Abstract
Redhorse, Moxostoma spp., are considered to be negatively affected by dams although this assertion is untested for Canadian populations. One hundred and fifty-one sites in the Grand River watershed were sampled to identify factors influencing the distribution of redhorse species. Individual species of redhorse were captured from 3 to 32% of sites. The most widespread species were golden redhorse, M. erythrurum (30%) and greater redhorse, M. valenciennesi (32%), while river redhorse, M. carinatum, was only found along the lower Grand River. Redhorse were absent from the highly fragmented Speed River sub-watershed and upper reaches of the Conestogo River and the Grand River. Redhorse species richness was positively correlated to river fragment size and upstream drainage area. Generalized additive models (GAMs) were applied to evaluate the influence of river fragment length, connectivity and habitat on species distribution. Principal component analysis reduced habitat data to three axes representing: channel structure, substrate, and pool, riffle and run habitats (PC1); gradient and drainage area (PC2); and cover (PC3). GAMs indicate that PC2 was important for predicting black redhorse and greater redhorse site occupancy and PC1 was important for golden redhorse. River fragment length was important for predicting site occupancy for shorthead redhorse, but not other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of species across fragmented landscapes has been explained in terms of the size, shape, quality and connectivity of habitats (Fahrig and Merriam 1994; Rieman and Dunham 2000). Accordingly, barriers that impede or prevent movement between habitat patches are a serious concern. For riverine fishes such as bull trout, Salvelinus confluentus, Lahontan cutthroat trout, Oncorhynchus clarkii henshawi (Rieman and Dunham 2000), Topeka shiner, Notropis topeka, (Schrank et al. 2001) and white-spotted charr, Salvelinus leucomaenis (Morita and Yamamoto 2002), factors such as patch area, duration of isolation, and connectivity to neighbouring populations have influenced local distribution patterns. Dams have a strong influence on all of these factors. Within watersheds, dams are considered responsible for extirpations of fish populations (Winston et al. 1991; Lutterall et al. 1999) and, at a larger spatial scale, the homogenization of fish faunas in North America (Rahel 2002). Hypothesized causes for extirpations include: (1) restricted access to, and/or alteration of spawning habitats; (2) increased numbers of predators due to the creation of lentic habitats; (3) the creation of small isolated populations that are more vulnerable to extinction events; and, (4) the prevention of re-colonization from other populations after local extinction events due to barriers (Winston et al. 1991; Rieman and Dunham 2000; Schrank et al. 2001; Hill et al. 2002).
Redhorse, Moxostoma spp., are relatively large, laterally compressed and superficially similar suckers that are typically found in large streams and rivers (Jenkins and Burkhead 1993; Scott and Crossman 1998). In southern Ontario rivers, redhorse comprise a large proportion of fish biomass (Cooke et al. 2005). Some species, such as shorthead redhorse, M. macrolepidotum, and silver redhorse, M. anisurum, are widely distributed across central Canada (Scott and Crossman 1998). Conversely, two of the six species found in southern Ontario are designated at risk by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC): black redhorse, M. duquesnei (Threatened) and river redhorse, M. carinatum (Special Concern) (COSEWIC 2005a). These two species and the greater redhorse, M. valenciennesi, are also imperiled in several neighbouring Great Lakes states (NatureServe 2005).
Many of the rivers in southern Ontario and other parts of the lower Great Lakes basin have been fragmented by dams. In the United States, conversion of riverine habitat into reservoirs, poor tailwater habitat conditions and the blockage of migratory routes by dams have been implicated in the decline of most redhorse species that occur in Canada: black redhorse (Bowman 1970; Travinchek and Maceina 1994), golden redhorse, M. erythrurum (Hughes 1994), river redhorse (Jenkins and Burkhead 1993; Quinn and Kwak 2003), shorthead redhorse (Santucci et al. 2005) and silver redhorse (Hughes 1994). In Canada, multi-species recovery strategies (Portt et al. 2006) and conservation status assessments (COSEWIC 2005b) contend that dams have negatively affected redhorse populations in Ontario. However, the hypothesized negative effect of dams on Canadian populations has not been tested through empirical studies. As well, many of the past studies have focused on effects at local (e.g. species absence in dam tailwaters) rather than larger spatial scales (i.e. distribution throughout a watershed). Other important factors that may limit redhorse distribution include watercourse size (Larimore and Smith 1963; Bowman 1970), natural barriers (Carlson and Daniels 2004), excessive siltation, and other forms of pollution (Jenkins and Burkhead 1993).
