Abstract
In species with restricted dispersal, traits may become genetically fixed leading to local adaptations. Therefore, predator avoidance in a prey species may differ between populations experiencing different predator regimes, but also between sexes within a population due to different vulnerability to predators. In this study we used male and female Gammarus pulex from two different predator regimes: fishless ponds, where invertebrates are the dominant predators and ponds with predatory fish. In the laboratory we examined refuge use, mortality, leaf decomposition rate and pair-formation in G. pulex when exposed to predator cues from either invertebrate predators or fish. Individuals from fish ponds spent more time in refuge and had a higher mortality than those from fishless ponds independent of predator cues. There was no effect of pond predator regime or predator cues on leaf decomposition rates. Further, fewer individuals formed pairs in G. pulex from fish ponds than from fishless ponds. Male G. pulex had a higher mortality and a higher decomposition rate than females independent of predator cues. However, there was no difference in refuge use between sexes. Our study shows that there are general differences in behaviour traits, both between predator regimes and sexes in G. pulex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predators are of great importance in freshwater ecosystems, since they may have a large impact upon prey community structure and dynamics (e.g. Lima & Dill, 1990; Carpenter & Kitchell, 1993; Wellborn et al., 1996; Åbjörnsson et al., 2004). In addition to having a direct lethal effect on prey abundance (e.g. Cooper et al., 1990; Holomuzki & Hatchett, 1994; Nyström et al., 2001) predators can also have an indirect effect by, e.g. inducing changes in prey behaviour (e.g. Holomuzki & Short, 1988, Turner et al., 1999, Nyström & Åbjörnsson, 2000). In aquatic habitats prey often use chemical cues to recognise predator presence, which enables them to respond to and lower the risk of predation (e.g. Brönmark & Hansson, 2000). For example, a number of studies have shown that prey species may change their activity level, habitat use, forage at different times or alter their foraging behaviour in response to predator cues (e.g. Douglas et al., 1994; Sih & Wooster, 1994; Nyström & Åbjörnsson, 2000). Predators may thus affect ecosystem processes, such as grazing and decomposition, by direct (density-mediated indirect effects; Peacor & Werner, 1997) and indirect (trait-mediated indirect effects; Peacor & Werner, 1997) effects on their prey (e.g. Obernborfer et al., 1984; McIntosh and Townsend, 1996; Turner, 1997; Peckarsky & Mcintosh, 1998).
In aquatic systems invertebrate prey may be exposed to a range of different predators (e.g. fish, invertebrates, waterfowl) that differ in foraging strategy. Hence, in order to survive prey species have to respond differently to different predators (Sih et al., 1998). If the composition of the predator assemblage differs between different habitats, for example, lakes and ponds, then the local predator regime may give rise to local adaptations in prey defences, given that migration rates among habitats are sufficiently low (e.g. Mccarthy & Fisher, 2000; Åbjörnsson et al., 2004). Further, responses to predators may differ not only between species and predator regimes but also between sexes within a species due to different vulnerability to predators. For example, male water striders (Aquarius remigis) shift to safer habitats (refuge) and decrease their activity when exposed to fish, whereas female water striders do not respond at all (Krupa & Sih, 1998). Also, as in many taxa, males tend to be larger than females (e.g. Goedmakers, 1981), and since larger individuals are more vulnerable to positive size selective predators males may react stronger to these predatory cues (Cothran, 2004).
Here, we investigate the influence of predation regime on behaviour and mortality in males and females of an ecologically important freshwater species, the amphipod Gammarus pulex. G. pulex are common in both lentic and lotic freshwater systems and occur in both fish and fishless systems. Although G. pulex are considered detritivorous, they are generalists and cannibalism has been documented (Dick, 1995, McGrath et al., 2007, Felten et al., 2008). To increase chances of mating, males search for females and then guard them until mating is possible (precopulatory mate guarding; Parker, 1974). This strategy is considered to be costly for both males and females, with some costs being sex-specific (Robinson & Doyle, 1985; Dick, 1995; Plaistow et al., 2003), while others (e.g. increased predation risk from fish) apply to both sexes (Ward, 1986, Cothran, 2004). Because G. pulex has a restricted ability to disperse, their exposure to predator types outside the habitat is limited, and they may therefore be adapted to the local predator regime (Åbjörnsson et al., 2004). Several Gammarus species respond to chemical cues released from different predators or from activity associated with predation (Williams & Moore, 1985; Wisenden et al., 1999; Åbjörnsson et al., 2000, 2004; Wisenden et al., 2001; Bollache et al., 2006; Kaldonski et al., 2008). This makes G. pulex an excellent organism to test predator induced behaviour under different predator regimes.
