Abstract
Round Goby Neogobius melanostomus invasion of the Grand River (Ontario, Canada) presents an opportunity to assess the role of abiotic gradients in mediating the establishment and impact of nonnative benthic fishes in rivers. In this system, sequential low-head dams delineate uninvaded and invaded river reaches and create upstream gradients of increasing water velocity. We hypothesized that flow refugia created by impounded reservoirs above low-head dams enhance local Round Goby abundance. Round Goby influence on the native fish community was determined by variance partitioning, and we used generalized additive models to identify small-bodied benthic fish species most likely to be impacted by Round Goby invasion. Round Goby abundance declined as the degree of reservoir effect decreased upstream. The distributions of four species (including the endangered Eastern Sand Darter Ammocrypta pellucida) in invaded reaches were best explained by inclusion of both reservoir-associated abiotic variables and Round Goby abundance as model terms. To determine establishment potential of the uninvaded reach immediately upstream, four environmental habitat characteristics were used in discriminant function analysis (DFA) to predict three potential outcomes of introduction: non-invaded and either lower or higher Round Goby abundance (low and high invasion status, respectively) than the median number of Round Goby at invaded sites. Our DFA function correctly classified non-invaded and high-abundance invasion status sites > 85% of the time, with lower (73%) success in classifying low-abundance invasion status sites, and the spatial pattern of our results suggest that likelihood of establishment is greatest in impounded habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 180 aquatic nonindigenous species are established in the Laurentian Great Lakes basin (GLB), and some of these species have caused substantial ecological and economic impacts (Mills et al. 1993; Ricciardi 2006). The GLB is a model system for studying how introduced species invade new environments, yet most attention has focused on the establishment of invaders in the lakes themselves, rather than their tributaries. Physical attributes of tributaries (e.g., water velocity, temperature) may initially inhibit establishment by aquatic invasive species, such as dreissenid (zebra and quagga) mussels and Eurasian Ruffe Gymnocephalus cernua, which have been slow to colonize flowing waters (Bauer et al. 2007). Round Goby Neogobius melanostomus, a small non-indigenous benthic fish of Eurasian origin, has recently expanded its range into tributaries after colonizing all five Great Lakes (Bronnenhuber et al. 2011; Poos et al. 2010) and was the fastest spreading vertebrate in the GLB in the years immediately following its introduction (Dopazo et al. 2008). Round Goby has become the most abundant benthic fish in some nearshore Great Lakes habitats (Kornis et al. 2012) and has been linked to local declines or extirpations of native fishes, including Logperch Percina caprodes and Mottled Sculpin Cottus bairdii (Balshine et al. 2005; Janssen and Jude 2001; Leino and Mensinger 2016). Documented impacts of Round Goby on native fish communities in tributaries appear not to have been as severe as in the Great Lakes proper (Kornis et al. 2013; Poos et al. 2010), but a broader suite of species may be affected. Fish communities in southwestern Ontario tributaries are the most speciose in Canada, with a number of nationally listed at-risk fishes confined to small ranges within this region (Staton and Mandrak 2005). For example, Northern Madtom Noturus stigmosus and Eastern Sand Darter Ammocrypta pellucida are two at-risk native benthic fishes found in tributaries colonized by Round Goby (Poos et al. 2010), and the conservation implications of these invasions is uncertain.
Tributaries in the GLB have frequently been hydrologically altered for navigation, flow management, hydroelectricity, and preventing the spawning migration of invasive Sea Lamprey Petromyzon marinus (Harford and McLaughlin 2007; Noakes et al. 2000; Porto et al. 1999). The majority of these dams are low-head barriers (< 4 m in height), which have less pronounced fragmentation, hydrologic, and water chemistry effects than large dams (Cumming 2004; Nilsson et al. 2005). Nonetheless, low-head dams restrict fish movement (Noakes et al. 2000; Porto et al. 1999) and share similarities with large dams by affecting river processes and biota at local and landscape scales (Dodd et al. 2003; Harford and McLaughlin 2007; Santucci et al. 2005). Reservoirs can facilitate invasions within a watershed by acting as ‘stepping-stones’ for spread and by supporting increased propagule pressure, altering hydrologic conditions, and altering native assemblages (Havel et al. 2005; Johnson et al. 2008). For example, low-head dams are believed to enhance downstream recruitment of dreissenid mussels in an inland river-reservoir system, by supporting high mussel abundance in the lentic sections above dams (Smith et al. 2015). On some GLB tributaries, low-head dams may impede Round Goby range expansion (Poos et al. 2010). Yet, on the Grand River in southern Ontario, Round Goby has been detected above dams that are naturally impassable to them, suggesting human-facilitated transport in sufficient numbers to overcome demographic barriers to establishment (Bronnenhuber et al. 2011). The slower water velocity of the impounded reservoir immediately upstream of the dam may provide a flow refuge for Round Goby, which cannot hold a fixed position in water velocities above 0.35 m s−1 and cannot sustain swimming speeds over 1.25 m s−1 (Tierney et al. 2011). Therefore, high water velocities could form an effective barrier to the upstream movement of Round Goby in hydrologically unmodified tributaries and in areas beyond the reservoir on modified tributaries. Reservoir sections affect more than just water velocity, with consequences for substrate size and embeddedness, channel width, and depth (Alexandre and Almeida 2010; Gillette et al. 2005; Santucci et al. 2005). We describe this suite of highly collinear variables acting in concert as the ‘reservoir effect’.
Compared to unmodified rivers, fish communities in dammed rivers may be more vulnerable to the effects of fish invasions, and there is considerable evidence that the reservoir effect tends to favour invaders (Alexandre and Almeida 2010; Falke and Gido 2006; Moyle and Marchetti 2006). The majority of research examining Round Goby invasion of GLB tributaries has not extended upstream of the first barrier (e.g., Abbett et al. 2013; Campbell and Tiegs 2012; Kornis et al. 2013; Krakowiak and Pennuto 2008) and has typically focused on relatively slow-moving stream reaches near lake inlets (Pennuto et al. 2010). Round Goby behaviour in slow-moving and reservoir habitat likely plays a role in the decline of native benthic fishes; aggression and competitive superiority has been observed experimentally where food and shelter are limiting (Balshine et al. 2005; Dubs and Corkum 1996; Janssen and Jude 2001; Kornis et al. 2012). Presence of Round Goby at low densities was experimentally shown to negatively affect the growth of a native darter in a slow-moving GLB tributary (average water velocity < 10 m s−1), which was attributed to interspecific competition (Kornis et al. 2014); however, the trend was not evident at high invader abundance, potentially a result of complex inter- and intraspecific interactions that were responsible for increased mortality. Where access to free-flowing riverine habitat is limited by impoundment and the creation of a reservoir effect, Round Goby are the likely winners of competition; when the reservoir effect is minimal, lotic-adapted species are likely to persist.