The objective of this study was to investigate the influence of dams and habitat condition on redhorse species distribution. Recovery planning for species at risk in Canada, such as the black redhorse and river redhorse, is limited by a lack of knowledge regarding species ecology, population size and factors that affect distribution and abundance. In contrast to sportfishes, the distribution and habitat requirements of most other fish species are poorly documented (Minns 2001). Between 2002 and 2004, a highly fragmented watershed in south-western Ontario, the Grand River, was sampled to identify factors affecting redhorse distribution. Generalized additive models were used to evaluate the relative influence of river fragment size, connectivity and habitat condition on the occurrence of individual redhorse species. As large differences in topography and surficial geology exist across the Grand River watershed, associations between habitat condition and species distribution with different physiographic regions were also tested.
Materials and methods
Study area
The Grand River watershed (43o21′ N, 80o18′ W) is the largest in southern Ontario, with an area of over 6500 km2. More than half of the 158 species of freshwater fishes known to occur in Ontario are found in the Grand River watershed (Mandrak and Crossman 1992). The Grand River and the adjacent Thames River are the only rivers that support all six Ontario redhorse species. The Conestogo, Speed and Nith rivers, the Grand River’s major tributaries, meet in its middle reaches. They drain the northwestern, eastern and western sub-basins, respectively. Land cover is primarily agricultural (79%) and woodland (17%). Approximately 136 public and private dams (1–31 m high) have been identified throughout the watershed (W. Yerex, Grand River Conservation Authority, personal communication). This includes eight major dams operated by the Grand River Conservation Authority (GRCA) to control flooding and augment low flows. Flows in the middle and lower parts of Grand River watershed are strongly influenced by the operations of upstream reservoirs.
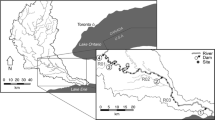
River channel morphology is a function of the quantities of water and sediment introduced into the channel. The physiography of the landscape through which a river flows determines the type of sediments available and establishes the topographic gradient down which the river flows (Church 2002). The Grand River Fisheries Management Plan (Ontario Ministry of Natural Resources and GRCA 1998) identified seven management units based on sub-watersheds and physiographic regions across the watershed. Using the physiographic information provided in the Grand River Fisheries Management Plan, the watershed was divided into four general physiographic regions that reflected differences in sediment and flow characteristics: (1) the upper watershed (upper reaches of the Conestogo, Grand and Nith rivers and Whitemans Creek) which flows through clay to silt/clay till plains and low moraines and displays poor infiltration, flashy flows and low base flows; (2) the middle watershed (middle reaches of the Grand River and the lower reaches of the Conestogo and Nith rivers and Whitemans Creek) characterized by extensive moraines with numerous groundwater sources and higher base flows; (3) the Speed River watershed with its rolling topography, complex mix of sand to sandy/silt till moraines, extensive glacial spillways and localized kame moraines that provide high groundwater input and baseflows, and its bedrock riverbed that limits pool development; and (4), the lower watershed (Grand River downstream of the City of Brantford and MacKenzie and Fairchild creeks) which flows through glaciolacustrine silt and clay deposits with very poor infiltration (Fig. 1).
Distribution of dams (left) and sampling sites (right) throughout the Grand River watershed. Sampling sites in the: (a) Upper Grand physiographic region are represented by white squares; (b) Middle Grand physiographic region by solid black circles; (c) Lower Grand physiographic region by grey circles; and, (d) Speed River sites by grey squares
Fish sampling
During the spring (May–June) and late summer-early fall (late August–early October) of 2002, 2003 and 2004, 151 sites across the Grand River watershed were sampled (Fig. 1). Sampling of lower Grand River sites was limited to the spring as low flows during late summer-early fall prevented sampling by boat electrofisher. Sites were distributed across physiographic regions, different watercourse sizes (1–239 m wide), land-uses (29 sites in urban or settled areas and 122 sites in agricultural areas), sub-watersheds affected by differing levels of river fragmentation (1–42 dams) and river fragment sizes (0.7–129 km). Site selection was not random but instead based on historical collection records, site access, and the distribution of watercourse sizes, land-uses and dams across the watershed. Water temperatures at sites sampled ranged between 6 and 27°C.
Depending on watercourse size, sites were sampled with either a single backpack electrofisher (93 sites), two backpack electrofishing crews working in tandem (44 sites), or a 5 KW pulsed DC boat-mounted electrofisher with a single boom anode and single netter (14 sites). For sites sampled with backpack electrofishers, site length was set at 10 times channel width, with a minimum length of 40 m (Stanfield 2005). Boat electrofishing effort was standardized at 2000 s. Sites typically included pool, riffle and run habitats. Capture probabilities for redhorse using these gear types are unknown. However, past gear type comparisons indicate that the likelihood of capturing redhorse with electrofishing equipment is much higher than with a seine net (Holm and Boehm 1997; Patton et al. 1998). As well, boat electrofishing surveys of Illinois (Retzer and Kowalik 2002) and Ohio (Yoder and Beaumier 1986, Sanders 1992) rivers have been effective at capturing rare redhorse species.
