves as food source (Fig. 2). The remarkable difference between consumption rates estimated under semi-natural conditions and under artificial conditions underlines the crucial importance of the applied method. Important experimental conditions of all studies, such as temperature and conditioning of the leaves, were very similar. Although the leaves were of different tree species, all tree species (especially alder, willow and sycamore leaves) are common along stream-sides and known to be palatable for amphipods (MacNeil and Platvoet 2005; Truhlar et al. 2014; Jourdan et al. 2016). We feel that the observed difference between the consumption estimates can be explained by no single factor alone but by a congregation of several factors which are described in the following. The first group of such factors originates from the principle of calculation and the second from the different surrounding conditions of the two methods.
The probably strongest factor in the first group is the dominant influence of the evacuation rate on the consumption estimation. Our applied method resulted probably in evacuation rates near the maximum, i.e., possible under optimal conditions. In laboratory (this study) and field experiments (unpublished data) we observed that D. villosus feeds more or less continuously and there are no extended feeding intermissions. Because such intermissions normally result in reduced gut evacuation as observed for other crustaceans (Daphnia magna, Gillis et al. 2005) and for fish (Thorpe 1977), we intended to avoid an underestimation of the evacuation rate by allowing the animals to feed continuously during gut evacuation experiments. In order to separate the experimental and the post-experimental food in the gut contents for evacuation rate estimation, the food source was changed at the start of the actual evacuation period. Although faeces were reported to differ according to the food source (Bärlocher and Kendrick 1975), the discrimination between leaf-based and chironomid-based gut contents proofed difficult. For this reason, we applied color coding dots as post-experimental food consisted of because they are clearly identifiable in the guts. Even if this method was used to avoid an underestimation of the evacuation rate, one could suspect the use of the post-experimental food to affect the evacuation rate of the experimental food. In fact, we observed a much lower gut evacuation rate of CCD compared to willow leaves or chironomid larvae, which could be, among others, a result of larger sampling intervals or harder digestibility. Nevertheless, CCD did not harm the animals because the number of dead animals during the CCD experiments was even lower than in the consumption experiments and nearly all CCD were evacuated after 24 h. We therefore do not expect experimental artifacts from using CCD. In consequence, CCD seem appropriate to determine the gut evacuation rate via gut content separation. Another factor influencing the estimate of food consumption could be that amphipods do not empty their gut completely (Bärlocher and Kendrick 1975). Consequently, by weighing the whole animal with its remaining gut content when using the subtraction method, the ratio of consumed food and body weight and thus indirectly the consumption rate could be underestimated. For these reasons, the investigation of gut contents and the measuring of the gut evacuation rate during constant feeding seems to be the more adequate method.
One possible influencing factor belonging to the second group (experimental conditions) is that consumption experiments under standardized artificial conditions do not represent an optimal environment. For the determination of feeding rates in ecotoxicological or physiological studies, a largely standardized and nearly one-factorial feeding assay is necessary (Agatz and Brown 2014). However, in this study, we aimed to determine evacuation and consumption rates which can be used as an estimate of field conditions and also applied to field research questions. Therefore we provided more natural conditions in our feeding experiment, which is why we use the specificaion ‘semi-natural’. Without sufficient refuges, D. villosus consumption rates might not benefit any more from its typical refuge affinity because feeding activity might decrease temporarily (e.g. during daytime). We expect that group-keeping of the animals during the semi-natural experiments increased the consumption rate, as was indicated by observations in preliminary experiments. Such a behavior is also known for other species, e.g. flatworms (Cash et al. 1995) or birds (Beauchamp 1998). However, keeping the animals in groups or individually did not seem to affect the food consumption rate in the ‘artificial’ experiments to a great extend [Fig. 2, group size either 1 or 5 animals except Boeker and Geist (2015) 20 animals].