Åbjörnsson et al. (2004, 2009) found differences in refuge use and life history strategies between G. pulex from fish and fishless ponds. Our aim with this experiment was to study if these differences are primarily driven by population level differences in trait values rather than plastic responses to predator cues. Further, in addition to previous studies looking only at effects from fish cues, we want to study chemical cues from both predatory fish and predatory invertebrates, and if they trigger different behavioural responses in G. pulex from fish and fishless ponds, respectively. Also, to quantify if these responses differed between sexes, we looked at males and females separately. Furthermore, we examined if there were differences in mortality rate between the two predator regimes and sexes. Since male and female G. pulex differ in both size and behaviour, we also measured decomposition rate of leaves, their main food source. Pair-formation might increase their vulnerability to predators, therefore, we also measured pair-formation frequency in G. pulex from different predator regimes.
Materials and method
Experimental animals
Gammarus pulex individuals were collected by handnet from three fishless ponds and three fish ponds near Lund, southern Sweden. Both the fish ponds and the fishless ponds were situated in an agricultural region and each ranged in size from 0.5–1 ha. All ponds were surveyed for the presence of fish using both net and electro-fishing. Crucian carp Carassius carassius (L.) were the most abundant fish species in the fish ponds. Due to low population densities in some ponds, we had to difficulties in finding enough experimental animals and were forced to pool individuals from fish and fishless ponds, respectively. Further, the focus of this study was to investigate differences due to predator regime, not differences among populations per se. The three populations from fish ponds were kept together in one aerated 15-l aquaria, whereas the three populations from fishless ponds were kept in another aerated 15-l aquaria. G. pulex were fed rinsed alder leafs (Alnus glutinosa) and frozen chironomids. The sex of G. pulex from fish/fishless ponds were determined according to Goedmakers (1981). Since both paired and unpaired individuals where collected, pairs where placed on paper for a few seconds, this is enough for the males to release the females. For each pond type, ~350 individuals of each sex were kept in separate 15-l aquaria for at least 3 weeks prior to the start of the experiment. Total length (mean ± SD) of the G. pulex used was for males 15.0 ± 1 mm and females 14.4 ± 2 mm.
A mixture of predatory invertebrates (Notonecta glauca, Aeshna sp., Agabus sp., and Acilius sulcatus, in total 30 individuals) were collected by handnet in the fishless ponds and kept in an aerated 15-l “invertebrate predator holding aquarium” and fed frozen chironomids and G. pulex. Crucian carp (20 individuals, mean length: 96 ± 4 mm) were collected by trap-netting in a pond in the University Park, Lund. Crucian carp were kept in an aerated 200-l “fish holding aquarium” and fed frozen chironomids and G. pulex. The day:night regime was set to 14:10 h and the water temperature was kept at 18 ± 1°C. All experimental animals were allowed to acclimatize to laboratory conditions for 3 weeks prior to the start of the experiment.
Cue preparation
The predator cues were prepared 3 days prior to the experiment by adding either 10 invertebrate predators (Notonecta glauca n = 4, Aeshna sp. n = 2, Agabus sp. n = 2, and Acilius sulcatus n = 2) or 3 crucian carps to an aerated 15-l “invertebrate cue aquarium” and “fish cue aquarium”, respectively. Both the invertebrates and the crucian carp were fed 10 G. pulex three times/week in their cue aquaria throughout the experiment. Concentrations of predator cues from cue aquaria, were strong enough to stimulate predator induced behaviours (Ahlgren, personal observations). Control water was prepared in the same way but without animals. Both the invertebrate predators and the crucian carp were exchanged once a week with new individuals from the invertebrate predator and the fish holding aquarium, respectively. The amount of water used as a cue was continuously replaced with the same amount of tap water.