We surveyed the Grand River as a model system to examine the role of abiotic gradients in mediating the colonization and impact of Round Goby. We hypothesized that impoundments above low-head dams create a reservoir effect that promote establishment of Round Goby, while negatively affecting the abundance of native small-bodied benthic fishes. Further, we predicted that Round Goby itself would negatively affect abundance of native benthic fishes where these species co-occur. Finally, we used invaded reaches to predict establishment risk to an uninvaded reach if Round Goby are introduced, a plausible scenario given strong angling activity in the region (Drake and Mandrak 2014).
Methods
Study area
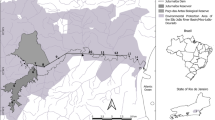
The Grand River (43°08′N, 80°17′W) is a species-rich tributary of Lake Erie and a species-at-risk hotspot (Poos et al. 2010). It is highly fragmented, with over 130 dams throughout the ~ 6500 km2 watershed (Reid et al. 2008). Three reaches of the middle and lower Grand River, bounded at their downstream and upstream limits by dams, were sampled between 2010 and 2014 (Fig. 1). Moving downstream in the watershed, the three reaches measure 15 km (reach 1; R01), 43 km (reach 2; R02), and 53 km (reach 3; R03), respectively. Stream gradient is highest in R01 (1.07 m km−1), followed by R02 (0.33 m km−1), and R03 (0.19 m km−1; Google Earth 2017). Low-head (< 4 m) dams have caused a detectable reservoir: an upstream impoundment of water with slower velocity and greater depth than the historical riffle-pool morphology of the river (Dextrase 2013). The Dunnville Dam, at Dunnville, Ontario, separates R03 from Lake Erie and is the first barrier to fish movement on the Grand River (Fig. 1). In all reaches the reservoir extends upstream of each dam until a transition to the riffle-pool morphology that we refer to as mainstem habitat. Round Goby is present in Lake Erie and has been present above lowhead dams in R02 and R03 since at least 2007 (Poos et al. 2010), likely introduced through human-mediated transfer (Bronnenhuber et al. 2011). Initial site selection was done with a stratified-random approach along each reach, provided that each potential site met the minimum depth criteria for the sampling gear (Fig. 1). Reaches R02 and R03 were sampled over 3 years to examine temporal changes in the invasion front, and R01 (upstream of the Round Goby invasion) was sampled once in 2014. R02 and R03 sites were established in 2010 and were revisited in 2011 and 2013; however, this was done with low precision as a result of inter-annual changes to trawling hazards (e.g., sunken timber) with up to 400 m separating sampling sites.
Location of the study area within the lower Grand River watershed, sampled from 2010 to 2014. Detail view: Study sites (circles) on three river reaches (R01–R03). Dams are indicated by numbered arrows: 1. Dunnville Dam, Dunnville, Ontario; 2. Caledonia Dam, Caledonia, Ontario; 3. Wilkes Dam, Paris, Ontario; 4. Paris Dam, Paris, Ontario
Habitat sampling
To delineate sites a linear transect of 100 m was marked, and global positioning system (GPS) coordinates of the upstream (top) and downstream (bottom) points of the fish sampling transect were taken. Transects were established through relatively homogeneous habitat, confirmed visually at the surface and using bottom scanning sonar. Stream depth and water velocity were measured at the top, midpoint, and bottom of 100 m transects, and a single mean value was computed for each transect. Depth was measured by a Humminbird sidescan unit and velocity was measured using a Swoffer Model 2100 current velocity meter held at 1 m depth, or when total depth was < 2 m, at total depth/2. Sediment composition was visually estimated as percent contribution of 11 dominant substrate types from a single Ponar grab sample at the midpoint of the transect and average sediment size was calculated by converting percentage values to the Phi scale (Inman 1952), which provides a measure of sediment fineness. Stream width was determined to the nearest meter using a laser rangefinder in the field or satellite imagery in Google Earth Pro v. 7.1.5. Distance of sites upstream from the nearest downstream dam was calculated in the GIS program GRASS (GRASS Development Team 2012).
Fish sampling
Benthic trawling was used to sample the fish community during a one- to two-week period, between June and August of 2010, 2011, 2013, and 2014. On arrival at each site transects were trawled in rapid succession by three passes of an Innovative Net Systems 2.44 m mini-Missouri trawl with 3.18 mm mesh. The mini-Missouri trawl targets benthic fishes (e.g., darter spp.) within 50 cm of the substrate and is useful in rivers where clarity, depth, and velocity exclude other techniques (Herzog et al. 2009). All captured fishes were enumerated, and maximum and minimum length recorded for each species. Voucher specimens were collected in the field and used to verify species identities in the lab. Fish data from the three trawl passes were pooled for each site to gain a relative measure of species abundances among sites.
Analytical approach
Analyses were focused on identifying the dominant abiotic gradients associated with low-head dams on the Grand River, relating these gradients to native benthic species and Round Goby abundance on invaded reaches and applying information from invaded reaches, to predict establishment likelihood upstream of a low-head dam in an uninvaded reach. All analyses were conducted in R (R Core Development Team 2015).
Environmental data
Nine physical and chemical variables representing the environmental conditions at study sites were selected for inclusion in analyses (Table 1). Six of the nine environmental variables had at least one missing value. Multivariate analyses can be sensitive to missing values, thus, these were imputed by multiple imputation using the missMDA function in the FactoMineR package in R (Husson et al. 2013), which uses a PCA model to impute multiple missing values in continuous data sets (Josse and Husson 2012). Habitat variables were tested for normality using the Shapiro–Wilk test statistic. Variables that did not meet normality (p < 0.01) were transformed by ln(x + 1) or, in the case of proportional data, by the Arcsin√x function. When data transformations were found to improve normality, transformed data were used in further analyses. Sediment fineness was not further normalized after its calculation as a Phi-scale value.
Strong velocity and depth gradients in the riverine environment are likely to cause collinearity of certain physical habitat variables among study sites. To address collinearity and reduce dimensionality in habitat variables, we used principal components analysis (PCA) on scaled and centered physical habitat variables. The optimal number of principal components was determined by visual examination of scree plots. PCA was conducted on pooled 2010, 2011, and 2013 data. PC scores were used in further analyses as a composite metric to describe the physical gradient caused by low-head dams on the Grand River. Water-column measurements (conductivity, temperature, dissolved oxygen, turbidity) were excluded from characterizing the reservoir effect due to the instantaneous nature of their collection during site visits, and relatively low variability among sites within a sampling year due to short residence time in shallow, well-mixed mainstem, and impounded sections.
Fish community and species distributions
Fish abundance data were highly right-skewed (Poisson distributed) and overdispersed due to an excess number of zeros. Species abundances were ln(x + 1) transformed to improve normality if included as explanatory variables in models. To determine if there were differences in the benthic fish community among reaches and years, we conducted a two-factor permutational multivariate analysis of variance (PERMANOVA, Anderson 2001) on Chord-transformed site-species matrices (Legendre and Gallagher 2001), with significance based on 9999 iterations using the Vegan package (Oksanen et al. 2011). To account for physical habitat gradients within reaches, a PCA-derived site score was included as a covariate in PERMANOVA analyses. Non-metric multidimensional scaling (NMS) was used to visualize community similarity among reaches, and abiotic variables were fitted to the NMS plot as vector overlays when significant (p < 0.01) based on 9999 iterations.