We conclude from our results that the assessment of food consumption under semi-natural conditions probably represents a maximum estimation. Therefore, we think that the consumption rates from our semi-natural experiments are close to what can be expected in real ecosystems at sufficient food availability, although our estimates are higher than those of former studies. In consequence, D. villosus is not necessarily feed less than native gammarid species, as was previously assumed, and may be a very efficient shredder under appropriate environmental conditions. The results of the present study also indicate that the daily food consumption of D. villosus does not decrease in presence of fish kairomones. We expect that its strong affinity to refuges which are used simultaneously for feeding apparently releases D. villosus from the trade-off between predator avoidance and feeding. Most probably, this behavior as well as compensatory feeding of low-quality food (when only one food source is available) results in higher consumption rates of willow leaves than of chironomids, as observed in our study. This enormous food consumption potential of D. villosus in addition to the described benefits for feeding (no disadvantages by predation) could support the invasion success of D. villosus in European Rivers.
References
Åbjörnsson K, Dahl J, Nyström P, Brönmark C (2000) Influence of predator and dietary chemical cues on the behaviour and shredding efficiency of Gammarus pulex. Aquat Ecol 34:379–387
Åbjörnsson K, Hansson L-A, Brönmark C (2004) Responses of prey from habitats with different predator regimes: local adaptation and heritability. Ecology 85:1859–1866
Agatz A, Brown CD (2014) Variability in feeding of Gammarus pulex: moving towards a more standardised feeding assay. Environ Sci Eur 26:1–9
Ahlgren J, Åbjörnsson K, Brönmark C (2011) The influence of predator regime on the behaviour and mortality of a freshwater amphipod, Gammarus pulex. Hydrobiologia 671:39–49
Amundsen P-A, Bergersen R, Huru H, Heggberget TG (1999) Diel feeding rhythms and daily food consumption of juvenile Atlantic salmon in the River Alta, Northern Norway. J Fish Biol 54:58–71
Andersson K, Brönmark C, Herrmann J, Malmqvist B, Otto C, Sjörström P (1986) Presence of sculpins (Cottus gobio) reduces drift and activity of Gammarus pulex (Amphipoda). Hydrobiologia 133:209–215
Bajkov AD (1935) How to estimate the daily food consumption of fish under natural conditions. Trans Am Fish Soc 65:288–289
Bärlocher F, Kendrick B (1975) Assimilation efficiency of Gammarus pseudolimnaeus (Amphipoda) feeding on fungal mycelium or autumn-shed leaves. Oikos 26:55–59
Baumgärtner D, Koch U, Rothhaupt K-O (2003) Alteration of kairomone-induced antipredator response of the freshwater amphipod Gammarus roeseli by sediment type. J Chem Ecol 29:1391–1401
Beauchamp G (1998) The effect of group size on mean food intake rate in birds. Biol Rev 73:449–472
Beggel S, Brandner J, Cerwenka AF, Geist J (2016) Synergistic impacts by an invasive amphipod and an invasive fish explain native gammarid extinction. BMC Ecol 32:1–13
Bentley CR, Hurd H (1995) Depressed protein and copper content of the midgut gland in an intermediate host, Gammarus pulex (Crustacea), infected with cystacanths of Pomphorhynchus laevis (Acanthocephala). J Invertebr Pathol 66:1–5
Bij de Vaate A, Klink AG (1995) Dikerogammarus villosus Sowinsky (Crustacea: Gammaridae) a new immigrant in the Dutch part of the Lower Rhine. Lauterbornia 20:51–54
Bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, Van der Velde G (2002) Geographical patterns in range extension of Ponto–Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174
Boeker C, Geist J (2015) Effects of invasive and indigenous amphipods on physico-chemical and microbial properties in freshwater substrates. Aquat Ecol 49:467–480