Experimental setup
We set up 60 aerated 2-l experimental aquaria (16 × 14 × 9 cm, l × w × h) with 3 ± 0.1 g of rinsed and dried alder leafs (as food source and refuge) in each. G. pulex were divided into treatment groups according to pond predator regime (fish- and fishless ponds), sex (male and female) and treatment (control, invertebrate- or fish cue) all replicated five times (Fig. 1). Twelve G. pulex were added to each of the 60 aerated 2-l experimental aquaria according to the categories above. Cue (0.05-l; either control, invertebrate- or fish cue) was added to the experimental aquaria in the morning 5 days a week, plus another three randomly chosen days in the afternoon to stimulate predator induced behaviours. The day:night regime was set to 14:10 h, the water temperature was kept at 18 ± 1°C and the experiment was run for 31 days. One replicate (female from fishless ponds, invertebrate cue) was removed due to technical problems. Three times a week the number of dead G. pulex was counted, removed and replaced with new individuals in all the experimental aquaria.
Refuge use
To determine if G. pulex refuge use was influenced by pond predator regime, sex and/or predatory cues, we quantified the number of G. pulex outside of the leaves 20 min after the addition of the cue. Since a few G. pulex were missing at the end (not replaced) of the experiment a mean number of individuals for each aquarium were calculated. The total number of individuals under the leaves (refuge) was divided by the mean number of individuals for each aquarium to get the proportion of G. pulex using the refuge. A three-way ANOVA (pond predator regime, sex and treatment) was used to analyse differences in refuge use.
Mortality
To assess if G. pulex pond predator regime, sex and exposure to predator cue had an effect on mortality, we counted the number of dead G. pulex three times a week in all the experimental aquaria. A three-way ANOVA was used to analyse the results (pond predator regime, sex and treatment).
Decomposition
We also wanted to determine if there were any treatment effects on decomposition rate. When terminating the experiment after 31 days, the remaining leaves in each aquarium were collected and dried at 60°C for 48 h and weighed. The remaining leaf mass was then subtracted from the initial leaf mass to get the total leaf consumption. To calculate the decomposition rate/individual we used the mean number of individuals for each aquarium. Differences in pond predator regime (fish pond and fishless pond), sex (male and female) and treatment (control, invertebrate- or fish cue) on decomposition rate were analysed by a three-way ANOVA.
Pair-formation
Six males and six females from either fish or fishless ponds were placed in thirty 2-l experimental aquaria (16 × 14 × 9 cm, l × w × h) with 3 ± 0.1 g of rinsed and dried alder leaves in each. We had three treatments (control, invertebrate- or fish cue) all replicated five times. Three days a week the number of individuals in pairs were counted. The mean number of pairs/possible number of pairs, were calculated using the mean number of males and females/aquarium. Differences in the proportion of pairs was analysed with a two-way ANOVA for effect of pond predator regime (fish pond and fishless pond) and treatment (control, invertebrate- or fish cue). Following the procedure described above differences in refuge use was tested with a two-way ANOVA.
Statistical methodology
When necessary, data were arcsine transformed to meet the assumptions of normality and equal variance. Non-significant interactions were removed from the analyses. Tukey’s HSD test was used to assess differences among treatments following a significant ANOVA. All statistics were carried out using PASW Statistics 18 for Mac OS X.
Results
Refuge use
Individuals from fish ponds had a significantly greater number of individuals in the refuge than those from fishless ponds (three-way ANOVA: Table 1; Fig. 2). Sex and treatment had no significant effect upon refuge use (Table 1).
Mortality
Gammarus pulex from fish ponds had a significantly higher mortality compared to G. pulex from fishless ponds (three-way ANOVA; Table 2; Fig. 3) and male G. pulex from both fish and fishless ponds had higher mortality compared to females (three-way ANOVA; Table 2). There was no effect of predator cue exposure on mortality (Table 2). There was no significant interaction between pond predator regime and sex (Table 2), however, there was a trend suggesting that males from fish ponds having the highest mortality.
Leaf decomposition rates
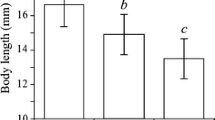
There was no difference in total decomposition rates between fish and fishless ponds (three-way ANOVA: Table 3). Also, decomposition rate did not differ between treatments (control, invertebrate- or fish cue) (three-way ANOVA, Table 3), but there was a significant effect of sex, with males having a higher decomposition rate than females (three-way ANOVA; Table 3, Fig. 4).