Round Goby establishment and impact
To examine native benthic species associations along environmental gradients while accounting for the influence of Round Goby invasion, we focused on the two reaches, R02 and R03, where the invasive fish is established. We first conducted transformation-based redundancy analysis (tb-RDA) in the Vegan package (Oksanen et al. 2011); the site-species matrix was transformed to Chord distance (Legendre and Gallagher 2001) and then RDA was performed on the transformed matrix, constrained by environmental variables. RDA can allow for the influence of certain environmental variables known to have strong gradients across sites to be controlled for in order to focus on variation across other relatively weak environmental gradients (Legendre and Legendre 2012). We identified three variable categories hypothesized to affect species community composition in the dammed river: physical variables associated with river morphology (PC1 from PCA analyses); water chemistry (WC); and, Round Goby abundance (RG). By conducting partial tb-RDA and selectively conditioning out all but one category at a time, we were able isolate the proportion of community variance explained by variable categories and shared variance that could not be attributed solely to one variable category. Significance of variance parts was tested using randomization with 9999 iterations. We show tb-RDA results in a Venn diagram to demonstrate total, shared, and category-explained variance.
Given strong gradients in abiotic conditions moving upstream from the reservoir sections of river reaches and the likelihood for species-specific responses to this gradient, this abiotic reservoir effect must be taken into account when isolating the influence of invasive species on the receiving community. To evaluate the response of individual benthic fish species across dominant abiotic gradients and Round Goby abundance, we used generalized additive mixed models (GAMM). Generalized additive models are useful to describe species distributions along habitat gradients, particularly in situations where there exist non-linear relationships between species abundances and predictors (e.g., Reid et al. 2008). Our full GAMMs predicted individual species abundance using the dominant abiotic gradients summarized along PC1, log-transformed Round Goby abundance, and we included year and reach as random effects. Less-complex models were selected when there was no significant increase in explained deviance with the addition of terms beyond PC1 alone. As we were interested in the effect of Round Goby presence on the native fish community, and Round Goby had not yet established at R01, this reach was excluded from our analyses.
Establishment risk
Given the history of, and future likelihood for, human-mediated introductions in the Grand River watershed, we sought to develop a discriminant function that could determine post-arrival Round Goby establishment and abundance at yet uninvaded sites. We used local abiotic data in discriminant function analysis (DFA) based on the following steps: (1) selection of variables that best explain Round Goby abundance; (2) classification of Round Goby invasion status on invaded reaches; (3) computation of discriminant function; (4) evaluation of discriminant function performance; and, (5) assessment of uninvaded reach for establishment outcomes. Forward step-wise variable selection identified which of the physical habitat variables best described Round Goby abundance in the invaded reaches, and the selected variables were then used to compute a discriminant function using the MASS package (Ripley et al. 2015). Round Goby invasion status on invaded reaches was determined statistically, in the absence of obvious breakpoints in the data or ecological rationale; median number of Round Goby across all sites on invaded reaches was chosen as the break point between low- and high-abundance invasion classes. Discriminant function performance was evaluated for the whole data set by leave-one-out cross-validation, to produce a percentage value for correct classifications. To estimate outcomes of establishment if Round Goby arrive upstream of the barrier between R01 and R02, the DFA used physical properties of the uninvaded reach to classify sites into non-, low-, and high-abundance invasion classes. To visualize outcomes of the DFA, we mapped observed and predicted invasion status for R02 and R03 based on 2013 data, as well as predicted invasion status for R01.
Results
Physical reservoir gradient
Of the nine measured habitat variables, five physical variables were used in PCA to characterize abiotic gradients in the Grand River study reaches (Table 1). The first two principal component (PC) axes together explained 71% of the total variance; PC1 was characterized by sediment fineness, water depth, and water velocity, all variables understood to be directly affected by low-head dams, while PC2 was characterized by bank slope and channel width, typically related to surficial geology (Table 2). As river distance upstream of dams (normalized by reach as a proportion of total distance upstream of dams) increased, PC1 decreased on all reaches (Fig. 2). R01 had a considerably less proportion of reservoir habitat than R02 and R03, each of which had at least 50% of total reach length affected by dams. Stream gradient varied across our three study reaches and R01, located in the higher-gradient middle Grand River, was characterized by overall higher average water velocity, shallower bank slope, and coarser sediment (Table 1).
Reservoir scores across reaches. Values represent site scores for principal components axis 1 (PC1), characterized by sediment fineness, water depth, and water velocity. PC1 explained 50% of the total variance in physical habitat variables. Sites from reaches 1 through 3 (R01–R03) are plotted at proportional location upstream of dams to normalize relative positions among reaches of different lengths. Points correspond to sites following: R01, 2014 = asterisk; R02 = black fill shapes; R03 = open shapes (2010 = triangle; 2011 = circle; 2013 = square)
Fish community and species distribution patterns
Benthic trawling surveys on R01, R02, and R03 between 2011 and 2014 captured 38 718 individuals, representing 47 fish species, including nine small-bodied benthic species (Table 3). Of these nine species, only Round Goby was considered invasive to the Grand River.
The benthic fish community differed significantly among years and between reaches for all sites sampled between 2010 and 2013 on R02 and R03 (PERMANOVA; n = 97). The majority of explained variation in the benthic fish community could be attributed to the covariate PC1 (20.6%, F 1,94 = 28.24, p < 0.001), which describes the physical habitat gradient on each reach caused by dams. Lesser variation was described by reach identity (9.8%, F 1,94 = 13.45, p < 0.001) and sampling year (4.0%, F 2,94 = 2.72, p = 0.014). To further examine differences among reaches while minimizing temporal spread, we compared 2014 survey data from R01 to 2013 survey data from R02 and R03. Fish community composition differed among reaches (PERMANOVA; n = 68). The majority of explainable variation in the fish community could be attributed to reach identity (36.4%, F 2,64 = 20.57, p < 0.001), whereas, the PC1 covariate has less of a structuring effect at this scale (9.6%, F 1,64 = 10.84, p < 0.001). When the analysis was run without Round Goby (which has not colonized the upstream reach), there was no change in the result.
To visualize community differences across years and reaches, we first plotted NMS axes 1 and 2 for sites on reaches R02 and R03 sampled 2010–2013 (Fig. 3, n = 97, final stress = 0.191 after 6 iterations). While inter-annual variation in the fish community may have been significant by PERMANOVA, the pattern in this variation was not apparent in the NMS biplot. However, there was evident separation of R02 and R03 across NMS axis 2; R02 sites were evenly spread across NMS axis 1 and placed high on NMS axis 2. Benthic species Rainbow Darter Etheostoma caeruleum, Greenside Darter Etheostoma blennioides, and Stonecat Noturus flavus were associated with high water-velocity sites on R02, whereas, benthic species Eastern Sand Darter, Brindled Madtom Noturus miurus, Blackside Darter Percina maculata, and Johnny Darter Etheostoma nigrum were found at deeper, lower velocity sites with fine sediment. R03 sites were found low on NMS axis 2, characterized by communities containing Round Goby and Logperch.