Boets P, Lock K, Messiaen M, Goethals PLM (2010) Combining data-driven methods and lab studies to analyse the ecology of Dikerogammarus villosus. Ecol Inform 5:133–139
Bollache L, Dick JTA, Farnsworth KD, Montgomery WI (2008) Comparison of the functional responses of invasive and native amphipods. Biol Lett 4:166–169
Borgmann U (1996) Systematic analysis of aqueous ion requirements of Hyalella azteca: a standard artificial medium including the essential bromide ion. Arch Environ Contam Toxicol 30:356–363
Bruijs MCM, Kelleher B, Van der Velde G, Bij de Vaate A (2001) Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indicators of further dispersal via ballast water transport. Fundam Appl Limnol 152:633–646
Cash KJ, Wrona FJ, Scrimgeour GJ (1995) The effects of group size on per capita ingestion in flatworms. Freshw Biol 34:477–483
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219
Devin S, Beisel J-N, Bachmann V, Moreteau J-C (2001) Dikerogammarus villosus (Amphipoda: Gammaridae): another invasive species newly established in the Moselle river and French hydrosystems. Int J Limnol 37:21–27
Devin S, Piscart C, Beisel J-N, Moreteau J-C (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56
Devin S, Piscart C, Beisel J-N, Moreteau J-C (2004) Life history traits of the invader Dikerogammarus villosus (Crustacea: Amphipoda) in the Moselle River, France. Int Rev Hydrobiol 89:21–34
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc R Soc B 267:977–983
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084
Dick JTA, Johnson MP, McCambridge S, Johnson J, Carson VEE, Kelly DE, MacNeil C (2005) Predatory nature of the littoral amphipod Echinogammarus marinus: gut content analysis and effects of alternative food and substrate heterogeneity. Mar Ecol Prog Ser 291:151–158
Dick JTA, Armstrong M, Clarke HC, Farnsworth KD, Hatcher MJ, Ennis M, Kelly A, Dunn AM (2010) Parasitism may enhance rather than reduce the predatory impact of an invader. Biol Lett 6:636–638
Dodd JA, Dick JTA, Alexander ME, MacNeil C, Dunn AM, Aldridge DC (2013) Predicting the ecological impacts of a new freshwater invader: functional responses and prey selectivity of the “killer shrimp”, Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw Biol 59:337–352
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, London, p 346
Elliott JM, Persson L (1978) The estimation of daily rates of food consumption for fish. J Anim Ecol 47:977–991
Emde S, Rueckert S, Palm HW, Klimpel S (2012) Invasive Ponto–Caspian amphipods and fish increase the distribution range of the acanthocephalan Pomphorhynchus tereticollis in the River Rhine. PLoS ONE 7:e53218
Gergs R, Rothhaupt K-O (2008) Feeding rates, assimilation efficiencies and growth of two amphipod species on biodeposited material from zebra mussels. Freshw Biol 53:2494–2503
Gillis PL, Chow-Fraser P, Ranville JF, Ross PE, Wood CM (2005) Daphnia need to be gut-cleared too: the effect of exposure to and ingestion of metal-contaminated sediment on the gut-clearance patterns of D. magna. Aquat Toxicol 71:143–154
Gollasch S (1996) Untersuchungen des Artbeitrages durch den internationalen Schiffsverkehr unter besonderer Berücksichtigung nichteinheimischer Arten. Ph.D. thesis, University of Hamburg
Grabowski M, Bacela K, Konopacka A (2007) How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia 590:75–84
Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams—a review. Int Rev Hydrobiol 86:383–393
Hellmann C, Worischka S, Mehler E, Becker J, Gergs R, Winkelmann C (2015) The trophic function of Dikerogammarus villosus (Sowinsky, 1894) in invaded rivers: a case study in the Elbe and Rhine. Aquat Invasions 10:385–397
Hellmann C, Schöll F, Worischka S, Becker J, Winkelmann C (2017) River-specific effects of the invasive amphipod Dikerogammarus villosus (Crustacea: Amphipoda) on benthic communities. Biol Invasions 19:381–398