Pair-formation experiments
Individuals from fishless ponds formed pairs more frequently compared to individuals from fish ponds (two-way ANOVA; Table 4; Fig. 5). Also, there was a significant effect of treatment on pair-formation (two-way ANOVA; Table 4; Fig. 5). Further, a Tukey HSD test revealed that it was the fish cue that decreased the number of pairs compared to control in G. pulex from fish ponds (P = 0.043) Table 5.
Discussion
Many taxa that spend their whole lifecycle in isolated ponds and lakes have a low dispersal rate (Bilton et al., 2001), which results in a low gene flow between populations. Low gene flow may lead to the evolution of local adaption in, e.g. predator defences to local predator regimes (Relyea, 2002; Åbjörnsson et al., 2004, 2009). Our study shows differences in refuge use, mortality rate and pair-formation between individuals from ponds with invertebrate versus fish predators. Individuals from ponds with non gape-limited fish predators used refuges to a higher extent, had higher mortality and formed fewer pairs than individuals from fishless ponds. However, there was no difference in leaf decomposition rate between individuals from the different predator regimes, as leaves may function as both food and refuge. Decomposition and mortality rates differed between sexes within predator regimes, with males having higher mortality rate and a higher decomposition rate per individual than females. Further, the differences in refuge use, mortality and pair-formation were found even in the absence of predator cues, indicating differences between predator regimes in individual’s baseline behaviour. Thus, it appears that these differences are primarily driven by predator regime level differences in trait values rather than plastic responses to predator cues. Below, we discuss refuge use, mortality, leaf decomposition rates and pair-formation one by one before merging these topics in a general discussion.
Refuge use
Gammarus pulex from the two predator regimes differed in their level of refuge use, with individuals from fish ponds being less willing to leave the refuge than individuals from fishless ponds, independent of the presence of predatory cues. This was found in both the experiment where males and females where kept separated and in the pair-formation experiment where males and females where kept together. This indicates that there are general differences in the baseline behaviour between G. pulex from the two predator regimes. When adding fish cues, Åbjörnsson et al. (2004) found short term (first 10 min) effects on the refuge use of fish pond individuals. However, we found no effect of predator cues on refuge use when measuring this behaviour 20 min after the addition of the cue. This indicates that G. pulex quickly return to their baseline behaviour most likely to minimise the costs of this predator avoidance behaviour (lost opportunity costs), e.g. due to increased competition within a refuge (e.g. Anholt & Werner, 1998).
The response to predators may also differ between sexes within a species due to, for example, size dependent differences in vulnerability (Krupa & Sih, 1998; Cothran, 2004), but in this study we found no difference in refuge use between male and female G. pulex. However, such behavioural responses may only occur and/or be detectable at a lower time resolution and thus, further studies should include investigation of rapid behavioural responses in males and females when exposed to predator cues.
Mortality
Behavioural responses to predator cues may indirectly affect prey growth rates due to reduced foraging efficiency and decreased access to high density food patches (Petranka, 1989, and references therein) and in the long term there may also be a negative effect on prey survival rates. We found that even in the absence of predator cues G. pulex from fish ponds suffered from higher mortality rates than fishless pond individuals; a potential explanation for this might be that G. pulex from fish ponds suffer higher costs of their local adaptations (Åbjörnsson et al., 2004) than G. pulex from fishless ponds. Further, G. pulex from a habitat with predatory fish have been found to aggregate and stay close to conspecifics when exposed to fish cue, a behaviour that may increase competition for food (Kullmann et al., 2008) and hence affect growth and survival. These aggregations may also result into more aggressive interactions and hence, lead to more injuries and cannibalism (Plaistow et al., 2003). Further, the results from this study suggest that aggregation among fish pond individuals is something that goes on even in the absence of predatory cues. Hence the potential negative effects of this behaviour may be stronger for individuals from fish ponds compared to individuals from fishless ponds.