Benthic fish community-habitat associations visualized by NMS on Round Goby-invaded reaches of the Grand River (R02–R03) sampled from 2010 to 2013 (n = 97, final stress = 0.191 after 6 iterations). Points represent discrete sampling events; positions of points are relative to Bray–Curtis dissimilarity matrix and positions of species are weighted mean scores. Abiotic habitat variables were fitted to the NMS plot as vector overlays when significant (p < 0.01) based on 9999 iterations. Points correspond to sites following: R02 = black fill shapes; R03 = open shapes (2010 = triangle; 2011 = circle; 2013 = square). Habitat variable codes follow Table 1. Fish species codes follow Table 3
To examine fish community variation across the entire study area (R01-R03), we plotted the first two NMS axes of 2013 and 2014 data (Fig. 4; n = 68, final stress = 0.133 after 13 iterations). There was a clear separation of the fish community in R01 from R02 and R03, likely driven by higher captures of Rainbow Darter, Greenside Darter, and Stonecat in R01, and higher abundances of Logperch, Eastern Sand Darter, and Round Goby in R02 and R03. Habitat vectors indicate Rainbow Darter and Greenside Darter inhabited faster flowing, shallower water with coarser sediment than sites at which Eastern Sand Darter, Blackside Darter, Logperch, or Round Goby was found. Logperch was associated with Round Goby at deeper, reservoir-affected sites. Water conductivity and water temperature habitat vector overlays clearly separate R01 from R02 and R03.
Benthic fish community-habitat associations visualized by NMS on three study reaches of the Grand River (R01–R03) sampled in 2013 and 2014 (n = 68, final stress = 0.133 after 13 iterations). Points represent discrete sampling events; positions of points are relative to Bray–Curtis dissimilarity matrix and positions of species are weighted mean scores. Abiotic habitat variables were fitted to the NMS plot as vector overlays when significant (p < 0.01) based on 9999 iterations. Points correspond to sites following: R01, 2014 = asterisk; R02, 2013 = black square; R03, 2013 = open square. Habitat variable codes follow Table 1. Fish species codes follow Table 3
Round Goby establishment and impact
The greatest explainable variation in the native benthic fish community was attributable to the reservoir (27%, p < 0.001), followed by Round Goby abundance (10%, p < 0.001), and water quality (5%, p = 0.002; Fig. 5). Considerable variation was shared among categories and variance partitioning revealed a small, but significant, amount of explainable variation attributed to Round Goby abundance alone (1%, p = 0.045).
Multivariate variance partitioning of the native benthic fish community on three reaches of the Grand River by tb-RDA. Variance is partitioned among three suites of variables: Reservoir, Water Quality, and Round Goby abundance. Total explained variance = 0.31 (n = 135). Asterisk indicates significance of variance part at p < 0.05
Significant predictors of abundance in GAM or GAMM models varied by species across R02 and R03 (Table 4). No significant predictors were identified for Stonecat, and this species was excluded from further analyses and visualization. Round Goby abundance showed an exponential increase along PC1, as the degree of reservoir effect increased (Fig. 6). PC1 and Round Goby abundance was a significant predictor of Eastern Sand Darter, Greenside Darter, Johnny Darter, and Logperch abundances, whereas, Blackside Darter and Rainbow Darter abundances were best described only by PC1 (Table 4). Species showed idiosyncratic responses to PC1, supporting the concept of niche partitioning driven by water velocity. As the reservoir effect increased along PC1, fishes such as Greenside Darter, Rainbow Darter and, to some degree, Logperch showed decreasing abundance, whereas Eastern Sand Darter, Johnny Darter, Blackside Darter, and Brindled Madtom increased in abundance. Logperch abundance did not show a negative relationship with Round Goby abundance when the latter was a significant predictor in the model. Year was significant as a random effect in all but the Blackside Darter model.
GAM-generated smoothing functions or linear regressions (black lines) and 95% confidence bands (shaded areas) showing the relationship between abundances of eight benthic fish species from the Grand River, Ontario, and either one or two significant predictors—reservoir gradient (PC1) and Round Goby abundance (RG). Model results are provided in Table 4. Straight regression lines indicate a linear model provided a better fit for the data than a smoothing function
Establishment risk
The variables selected for DFA were water velocity, sediment fineness, water depth, and channel width. The median number of Round Goby at sites on invaded reaches was 7 (R02 range 0–840; R03 range 0–520), and this represented the chosen breakpoint between low- and high-abundance invasion classes; sites with 1–7 Round Goby detected were considered low-abundance invasion status. The discriminant function reclassified invasion status on invaded reaches R02 and R03 with a high degree of fidelity, indicating good model performance. We correctly predicted absence (n = 30) in 86.5% of cases, low abundance (n = 30) in 72.7% of cases, and high abundance (n = 38) in 85.7% of cases (Table 5). Spatially, there was little change to upstream invasion edge between observations and predictions, and the principal differences were in assignment of the farthest downstream sites on R03 to high invasion status, when the observed status was either low, or non-invaded (Fig. 7). When applied to habitat data from uninvaded R01 to forecast invasion risk, the discriminant function identified 25 sites where Round Goby is not likely to establish, eight sites that could support low abundance, and five sites that could support high abundance. All sites that would reach high Round Goby abundance (high invasion status) were < 1 km upstream of the low-head dam at the downstream end of the reach (Fig. 7).
Invasion status and risk on the lower Grand River. Points correspond to sites following: non-invaded = open triangle; low-invasion status = grey inverted triangle; high-invasion status = black triangle. Continuous black line with grey shading represents river thalweg; flow direction is from top left to bottom right. Black arrows indicate dam locations (see Fig. 1 for dam identities). a Discriminant function-predicted invasion status on invaded reaches R02 and R03 based on 2013 habitat measurements. b Current invasion status on invaded reaches R02 and R03 from 2013 benthic trawl surveys. c Discriminant function-predicted invasion status on R01 based on 2014 habitat measurements
Discussion
Round Goby invasion appears to be facilitated by the presence of low-head dams on the lower Grand River. Impounded sections above low-head barriers have contributed to the maintenance of wider channels, slow-moving deep water, and fine substrate. The water-velocity gradient appears to promote Round Goby establishment upstream of dams and, at the same time, negatively affects the abundances of certain native benthic fishes. Introduction of Round Goby in the study reaches above low-head dams is suspected to be of human origin (Bronnenhuber et al. 2011). Given that such transport is all but inevitable (Drake and Mandrak 2014), we must accurately characterize the receiving environment to estimate risk of establishment. Previous studies that have focused primarily on lower reaches of rivers below the first upstream barrier (e.g., Abbett et al. 2013; Campbell and Tiegs 2012; Kornis et al. 2013; Krakowiak and Pennuto 2008) do not address the majority of tributary habitat currently at risk of invasion by human-mediated introductions.