Héroux D, Magnan P (1996) In situ determination of food daily ration in fish: review and field evaluation. Environ Biol Fish 46:61–74
Huhta A, Muotka T, Tikkanen P (2000) Nocturnal drift of mayfly nymphs as a post-contact antipredator mechanism. Freshw Biol 45:33–42
Jermacz Ł, Kobak J (2017) Keep calm and don’t stop growing: non-consumptive effects of a sympatric predator on two invasive Ponto–Caspian gammarids Dikerogammarus villosus and Pontogammarus robustoides. PLoS ONE 12:e0182481
Jermacz Ł, Dzierżyńska A, Kakareko T, Poznańska M, Kobak J (2015) The art of choice: predation risk changes interspecific competition between freshwater amphipods. Behav Ecol 26:656–664
Jermacz Ł, Dzierżyńska-Białończyk A, Kobak J (2017) Predator diet, origin or both? Factors determining responses of omnivorous amphipods to predation cues. Hydrobiologia 785:173–184
Jobling M (1981) Mathematical models of gastric emptying and the estimation of daily rates of food consumption for fish. J Fish Biol 19:245–257
Jourdan J, Westerwald B, Kiechle A, Chen W, Streit B, Klaus S, Oetken M, Plath M (2016) Pronounced species turnover, but no functional equivalence in leaf consumption of invasive amphipods in the River Rhine. Biol Invasions 18:763–774
Kaldonski N, Lagrue C, Motreuil S, Rigaud T, Bollache L (2008) Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften 95:839–844
Kinzelbach R (1995) Neozoans in european waters—exemplifying the worldwide process of invasion and species mixing. Experientia 51:526–538
Kobak J, Jermacz Ł, Dzierżyńska-Białończyk A (2015) Substratum preferences of the invasive killer shrimp Dikerogammarus villosus: substratum preferences of Dikerogammarus villosus. J Zool 297:66–76
Kobak J, Rachalewski M, Bącela-Spychalska K (2016) Conquerors or exiles? Impact of interference competition among invasive Ponto–Caspian gammarideans on their dispersal rates. Biol Invasions 18:1953–1965
Koester M, Gergs R (2014) No evidence for intraguild predation of Dikerogammarus villosus (Sowinsky, 1894) at an invasion front in the Untere Lorze, Switzerland. Aquat Invasions 9:489–497
Koester M, Bayer B, Gergs R (2016) Is Dikerogammarus villosus (Crustacea, Gammaridae) a “killer shrimp” in the River Rhine system? Hydrobiologia 768:299–313
Krisp H, Maier G (2005) Consumption of macroinvertebrates by invasive and native gammarids: a comparison. J Limnol 64:55–59
Lampert W (1989) The adaptive significance of diel vertical migration of zooplankton. Funct Ecol 3:21–27
Lehtiniemi M (2005) Swim or hide: predator cues cause species specific reactions in young fish larvae. J Fish Biol 66:1285–1299
Lockwood SJ (1980) The daily food intake of 0-group plaice (Pleuronectes platessa L.) under natural conditions. ICES J Mar Sci 39:154–159
Maazouzi C, Masson G, Izquierdo MS, Pihan J-C (2007) Fatty acid composition of the amphipod Dikerogammarus villosus: feeding strategies and trophic links. Comp Biochem Physiol A Mol Integr Physiol 147:868–875
Maazouzi C, Piscart C, Pihan J-C, Masson G (2009) Effect of habitat-related resources on fatty acid composition and body weight of the invasive Dikerogammarus villosus in an artificial reservoir. Fundam Appl Limnol 175:327–338
MacNeil C, Platvoet D (2005) The predatory impact of the freshwater invader Dikerogammarus villosus on native Gammarus pulex (Crustacea: Amphipoda); influences of differential microdistribution and food resources. J Zool 267:31–38
MacNeil C, Platvoet D (2013) Could artificial structures such as fish passes facilitate the establishment and spread of the “killer shrimp” Dikerogammarus villosus (Crustacea: Amphipoda) in river systems? Aquat Conserv 23:667–677
MacNeil C, Platvoet D, Dick JTA, Fielding N, Constable A, Hall N, Aldridge DC, Renals T, Diamond M (2010) The Ponto–Caspian “killer shrimp”, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Invasions 5:441–445