In both fish and fishless ponds females had lower mortality rate than males. Since there was no direct predation or any effect of predator cues on mortality it seems to be a general difference between sexes affecting mortality. This pattern is found in a broad range of taxa, and might be due to a “live fast, die young” life history strategy among males (Bonduriansky et al., 2008). Further, both sexes have been found to be cannibalistic, however, when females are close to the stage when their own offspring hatch they decrease their cannibalistic behaviour in order to avoid consuming their own offspring (Lewis et al., 2010). Males may also be more aggressive than females due to mate searching (Krupa & Sih, 1998) and mate guarding behaviour (Dunn et al., 2008). In our experiment, males were kept separate from females, potentially leading to a higher frequency of aggressive male-male interactions, which may have adversely affected male mortality, due to cannibalism and increased injuries from interference with other males (Plaistow et al., 2003). Further, since males may be more active than females their encounter rates with conspecifics, but also with predators, increases (Krupa & Sih, 1998; Peeters et al., 2009). Increased encounter rate may lead to increased risk of being eaten or injured by conspecifics. Also males are bigger than females and are thus more easily detected by visually hunting predators (Mcintosh and Peckarsky, 1999). Hence, males may be under greater stress than females, which may have a negative effect on their survival.
Leaf decomposition rates
Many prey species decrease their foraging activity when exposed to high predation risk (Abrams & Rowe, 1996; Benard, 2004). However, the difference in refuge use between G. pulex from fish and fishless ponds had no effect on decomposition rate, which supports the idea that G. pulex use leaves both as a refuge and as a food source (Åbjörnsson et al., 2000). Hence, G. pulex from fish ponds may forage on leaves at the same rate as those from fishless ponds independent of predatory fish presence. In the light of foraging rates, this strategy seems to be an optimal predator defence, as there then is no trade-off between foraging and refuge use.
We found that males shred leaves at a higher rate than females. This could be explained by body size differences between sexes, with bigger individuals (males) feeding more than small individuals (females). Also, there may be differences in metabolic rates between males and females (Fox et al., 2003), due to the “live fast, die young” life history strategy among males (Bonduriansky et al., 2008). This points out the importance of considering male–female differences when studying foraging rate.
Pair formation
Local adaptation in prey species is highly influenced by the main predator type in the habitat (McPeek, 1990; Wellborn et al., 1996). In our fish ponds crucian carp, which do not suffer from gape limitation when feeding on G. pulex, is the main predator, whereas gape-limited invertebrate predators dominate in fishless ponds. Hence, in fish ponds G. pulex should experience an increased risk of predation when they form pairs due to larger size and reduced mobility, whereas the opposite pattern should occur in fishless ponds (Ward, 1986, Cothran, 2004). As with refuge use and mortality rate, there seems to be a general difference in pair-formation between fish and fishless pond individuals. Individuals from fish ponds formed fewer pairs compared to individuals from fishless ponds. This is in line with a previous study, showing that individuals from fish ponds have a different life history strategy, with being bigger in size, having lower mating success but producing more offspring than individuals from fishless ponds (Åbjörnsson et al., 2009). Further, both male and female Gammarus duebeni have been found to decrease their activity when exposed to predator cues from fish, which was argued to decrease encounter rates between sexes and hence decreases pair-formation (Dunn et al., 2008). Further, males left females faster in the presence of fish cues due to a trade-off between mate guarding and predator avoidance (Dunn et al., 2008). We found the same pattern in our study, with a reduction of the number of pairs in G. pulex from fish ponds when exposed to fish cue compared to the control. On the other hand, there was no effect of invertebrate predatory cues on pair-formation in G. pulex from fishless ponds, probably because pairs are bigger then singular G. pulex and this should decrease the predation pressure from gape-size-limited invertebrate predators (Cothran, 2004; Åbjörnsson et al., 2009). The reason that there is an effect of predator cues in pair-formation even after 20 min, but not in the other traits, is probably due to finding a partner being a longer/slower process than other behaviours assayed.
Even though the shorter duration of pair-formation in G. pulex from fish ponds may release them from some of the costs of pair-formation (Ward, 1986; Cothran, 2004) they will also miss the benefits of pair-formation. For example, at high male:female ratios males have mating advantages through mate-guarding (Parker, 1974; Härdling et al., 2004), whereas females may gain from pair-formation by shortening their intermoult duration (Galipaud et al., 2010).
General discussion
Theoretical models suggest that plasticity is favoured in heterogeneous or fluctuating environments whereas in stable environments there will be a loss of plasticity and genetic assimilation of traits, i.e. due to directional selection traits will become genetically determined and canalized (Van Tienderen, 1991, Sultan & Spencer, 2002). G. pulex that live in isolated ponds should experience low gene flow from other populations, due to low dispersal rates. Hence, this may lead to that life history strategies and behavioural responses to predators become genetically fixed. And indeed, the differences in refuge use, mortality and pair-formation suggest that G. pulex have become locally adapted to their prevailing predator regime. Also, these local adaptations are not exclusive for a specific pond, since we found this pattern even after pooling three populations of the same predator regime. Hence, selection for these adaptations should have occurred in parallel in the different ponds.