Physical reservoir gradient
Low-head dams on the Grand River effectively interrupt the river continuum, creating a repeating pattern of lentic-to-lotic transition seen elsewhere in rivers with successive low-head barriers (Alexandre and Almeida 2010; Gillette et al. 2005). Although lacking the conspicuous lacustrine reservoirs of large dams, small in-line low-head dams may nonetheless alter flow and depth along upstream reaches (Alexandre and Almeida 2010; Dodd et al. 2003). The widely observed ‘lentification’ (the slowing, deepening, and fine sediment deposition) of stream sections immediately upstream of small natural or anthropogenic barriers (e.g., Alexandre and Almeida 2010; Cumming 2004; Gillette et al. 2005; Tiemann et al. 2004; Wang et al. 2011) is, along with loss of habitat connectivity (Nilsson et al. 2005; Rahel et al. 2009), frequently cited as a principal mechanism by which small barriers structure the native fish community. Our results indicate that the physical stream environment on the Grand River is highly modified by the presence of dams. Habitat variables that loaded on principal component axis PC1 were those we understood to be the reservoir effect of low-head dams (water velocity, water depth, and sediment size) that diminished with distance upstream of the low-head structures. This repeated gradient of river morphology, in which higher quality habitat is characterized by larger sediment size and a greater magnitude of flow, has been noted in previous work examining low-head dams on the Grand River (Reid et al. 2008) and elsewhere (Alexandre and Almeida 2010; Gillette et al. 2005). A large rainfall event occurred in 2014 during the sampling period for R01 and we suspect this event was responsible for lower average water temperature, lower water conductivity, and higher dissolved oxygen on R01 versus R02 and R03, which were sampled in previous summers. This event highlights the importance of selecting variables that are relatively unaffected by such instantaneous physicochemical measurements of river condition when describing reservoir effects in shallow dam-affected tributaries (Alexandre and Almeida 2010). Water velocity, which was on average higher in R01, was also likely affected by the rainfall event; however, this is confounded by the fact that R01 had a higher stream gradient than R02 and R03.
Fish species distribution patterns
The benthic fish community within invaded reaches was clearly structured by abiotic habitat variables, with reservoir-associated variables explaining the majority of variance. Low-head dams affect fish assemblages by altering the physical environment and the context of their interactions (Alexandre and Almeida 2010; Gillette et al. 2005; Porto et al. 1999); thus, the reservoir effect was a useful predictor across which to examine individual species abundances, as the complex interaction of habitat variables in partitioning riverine niches warrants a multivariate approach (Pratt and Lauer 2013; Stauffer et al. 1996). In general, intact, small-bodied benthic fish communities should conform to assembly rules with respect to microhabitat partitioning (Paine et al. 1982; Pratt and Lauer 2013; Stauffer et al. 1996; Thompson and Stallsmith 2016), which is consistent with what we have found across our study reaches.
Contrary to our predictions, however, native benthic fishes demonstrated a variety of responses to the reservoir gradient, evident both in the multivariate plots of site-species associations and the individual species models across the reservoir gradient and Round Goby abundance. Greenside Darter, Rainbow Darter, and Stonecat occurred in faster flowing water; with the exception of Stonecat, their abundances decreased as the degree of reservoir effect increased, suggesting that the dam legacy limited their habitat on the Grand River prior to the introduction of Round Goby. The high-flow large-substrate association of Rainbow Darter and Greenside Darter was consistent with research examining habitat partitioning in non-invaded rivers (Paine et al. 1982; Pratt and Lauer 2013). Eastern Sand Darter, Johnny Darter, Brindled Madtom, and Blackside Darter exhibited higher abundance with a higher degree of reservoir effect and were relatively closely clustered in multivariate species space. Endangered Eastern Sand Darter may present a special case as it selects sandy substrate typical of slightly lower water velocities (Drake et al. 2008). Logperch abundance varied non-linearly across the reservoir gradient of our study reaches, declining sharply past a threshold in the degree of reservoir effect, which may represent avoidance of habitat with more embedded and finer substrate, as well as areas with high Round Goby abundance. Logperch is often associated with gravelly and rocky shorelines in lakes and large rivers (Balshine et al. 2005; Ray and Corkum 2001). In smaller lotic systems, it selects riffle- and pool-edge habitats (Aadland 1993) where its preferred substrate is maintained by higher water velocities.
Round Goby was more often found in dam-modified deeper, slower-flowing habitat than small-bodied native benthic fishes on the Grand River. For shelter and reproduction, Round Goby requires interstitial spaces typically provided by hard substrate such as cobble (Kornis et al. 2012); yet impounded sections were characterized by fine sediment. Reproduction was clearly occurring in each invaded reach, with young-of-year gobies (< 15 mm total length) captured in all years. In Michigan GLB tributaries, it was hypothesized that Round Goby may migrate seasonally out of areas with fine substrate to find suitable spawning locations (Campbell and Tiegs 2012; Pennuto et al. 2010) and, in the St. Clair River, Round Goby was suspected to utilize riprapped banks as spawning habitat in reaches of otherwise unsuitably fine substrate (Campbell and Tiegs 2012). Such movement and alternate habitat use within the Grand River is plausible, and may create temporal variation in the distribution of Round Goby.
Logperch and Round Goby grouped closely in species space (Fig. 3); such overlap in habitat use has been similarly reported in many GLB tributaries (Campbell and Tiegs 2012; Kornis et al. 2013; Phillips et al. 2003) and may promote competitive interactions between the two species. The outcomes of this competition were tested in situ in Duluth-Superior Harbour (Leino and Mensinger 2017), where habitat would be similar to that of impounded sections of the Grand River. Logperch were out-competed by Round Goby on soft-bottomed substrate but the larger physical area of this substrate type compared to preferred rocky habitat was hypothesized to promote coexistence in the harbor (Leino and Mensinger 2017). This may be the situation in the Grand River and explain the trends we see in the Logperch GAM and NMS visualizations.
Invasion impacts on native fishes
Round Goby abundance explained changes to native benthic-fish community composition and reduced abundances of at least three species beyond what could be attributed to the physical reservoir gradient. Abundances of Greenside Darter, Johnny Darter, and Eastern Sand Darter showed significant inverse correlations with Round Goby abundance. Elsewhere, Johnny Darter and Rainbow Darter are only found in New York tributaries of the St. Lawrence River that lacked Round Goby (Abbett et al. 2013), but we suspect that the Grand River low-head dams may mediate this impact by: (1) maintaining large substrate size preferred by Rainbow Darter in high flow areas immediately downstream of dams; and, (2) creating barriers to Round Goby upstream movement, thus protecting uninvaded pool habitat preferred by Johnny Darter. Although small-bodied native benthic fishes are adapted to variable flow and water levels of tributaries, Round Goby may be better able to tolerate hydrologically modified conditions in an impounded tributary.