MacNeil C, Dick JTA, Platvoet D, Briffa M (2011) Direct and indirect effects of species displacements: an invading freshwater amphipod can disrupt leaf-litter processing and shredder efficiency. J N Am Benthol Soc 30:38–48
Metcalfe NB, Fraser NHC, Burns MD (1999) Food availability and the nocturnal versus diurnal foraging trade-off in juvenile salmon. J Anim Ecol 68:371–381
Mills CA, Mann RHK (1983) The bullhead Cottus gobio, a versatile and successful fish. In: Fifty-first annual report for the year ended 31st March 1983. Rep Freshw Biol Ass 51:76–88
Moore PG, Eastman LB (2015) The tube-dwelling lifestyle in crustaceans and its relation to feeding. In: Thiel M, Watling L (eds) Lifestyles and feeding biology. The natural history of the crustacea. Oxford University Press, New York, pp 35–77
Naylor C, Maltby L, Calow P (1989) Scope for growth in Gammarus pulex, a freshwater benthic detritivore. Hydrobiologia 188–189:517–523
Normant M, Lamprecht I (2006) Does scope for growth change as a result of salinity stress in the amphipod Gammarus oceanicus? J Exp Mar Biol Ecol 334:158–163
Pellan L, Médoc V, Renault D, Spataro T, Piscart C (2016) Feeding choice and predation pressure of two invasive gammarids, Gammarus tigrinus and Dikerogammarus villosus, under increasing temperature. Hydrobiologia 781:43–54
Pettersson LB, Brönmark C (1993) Trading off safety against food: state dependent habitat choice and foraging in crucian carp. Oecologia 95:353–357
Pettersson LB, Andersson K, Nilsson K (2001) The diel activity of crucian carp, Carassius carassius, in relation to chemical cues from predators. Environ Biol Fish 61:341–345
Piscart C, Mermillod-Blondin F, Maazouzi C, Merigoux S, Marmonier P (2011) Potential impact of invasive amphipods on leaf litter recycling in aquatic ecosystems. Biol Invasions 13:2861–2868
Platvoet D, Dick JTA, Konijnendijk N, Van der Velde G (2006) Feeding on micro-algae in the invasive ponto-caspian amphipod Dikerogammarus villosus (Sowinsky, 1894). Aquat Ecol 40:237–245
Platvoet D, Dick JTA, MacNeil C, Van Riel MC, Van der Velde G (2009a) Invader–invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus—Sexton: amphipod pilot species project (AMPIS) report 6. Biol Invasions 11:2085–2093
Platvoet D, Van der Velde G, Dick JTA, Li S (2009b) Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda)—amphipod pilot species project (AMPIS) report 5. Crustaceana 82:703–720
Pöckl M (2007) Strategies of a successful new invader in European fresh waters: fecundity and reproductive potential of the Ponto–Caspian amphipod Dikerogammarus villosus in the Austrian Danube, compared with the indigenous Gammarus fossarum and G. roeseli. Freshw Biol 52:50–63
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computin, Vienna
Rewicz T, Grabowski M, MacNeil C, Bacela-Spychalska K (2014) The profile of a “perfect” invader—the case of killer shrimp, Dikerogammarus villosus. Aquat Invasions 9:267–288
Rossano C, Di Cristina G, Scapini F (2013) Life cycle and behavioural traits of Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda, Gammaridae) colonising an artificial fresh water basin in Tuscany (central Italy). Crustaceana 86:908–931
Rusak B, Zucker I (1975) Biological rhythms and animal behavior. Annu Rev Psychol 26:137–171
Schäffer M, Winkelmann C, Hellmann C, Benndorf J (2013) Reduced drift activity of two benthic invertebrate species is mediated by infochemicals of benthic fish. Aquat Ecol 47:99–107
Sornom P, Gismondi E, Vellinger C, Devin S, Férard J-F, Beisel J-N, Sakamoto KQ (2012) Effects of sublethal cadmium exposure on antipredator behavioural and antitoxic responses in the invasive amphipod Dikerogammarus villosus. PLoS ONE 7:e42435
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Szokoli F, Winkelmann C, Berendonk TU, Worischka S (2015) The effects of fish kairomones and food availability on the predator avoidance behaviour of Gammarus pulex. Fundam Appl Limnol 186:249–258