These adaptations may come with a cost, since G. pulex have to trade-off fitness related traits against predator threat. Even though the fish pond individuals need to spend more time in a refuge, there seems to be no trade-off between foraging rate and refuge use, indicating that this is an optimal refuge that provides both food and cover. However, spending more time aggregated in refuge increases the aggressive interactions with conspecifics, which increase morality rates for fish pond individuals (Plaistow et al., 2003; Kullmann et al., 2008). On the other hand, in fishless ponds, G. pulex aggregate and hide to a lesser extent and hence do not pay the costs associated with these behaviours. Further, since forming pairs increases the risk of predation from fish, G. pulex from fish ponds form fewer pairs and hence, they miss the benefits, but also the costs of pair-formation. Also, both sexes have preference for bigger partners, and whilst males may ignore small females, females can resist copulation attempts from small males leading to a selection for bigger size (Ward, 1984; Elwood et al., 1987). At the same time size selective predation selects for smaller individuals leading to a conflict between the optimum size for mating and predation risk in fish pond individuals. However, according to Åbjörnsson et al. (2009), fish pond individuals are actually bigger then individuals from fishless ponds. It seems as fish pond individuals allocate resources towards growth and one or few breeding occasions (Åbjörnsson et al., 2009), since they are more vulnerable to predation when in pair then unpaired (Ward, 1986; Cothran, 2004). Further, this strategy increases their risk of predation from positive size selective predators, but it may also reduce their life time fecundity compared to G. pulex from fishless ponds (Åbjörnsson et al., 2009). If so, fish pond G. pulex might pay a high cost for their adaptations to the local predator regime.
In summary, we found general differences in baseline behaviour, predator avoidance and mortality rate both between predator regimes and sexes in G. pulex. Based on our results, we suggest that fish pond individuals have to make a trade-off between predator avoidance and fitness-related behaviour (e.g. pair-formation) to a higher extent than fishless pond individuals. Also, males seem to have a “live fast, die young” life history strategy, which lowers their life span compared to females. In addition, we suggest that these differences between individuals originating from fishless systems, where invertebrates are the dominant predators, and systems with predatory fish are primarily driven by predator regime level differences in trait values rather than plastic responses to predator cues.
References
Åbjörnsson, K., J. Dahl, P. Nyström & C. Brönmark, 2000. Influence of predator and dietary chemical cues on the behaviour and shredding efficiency of Gammarus pulex. Aquatic Ecology 34: 379–387.
Åbjörnsson, K., L.-A. Hansson & C. Brönmark, 2004. Responses of prey from habitats with different predator regimes: local adaptation and heritability. Ecology 85: 1859–1866.
Åbjörnsson, K., L.-A. Hansson & C. Brönmark, 2009. The influence of predator regime on reproductive traits in Gammarus pulex populations. Hydrobiologia 635: 215–225.
Abrams, P. A. & L. Rowe, 1996. The effects of predation on the age and size of maturity of prey. Evolution 50: 1052–1061.
Anholt, B. R. & E. E. Werner, 1998. Predictable changes in predation mortality as a consequence of changes in food availability and predation risk. Evolutionary Ecology 12: 729–738.
Benard, M. F., 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annual Review of Ecology Evolution and Systematics 35: 651–673.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Bollache, L., N. Kaldonski, J.-P. Troussard, C. Lagrue & T. Rigaud, 2006. Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Animal Behaviour 72: 627–633.
Bonduriansky, R., A. Maklakov, F. Zajitschek & R. Brooks, 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Functional Ecology 22: 443–453.
Brönmark, C. & L. A. Hansson, 2000. Chemical communication in aquatic systems: an introduction. Oikos 88: 103–109.
Carpenter, S. R. & J. F. Kitchell, 1993. Titel. Cambridge University Press, Cambridge.
Cooper, S. D., S. J. Walde & B. L. Peckarsky, 1990. Prey exchange rates and the impact of predators on prey populations in streams. Ecology 71: 1503–1514.