Round Goby presence and assumed competitive superiority have been implicated in the precipitous decline or extirpation of Logperch from New York tributaries to Lake Erie (Krakowiak and Pennuto 2008), Hamilton Harbour (Balshine et al. 2005), Duluth-Superior Harbour (Lynch and Mensinger 2012), and inner Long Point Bay, Lake Erie (Reid and Mandrak 2008). On the Grand River, however, there was little evidence to suggest Logperch is in decline because of Round Goby; the complex riverine environment may be acting to allow coexistence not observed in lentic systems. We hypothesize that a number of factors explain the nonlinear bimodal response of Logperch to increasing Round Goby abundance, including differences in population size structure of Round Goby and native species, which could mediate competitive interactions. Round Goby has multiple spawning events per year (Corkum et al. 2004; Kornis et al. 2012), resulting in a relatively continuous size structure (Brandner et al. 2013; MacInnis and Corkum 2000; Taraborelli et al. 2010), in contrast to species that spawn once per year and mature in distinct cohorts, such as Roanoke Logperch Percina rex (Rosenberger and Angermeier 2002). The continuous size structure of Round Goby relative to Logperch may allow more efficient resource use along habitat continua (e.g., PC1) and Round Goby impacts could vary across the reservoir gradient depending on where particular size classes of each species overlap. It is also possible that, at the highest densities of Round Goby in the impounded sections, interspecific competition drops, reflected in the secondary increase in Logperch abundance as invader abundance reaches its highest levels. Indeed, experimental work on Round Goby and native fishes in tributaries to Lake Michigan supports a shift from inter- to intra-specific competition at highest invader abundances, which may release native fishes from competition with the invader; however, low recapture of native fishes the highest invader abundances may have been due to increased mortality in this treatment, thus confounding results (Kornis et al. 2014).
The synergistic interaction of habitat modification and species invasions can severely impact native aquatic species (e.g., Clavero et al. 2013; Didham et al. 2007; Hermoso et al. 2011; Marchetti et al. 2004; Marks et al. 2010), and there is considerable uncertainty as to the magnitude of effect Round Goby invasion of impounded tributaries will have on riverine small-bodied fishes (Poos et al. 2010). The role of lentic refugia in mediating coexistence among native and invasive benthic fishes in tributaries remains an important research focus.
Low-head dams: barriers or beachheads?
Small riverine impoundments on the Grand River appear to be acting beachheads for Round Goby establishment—an effect of low-head dams that has largely been overlooked in the GLB, where research has primarily focused on their isolating effect (Dodd et al. 2003; McLaughlin et al. 2003). Using information from invaded reaches of the Grand River, we predicted that Round Goby are likely to establish if introduced in the yet uninvaded impoundment on R01. However, despite good performance overall, our model failed most often to correctly separate low-abundance sites from non-invaded and high-abundance sites. This may be an artifact of our necessarily arbitrary invasion status assignment criteria, due to the structure of the data and paucity of biological rationale in the literature. Post-invasion dynamics may also affect model discrimination between low and high abundance sites. Modeling Round Goby invasion dynamics over the first decade following introduction in Hamilton Harbour, Ontario, demonstrated a sharp population decline as Round Goby reached its peak density, likely due to high competition and predation (Vélez-Espino et al. 2010). If the sampling period used to develop our model was early in the population expansion phase, or followed a population decline, this could introduce uncertainty in calculating invasion outcomes for uninvaded habitat. While Round Goby was consistently the most abundant species in R02 and R03, there was a peak in 2011, with fewer individuals captured in 2010 and 2013. Whether this represented cyclic fluctuation, or a true population peak, was unknown. Nevertheless, accounting for this variation is necessary for generating realistic estimates of uncertainty when assessing the performance of a predictive model. Prediction is integral to risk assessment by facilitating early detection and intervention in the event of species invasions, and our work provides a basis for evaluating establishment risk as Round Goby continues to spread in tributaries.
Completion of the Welland Canal and the subsequent invasion by Sea Lamprey into the upper GLB has required intensive management to mitigate its impact on native fishes (Lavis et al. 2003; Smith and Tibbles 1980), including the maintenance or installation of low-head barriers to lamprey spawning habitat, which have affected habitat connectivity and fish community structure (Dodd et al. 2003). The potential establishment of Asian carps (which require a minimum length of free-flowing river to complete their breeding cycle) in the GLB has renewed attention on low-head dams as potentially important interruptions in otherwise free-flowing river sections (Kocovsky et al. 2012; Murphy and Jackson 2013). On the other hand, impoundments are popular locations for angling and can act as hubs for the movement of invasive species in a way similar to shipping ports (Floerl et al. 2009). Human-mediated movement upstream of barriers has already been suspected in the dispersal of Greenside Darter in the Grand River, a species not found in the watershed until 1990 (Beneteau et al. 2009). This mode of inland dispersal is similarly suspected for Round Goby (Bronnenhuber et al. 2011), as beachheads become stepping-stones in the invasion process.
Dam removal is an increasingly common focus of river restoration efforts (Bednarek 2001) and can have unpredictable outcomes in the short term (Orr et al. 2008). The physical environment mediates interactions among native and invasive species, rendering ecological impacts of invasion highly context dependent (Byers 2002; Didham et al. 2007; Kestrup and Ricciardi 2009; McLaughlin et al. 2013). When assessing trade-offs in dam removal and fish-passage restoration, it is critical to account for the interaction of the abiotic environment and life history of invading organisms to develop management strategies (Fausch et al. 2009). Small hydrologic modifications, such as low-head dams, change fish assemblages (Alexandre and Almeida 2010; Gillette et al. 2005; Porto et al. 1999), and the alteration of hydrologic regimes increase local susceptibility to the establishment of aquatic invasive species (Moyle and Marchetti 2006; Rahel 2002; Scott et al. 2016). Many dams remain in place only as legacies from past use, although their ecological effects may evolve as the threat of anthropogenic species introduction moves inland. Our study adds to an emerging body of literature that challenges the idea that dam removal represents a straightforward tradeoff between habitat connectivity and invasive species containment.