Thorpe JE (1977) Daily ration of adult perch, Perca fluviatilis L. during summer in Loch Leven, Scotland. J Fish Biol 11:55–68
Tittizer T (1997) Ausbreitung aquatischer Neozoen (Makrozoobenthos) in den europäischen Wasserstrassen, erläutert am Beispiel des Main-Donau-Kanals. Schriftenreihe Bundesamtes Für Wasserwirtschaft 4:113–134
Tricarico E, Mazza G, Orioli G, Rossano C, Scapini F, Gherardi F (2010) The killer shrimp, Dikerogammarus villosus (Sowinsky, 1894), is spreading in Italy. Aquat Invasions 5:211–214
Truhlar AM, Dodd JA, Aldridge DC (2014) Differential leaf-litter processing by native (Gammarus pulex) and invasive (Dikerogammarus villosus) freshwater crustaceans under environmental extremes: leaf-litter processing by crustaceans under environmental extremes. Aquat Conserv Mar Freshw Ecosyst 24:56–65
Van Riel MC, Van der Velde G, Bij de Vaate A (2006a) To conquer and persist: colonization and population development of the Ponto–Caspian amphipods Dikerogammarus villosus and Chelicorophium curvispinum on bare stone substrate in the main channel of the River Rhine. Arch Hydrobiol 166:23–39
Van Riel MC, Van der Velde G, Rajagopal S, Marguillier S, Dehairs F, Bij de Vaate A (2006b) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto–Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58
Viherluoto M, Viitasalo M (2001) Effect of light on the feeding rates of pelagic and littoral mysid shrimps: a trade-off between feeding success and predation avoidance. J Exp Mar Biol Ecol 261:237–244
Wagner R (1991) The influence of the diel activity pattern of the larvae of Sericostoma personatum (Trichoptera) on organic matter distribution in stream-bed sediments? A laboratory study. Hydrobiologia 224:65–70
Weisberg SB, Whalen R, Lotrich VA (1981) Tidal and diurnal influence on food consumption of a salt marsh killifish Fundulus heteroclitus. Mar Biol 61:243–246
Werner EE, Gilliam JF, Hall DJ, Mittelbach GG (1983) An experimental test of the effects of predation risk on habitat use in fish. Ecology 64:1540–1548
Wijnhoven S, Van Riel MC, Van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158
Willoughby LG, Earnshaw R (1982) Gut passage times in Gammarus pulex (Crustacea, Amphipoda) and aspects of summer feeding in a stony stream. Hydrobiologia 97:105–117
Wisenden BD, Cline A, Sparkes TC (1999) Survival benefit to antipredator behavior in the amphipod Gammarus minus (Crustacea: Amphipoda) in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology 105:407–414
Worischka S, Mehner T (1998) Comparison of field-based and indirect estimates of daily food consumption in larval perch and zander. J Fish Biol 53:1050–1059
Wudkevich K, Wisenden BD, Chivers DP, Smith RJF (1997) Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics. J Chem Ecol 23:1163–1173
Acknowledgements
This study was financially supported by the German Research Foundation (DFG) as a part of the Emmy Noether project WI 3592/1. We would like to thank Thomas Berendonk, Kristin Berg, Anne Großmann, Stephanie Graumnitz, Felix Grunicke, Fanny Hempel, Steffen Kunze, Ulrike Mogck, Thomas Petzoldt, Thomas Schendel, Ulrike Schmalfuß, Janno Worischka and Christiane Zschornack for their help in field and laboratory, for technical support and constructive discussions of statistical analysis. We greatly acknowledge the support of René Gergs and Jonas Jourdan by providing us with original data contributing to our comparison of leaf consumption rates. Many thanks are also due to two anonymous reviewers for their comments improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richter, L., Schwenkmezger, L., Becker, J. et al. The very hungry amphipod: the invasive Dikerogammarus villosus shows high consumption rates for two food sources and independent of predator cues. Biol Invasions 20, 1321–1335 (2018). https://doi.org/10.1007/s10530-017-1629-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1629-4