Cothran, R. D., 2004. Precopulatory mate guarding affects predation risk in two freshwater amphipod species. Animal Behaviour 68: 1133–1138.
Dick, J. T. A., 1995. The cannibalistic behaviour of two Gammarus species (Crustacea: Amphipoda). Journal of Zoology (London) 236: 697–706.
Douglas, P. L., G. E. Forrester & S. D. Cooper, 1994. Effects of trout on the diel periodicity of drifting in baetid mayflies. Oecologia (Berlin) 98: 48–56.
Dunn, A. M., J. T. A. Dick & M. J. Hatcher, 2008. The less amorous Gammarus: predation risk affects mating decisions in Gammarus duebeni (Amphipoda). Animal Behaviour 76: 1289–1295.
Elwood, R., J. Gibson & S. Neil, 1987. The amorous Gammarus: size assortative mating in G. pulex. Animal Behaviour 35: 1–6.
Felten, V., G. Tixier, F. Guerold, V. D. C. De Billy & O. Dangles, 2008. Quantification of diet variability in a stream amphipod: implications for ecosystem functioning. Fundamental and Applied Limnology 170: 303–313.
Fox, C. W., L. Dublin & S. J. Pollitt, 2003. Gender differences in lifespan and mortality rates in two seed beetle species. Functional Ecology 17: 619–626.
Galipaud, M., F. Dechaume-Moncharmont, A. Oughadou & L. Bollache, 2010. Does foreplay matter? Gammarus pulex females may benefit from long-lasting precopulatory mate guarding. Biology Letters. doi:10.1098/rsbl.2010.0924
Goedmakers, A., 1981. Population dynamics of three Gammarid species (Crustacea, Amphipoda) in a French chalk stream, Part II Standing crop. Bijdragen tot de Dierkunde 51: 31–69.
Härdling, R., H. Kokko & R. W. Elwood, 2004. Priority versus brute force: when should males begin guarding resources? American Naturalist 163: 240–252.
Holomuzki, J. R. & L. A. Hatchett, 1994. Predator avoidance costs and habituation to fish chemicals by a stream isopod. Freshwater Biology 32: 585–592.
Holomuzki, J. R. & T. M. Short, 1988. Habitat use and fish avoidance behaviors by the stream-dwelling isopod Lirceus fontinallis. Oikos 52: 79–86.
Kaldonski, N., C. Lagrue, S. Motreuil, T. Rigaud & L. Bollache, 2008. Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften 95: 839–844.
Krupa, J. J. & A. Sih, 1998. Fishing spiders, green sunfish, and a stream-dwelling water strider: male-female conflict and prey responses to single versus multiple predator environments. Oecologia (Berlin) 117: 258–265.
Kullmann, H., T. Thunken, S. A. Baldauf, T. C. M. Bakker & J. G. Frommen, 2008. Fish odour triggers conspecific attraction behaviour in an aquatic invertebrate. Biology Letters 4: 458–460.
Lewis, S. E., J. T. A. Dick, E. K. Lagerstrom & H. C. Clarke, 2010. Avoidance of filial cannibalism in the Amphipod Gammarus pulex. Ethology 116: 138–146.
Lima, S. L. & L. M. Dill, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68: 619–640.
Mccarthy, T. M. & W. A. Fisher, 2000. Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Freshwater Biology 44: 387–397.
McGrath, K. E., E. T. H. M. Peeters, T. H. M. Edwin, J. A. J. Beijer & M. Scheffer, 2007. Habitat-mediated cannibalism and microhabitat restriction in the stream invertebrate Gammarus pulex. Hydrobiologia 589: 155–164.
Mcintosh, A. R. & B. L. Peckarsky, 1999. Criteria determining behavioural responses to multiple predators by a stream mayfly. Oikos 85: 554–564.
Mcintosh, A. R. & C. R. Townsend, 1996. Interactions between fish, grazing invertebrates and algae in a New Zealand stream: a trophic cascade mediated by fish-induced changes to grazer behaviour? Oecologia (Berlin) 108: 174–181.
Mcpeek, M. A., 1990. Behavioral differences between enallagma species odonata influencing differential vulnerability to predators. Ecology (Washington DC) 71: 1714–1726.
Nyström, P. & K. Åbjörnsson, 2000. Effects of fish chemical cues on the interactions between tadpoles and crayfish. Oikos 88: 181–190.