References
Aadland LP (1993) Stream habitat types: their fish assemblages and relationship to flow. N Am J Fish Manag 13:790–806
Abbett R, Waldt EM, Johnson JH, McKenna JE, Dittman DE (2013) Interactions between invasive round gobies (Neogobius melanostomus) and fantail darters (Etheostoma flabellare) in a tributary of the St. Lawrence River, New York, USA. J Freshwat Ecol 28:529–537
Alexandre CM, Almeida PR (2010) The impact of small physical obstacles on the structure of freshwater fish assemblages. River Res Appl 26:977–994
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. J Great Lakes Res 31:68–77
Bauer CR, Bobeldyk AM, Lamberti GA (2007) Predicting habitat use and trophic interactions of Eurasian ruffe, round gobies, and zebra mussels in nearshore areas of the Great Lakes. Biol Invasions 9:667–678
Bednarek AT (2001) Undamming rivers: a review of the ecological impacts of dam removal. Environ Manag 27:803–814
Beneteau CL, Mandrak NE, Heath DD (2009) The effects of river barriers and range expansion of the population genetic structure and stability in Greenside Darter (Etheostoma blennioides) populations. Conserv Genet 10:477–487
Brandner J, Cerwenka AF, Schliewen UK, Geist J (2013) Bigger Is better: characteristics of round gobies forming an invasion front in the Danube River. PLoS ONE 8:e73036
Bronnenhuber JE, Dufour BA, Higgs DM, Heath DD (2011) Dispersal strategies, secondary range expansion and invasion genetics of the nonindigenous round goby, Neogobius melanostomus, in Great Lakes tributaries. Mol Ecol 20:1845–1859
Byers JE (2002) Physical habitat attribute mediates biotic resistance to non-indigenous species invasion. Oecologia 130:146–156
Campbell TB, Tiegs SD (2012) Factors governing the distribution and fish-community associations of the round goby in Michigan tributaries of the Laurentian Great Lakes. J Great Lakes Res 38:569–574
Clavero M, Hermoso V, Aparicio E, Godinho FN (2013) Biodiversity in heavily modified waterbodies: native and introduced fish in Iberian reservoirs. Freshw Biol 58:1190–1201
Corkum LD, Sapota MR, Skora KE (2004) The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biol Invasions 6:173–181
Cumming GS (2004) The impact of low-head dams on fish species richness in Wisconsin, USA. Ecol Appl 14:1495–1506
Dextrase AJ (2013) Modelling occupancy and abundance of eastern sand darter (Ammocrypta pellucida) while accounting for imperfect detection. Environmental and Life Sciences Department. Trent University, Peterborough, Ontario, Canada, pp 352
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496
Dodd HR, Hayes DB, Baylis JR, Carl LM, Goldstein JD, McLaughlin RL, Noakes DLG, Porto LM, Jones ML (2003) Low-head sea lamprey barrier effects on stream habitat and fish communities in the Great Lakes basin. J Great Lakes Res 29:386–402
Dopazo SN, Corkum LD, Mandrak NE (2008) Fish assemblages and environmental variables associated with Gobiids in nearshore areas of the lower Great Lakes. J Great Lakes Res 34:450–460
Drake DAR, Mandrak NE (2014) Bycatch, bait, anglers, and roads: quantifying vector activity and propagule introduction risk across lake ecosystems. Ecol Appl 24:877–894
Drake DAR, Power M, Koops MA, Doka SE, Mandrak NE (2008) Environmental factors affecting growth of eastern sand darter (Ammocrypta pellucida). Can J Zool 86:714–722
Dubs DOL, Corkum LD (1996) Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J Great Lakes Res 22:838–844
Falke JA, Gido KB (2006) Spatial effects of reservoirs on fish assemblages in great plains streams in Kansas, USA. River Res Appl 22:55–68
Fausch KD, Rieman BE, Dunham JB, Young MK, Peterson DP (2009) Invasion versus isolation: trade-offs in managing native salmonids with barriers to upstream movement. Conserv Biol 23:859–870
Floerl O, Inglis GJ, Dey K, Smith A (2009) The importance of transport hubs in stepping-stone invasions. J Appl Ecol 46:37–45
Gillette DP, Tiemann JS, Edds DR, Wildhaber ML (2005) Spatiotemporal patterns of fish assemblage structure in a river impounded by low-head dams. Copeia 2005:539–549
Google Earth (2017) CNES, Airbus, DigitalGlobe, United States Geological Survey, First Base Solutions 2017 imagery. http://www.earth.google.com (June 19, 2017)
GRASS Development Team (2012) Geographic Resources Analysis Support System (GRASS) Software v6.4. Open Source Geospatial Foundation Project
Harford WJ, McLaughlin RL (2007) Understanding uncertainty in the effect of low-head dams on fishes of great lakes tributaries. Ecol Appl 17:1783–1796
Havel JE, Lee CE, Vander Zanden MJ (2005) Do reservoirs facilitate invasions into landscapes? Bioscience 55:518–525
Hermoso V, Clavero M, Blanco-Garrido F, Prenda J (2011) Invasive species and habitat degradation in Iberian streams: an analysis of their role in freshwater fish diversity loss. Ecol Appl 21:175–188
Herzog DP, Ostendorf DE, Hrabik RA, Barko VA (2009) The mini-Missouri trawl: a useful methodology for sampling small-bodied fishes in small and large river systems. J Freshw Ecol 24:103–108
Husson F, Josse J, Le S, Mazet J (2013) FactoMineR: multivariate exploratory data analysis and data mining with R. R Package, 1.15 edn.
Inman DL (1952) Measures for describing the size distribution of sediments. J Sediment Res 22:125–145
Janssen J, Jude DJ (2001) Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J Great Lakes Res 27:319–328
Johnson PTJ, Olden JD, Vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:359–365
Josse J, Husson F (2012) Selecting the number of components in principal component analysis using cross-validation approximations. Comput Stat Data Anal 56:1869–1879
Kestrup AM, Ricciardi A (2009) Environmental heterogeneity limits the local dominance of an invasive freshwater crustacean. Biol Invasions 11:2095–2105
Kocovsky PM, Chapman DC, McKenna JE (2012) Thermal and hydrologic suitability of Lake Erie and its major tributaries for spawning of Asian carps. J Great Lakes Res 38:159–166
Kornis MS, Mercado-Silva N, Vander Zanden MJ (2012) Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. J Fish Biol 80:235–285
Kornis MS, Sharma S, Vander Zanden MJ (2013) Invasion success and impact of an invasive fish, round goby, in Great Lakes tributaries. Divers Distrib 19:184–198
Kornis M, Carlson J, Lehrer-Brey G, Vander Zanden MJ (2014) Experimental evidence that ecological effects of an invasive fish are reduced at high densities. Oecologia 175:325–334
Krakowiak PJ, Pennuto CM (2008) Fish and macroinvertebrate communities in tributary streams of Eastern Lake Erie with and without Round Gobies (Neogobius melanostomus, Pallas 1814). J Great Lakes Res 34:675–689
Lavis DS, Hallett A, Koon EM, McAuley TC (2003) History of and advances in barriers as an alternative method to suppress sea lampreys in the Great Lakes. J Great Lakes Res 29:362–372
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Legendre P, Legendre LFJ (2012) Numerical ecology. Elsevier, Amsterdam
Leino JR, Mensinger AF (2016) The benthic fish assemblage of the soft-bottom community of the Duluth-Superior Harbor before and after round goby invasion (1989–2011). J Great Lakes Res 42:829–836
Leino JR, Mensinger AF (2017) Interspecific competition between the round goby, Neogobius melanostomus, and the logperch, Percina caprodes, in the Duluth-Superior Harbour. Ecol Freshw Fish 26:34–41
Lynch MP, Mensinger AF (2012) Seasonal abundance and movement of the invasive round goby (Neogobius melanostomus) on rocky substrate in the Duluth-Superior Harbor of Lake Superior. Ecol Freshwat Fish 21:64–74
MacInnis AJ, Corkum LD (2000) Age and growth of Round Goby Neogobius melanostomus in the upper Detroit River. Trans Am Fish Soc 129:852–858
Marchetti MP, Light T, Moyle PB, Viers JH (2004) Fish invasions in California watersheds: testing hypotheses using landscape patterns. Ecol Appl 14:1507–1525
Marks JC, Haden GA, O’Neill M, Pace C (2010) Effects of flow restoration and exotic species removal on recovery of native fish: lessons from a dam decommissioning. Restor Ecol 18:934–943
McLaughlin RL, Marsden JE, Hayes DB (2003) Achieving the benefits of sea lamprey control while minimizing effects on nontarget species: Conceptual synthesis and proposed policy. J Great Lakes Res 29:755–765
McLaughlin RL, Smyth ERB, Castro-Santos T, Jones ML, Koops MA, Pratt TC, Velez-Espino LA (2013) Unintended consequences and trade-offs of fish passage. Fish Fish 14:580–604
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. J Great Lakes Res 19:1–54
Moyle PB, Marchetti MP (2006) Predicting invasion success: freshwater fishes in California as a model. Bioscience 56:515–524
Murphy EA, Jackson PR (2013) Hydraulic and water-quality data collection for the investigation of Great Lakes tributaries for Asian carp spawning and egg-transport suitability In: Survey USG (ed). pp 30
Nilsson C, Reidy CA, Dynesius M, Revenga C (2005) Fragmentation and flow regulation of the world’s large river systems. Science 308:405–408
Noakes DLG, McLaughlin RL, Baylis JR, Carl LM, Hayes DB, Randall RG (2000) Biological impact of low-head barrier dams (BILD) Project completion report (1999) to the Great Lakes Fishery Commission. Michigan, Ann Arbor
Oksanen J, Blanchet B, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H (2011) VEGAN: Community ecology package. R Package, 1.17-0 edn.