Nyström, P., O. Svensson, B. Lardner, C. Brönmark & W. Graneli, 2001. The influence of multiple introduced predators on a littoral pond community. Ecology 82: 1023–1039.
Obernborfer, R. Y., J. V. Mcarthur, J. R. Barnes & J. Dixon, 1984. The effect of invertebrate predators on leaf litter processing in an alpine stream. Ecology 65: 1325–1331.
Parker, G. A., 1974. Courtship persistence and female guarding as male time investment strategies. Behaviour 48: 157–184.
Peacor, S. D. & E. E. Werner, 1997. Trait-mediated indirect interactions in a simple aquatic food web. Ecology 78: 1146–1156.
Peckarsky, B. L. & A. R. Mcintosh, 1998. Fitness and community consequences of avoiding multiple predators. Oecologia (Berlin) 113: 565–576.
Peeters, E. T. H. M., H. J. De Lange & M. Lurling, 2009. Variation in the behavior of the amphipod Gammarus pulex. Human and Ecological Risk Assessment 15: 41–52.
Petranka, J. W., 1989. Response of toad tadpoles to conflicting chemical stimuli: predator avoidance versus “optimal” foraging. Herpetologica 45: 283–292.
Plaistow, S. J., L. Bollache & F. Cezilly, 2003. Energetically costly precopulatory mate guarding in the amphipod Gammarus pulex: causes and consequences. Animal Behaviour 65: 683–691.
Relyea, R. A., 2002. Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecological Monographs 72: 77–93.
Robinson, B. W. & R. W. Doyle, 1985. Trade-off between male reproduction amplexus and growth in the amphipod Gammarus-lawrencianus. Biological Bulletin (Woods Hole) 168: 484–488.
Sih, A. & E. D. Wooster, 1994. Prey behavior, prey dispersal, and predator impacts on stream prey. Ecology 75: 1199–1207.
Sih, A., G. Englund & D. Wooster, 1998. Emergent impacts of multiple predators on prey. Trends in Ecology and Evolution 13: 350–355.
Sultan, S. E. & H. G. Spencer, 2002. Metapopulation structure favors plasticity over local adaptation. American Naturalist 160: 271–283.
Turner, A. M., 1997. Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behavioral Ecology 8: 120–125.
Turner, A. M., S. A. Fetterolf & R. J. Bernot, 1999. Predator identity and consumer behavior: differential effects of fish and crayfish on the habitat use of a freshwater snail. Oecologia (Berlin) 118: 242–247.
Van Tienderen, P. H., 1991. Evolution of generalists and specialists in spatially heterogeneous enviroments. Evolution 45: 1317–1331.
Ward, P. I., 1984. The effects of size on the mating decisions of Gammarus pulex (Crustacea, Amphipoda). Zeitschrift für Tierpsychologie 64: 174–184.
Ward, P. I., 1986. A comparative field study of the breeding behaviour of a stream and a pond population of Gammarus pulex (amphipoda). Oikos 46: 29–36.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363.
Williams, D. D. & K. A. Moore, 1985. The role of semiochemicals in benthic community relationships of the lotic amphipod Gammarus pseudolimnaeus: a laboratory analysis. Oikos 44: 280–286.
Wisenden, B. D., A. Cline & T. C. Sparkes, 1999. Survival benefit to antipredator behavior in the amphipod Gammarus minus (Crustacea: Amphipoda) in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology 105: 407–414.
Wisenden, B. D., S. G. Pohlman & E. E. Watkin, 2001. Avoidance of conspecific injury-released chemical cues by free-ranging Gammarus lacustris (Crustacea: Amphipoda). Journal of Chemical Ecology 27: 1249–1258.
Acknowledgements
We thank Ben Chapman and two anonymous referees for insightful comments on the manuscript and for improving the language, and Anders Nilsson for discussing statistical aspects. This research was supported by Formas (to K.Å.) and by The Swedish Research Council (to C.B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Rights and permissions
About this article
Cite this article
Ahlgren, J., Åbjörnsson, K. & Brönmark, C. The influence of predator regime on the behaviour and mortality of a freshwater amphipod, Gammarus pulex . Hydrobiologia 671, 39–49 (2011). https://doi.org/10.1007/s10750-011-0702-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0702-8