Orr CH, Kroiss SJ, Rogers KL, Stanley EH (2008) Downstream benthic responses to small dam removal in a coldwater stream. River Res Appl 24:804–822
Paine MD, Dodson JJ, Power G (1982) Habitat and food resource partitioning among four species of darters (Percidae: Etheostoma) in a southern Ontario stream. Can J Zool 60:1635–1641
Pennuto CM, Krakowiak PJ, Janik CE (2010) Seasonal abundance, diet, and energy consumption of round gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol Freshw Fish 19:206–215
Phillips EC, Washek ME, Hertel AW, Niebel BM (2003) The round goby (Neogobius melanostomus) in Pennsylvania tributary streams of Lake Erie. J Great Lakes Res 29:34–40
Poos M, Dextrase AJ, Schwalb AN, Ackerman JD (2010) Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: potential new concerns for endangered freshwater species. Biol Invasions 12:1269–1284
Porto LM, McLaughlin RL, Noakes DLG (1999) Low-head barrier dams restrict the movements of fishes in two Lake Ontario streams. N Am J Fish Manag 19:1028–1036
Pratt AE, Lauer TE (2013) Habitat use and separation among congeneric darter species. Trans Am Fish Soc 142:568–577
R Core Development Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33:291–315
Rahel RF, Rahel FJ, Hubert WA (2009) Complex influences of low-head dams and artificial wetlands on fishes in a Colorado River tributary system. Fish Manag Ecol 16:457–467
Ray WJ, Corkum LD (2001) Habitat and site affinity of the round goby. J Great Lakes Res 27:329–334
Reid SM, Mandrak NE (2008) Historical changes in the distribution of threatened channel darter (Percina copelandi) in Lake Erie with general observations on the beach fish assemblage. J Great Lakes Res 34:324–333
Reid SM, Mandrak NE, Carl LM, Wilson CC (2008) Influence of dams and habitat condition on the distribution of redhorse (Moxostoma) species in the Grand River watershed, Ontario. Environ Biol Fish 81:111–125
Ricciardi A (2006) Patterns of invasion in the Laurentian Great Lakes in relation to changes in vector activity. Divers Distrib 12:425–433
Ripley B, Venables R, Bates DM, Hornik K, Gebhardt A, Firth D (2015) Support functions and datasets for Venables and Ripley’s MASS. 7.3-43 edn.
Rosenberger AE, Angermeier PL (2002) Roanoke logperch (Percina rex) population structure and habitat use. Final Report submitted to the Virginia Department of Game and Inland Fisheries, Blacksburg, VA:110
Santucci VJ, Gephard SR, Pescitelli SM (2005) Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the fox river, Illinois. N Am J Fish Manag 25:975–992
Scott DC, Arbeider M, Gordon J, Moore JW (2016) Flood control structures in tidal creeks associated with reduction in nursery potential for native fishes and creation of hotspots for invasive species. Can J Fish Aquat Sci 73:1138–1148
Smith BR, Tibbles JJ (1980) Sea lamprey (Petromyzon marinus) in Lakes Huron, Michigan, and Superior: history of invasion and control, 1936–78. Can J Fish Aquat Sci 1780–1801
Smith BR, Edds DR, Goeckler JM (2015) Lowhead dams and the downstream dispersal of zebra mussels. Hydrobiologia 755:1–12
Staton SK, Mandrak NE (2005) Focusing conservation efforts for freshwater biodiversity. Protected areas and species and ecosystems at risk: research and planning challenges. In: Proceedings of the parks research forum of ontario annual meeting. pp 197–204
Stauffer JR, Boltz JM, Kellogg KA, van Snik ES (1996) Microhabitat partitioning in a diverse assemblage of darters in the Allegheny River system. Environ Biol Fish 46:37–44
Taraborelli AC, Fox MG, Johnson TB, Schaner T (2010) Round Goby (Neogobius melanostomus) population structure, biomass, prey consumption and mortality from predation in the Bay of Quinte, Lake Ontario. J Great Lakes Res 36:625–632
Thompson B, Stallsmith B (2016) Microhabitat partitioning of an assemblage of darter species within two tributaries of the Tennessee River in Alabama. River Res Appl 32:1232–1241
Tiemann JS, Gillette DP, Wildhaber ML, Edds DR (2004) Effects of lowhead dams on riffle-dwelling fishes and macroinvertebrates in a midwestern river. Trans Am Fish Soc 133:705–717
Tierney KB, Kasurak AV, Zielinski BS, Higgs DM (2011) Swimming performance and invasion potential of the round goby. Environ Biol Fish 92:491–502
Vélez-Espino LA, Koops MA, Balshine S (2010) Invasion dynamics of round goby (Neogobius melanostomus) in Hamilton Harbour, Lake Ontario. Biol Invasions 12:3861–3870
Wang LZ, Infante D, Lyons J, Stewart J, Cooper A (2011) Effects of dams in river networks on fish assemblages in non-impoundment sections of rivers in Michigan and Wisconsin, USA. River Res Appl 27:473–487
Acknowledgements
We thank the Fisheries and Oceans Canada Great Lakes Laboratory for Fisheries and Aquatic Science, in particular J. Barnucz and L. Bouvier for field support and provision of historical data. We also thank G. Larocque at the Quebec Centre for Biodiversity Science for statistical advice. Thorough critique by two anonymous reviewers significantly improved an early version of this manuscript. The Ontario Ministry of Natural Resources Species at Risk Research Fund, the Invasive Species Centre, the Quebec Centre for Biodiversity Science, and the Natural Sciences and Engineering Research Council of Canada funded this work through grants to D.R., A.R., and N.E.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Animal handling by D.R. was conducted in accordance to McGill University Animal Care Committee guidelines for field studies to minimize discomfort to animals, under animal care protocol 2014-7493.
Rights and permissions
About this article
Cite this article
Raab, D., Mandrak, N.E. & Ricciardi, A. Low-head dams facilitate Round Goby Neogobius melanostomus invasion. Biol Invasions 20, 757–776 (2018). https://doi.org/10.1007/s10530-017-1573-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1573-3