Habitat data collection and analysis
Habitat condition has been shown to influence species composition, abundance and productivity within river segments (Harper and Everard 1998). Therefore, habitat conditions at each site were scored using the Qualitative Habitat Evaluation Index (QHEI), a visual habitat index composed of seven principal metrics (Rankin 1989; Table 1). The QHEI approach was applied because it has been shown to generate scores that are strongly correlated with fisheries assessment data (Rankin 1989; Frimpong et al. 2005; Santucci et al. 2005) and successful in differentiating habitat characteristics at free-flowing and impounded sites (Santucci et al. 2005). Instead of QHEI scores for gradient, channel gradient (m/km) at each site was estimated from topographic maps (1: 50,000 scale). River fragment length and upstream drainage area were calculated using the Ontario Flow Assessment Tool (Version 1.0) (Chang et al. 2002). Geographic co-ordinates of dams were provided by the GRCA. Upstream drainage area was used as a surrogate in the analysis for watercourse size (Morita and Yamamoto 2002). Upstream drainage area was significantly correlated with field channel width measurements (Pearson correlation coefficient, r = 0.9; P < 0.001).
Principal component analysis (PCA) with varimax rotation was used to reduce dimensionality and eliminate collinearity in the habitat data. As variables were measured with different units, variables were standardized by subtracting the mean and dividing by the standard deviation (Legendre and Legendre 1998). Principal components (PC) with eigenvalues greater than 1 and loadings greater than |0.6| were retained for further analysis. PC scores were subsequently used as independent variables in generalized additive models (GAM) to predict species occurrence, and as dependent variables in Multivariate Analysis of Variance (MANOVA) to test for habitat differences among the four physiographic regions. Analysis of variance (ANOVA) and Tukey HSD tests were used to test for differences in individual PCs among physiographic regions.
Distribution modelling
At spatial scales beyond that of most experiments, empirical models provide one of the few options to evaluate how environmental factors influence species occurrence (Manel et al. 2001). Generalized additive models (GAM) were used to evaluate the relative influence of river fragmentation by dams and local habitat on the probability of site occupancy by redhorse species. River fragmentation was characterized using two metrics: (1) the length of each river fragment (a measure of habitat area); and, (2) the number of dams separating the site sampled from the next fragment where species was also captured (a measure of connectivity). Habitat quality at each site was based on PC factor scores. Descriptions of predictor variables are provided in Table 2.
GAMs are similar to logistic regression, but relax the assumption that the relationships between the dependent variable and predictor variables are linear. The relationship is described by estimating a nonparametric smooth function (Knapp and Preisler 1999). Relaxation of the linearity assumption is attractive as a number of shapes for individual relationships are plausible and unlikely to be known before analysis. GAMs are also well suited for examining species patterns across landscapes as spatial co-ordinates can be incorporated into models. Species distribution and habitat data are often spatially autocorrelated (Hinch et al. 1994), which can create problems for statistical tests that assume independence of error terms (Legendre and Legendre 1998). To address the influence of spatial autocorrelation, geographic co-ordinates were included as predictor variables (Preisler et al. 1997).
For each Grand River redhorse species, the specific relationship used for θ i (i.e. logit line) was:
In Eq. 1, s x represents the nonparametric smooth function that characterizes the effect of each independent variable on the probability of response. Regression calculations were done using S-Plus (Mathsoft 2002) and nonparametric functions were estimated using a spline smoothing function.
In multiple regression analysis, multi-collinearity between predictor variables may confound individual effects. Therefore, Pearson correlation coefficients (r) were calculated for all pairwise combinations of predictor variables. Correlation coefficients ranged between −0.6 and 0.5, all well below the recommended cutoff of |r| ≥ 0.85 (Berry and Felman 1985). The best combination of independent variables was determined by evaluating the change in deviance resulting from dropping each variable from the model in the presence of all other variables. The significance of each variable on the probability of occurrence was tested with analysis of deviance and likelihood ratio tests (based on the binomial distribution) (Guisan et al. 2002). Relative importance of significant variables was determined by calculating Akaike Information Criteria (AIC) (Burnham and Anderson 1998; Guisan et al. 2002).
For each species, response curves describing the contribution of the significant predictor variables to the probability of occurrence were developed. Response curves are based on partial residuals standardized to have an average value of 0. The vertical axis of the plots represents the variable contribution of each predictor to the response. Influential variables exhibit a high range of values and values above and below the zero indicate positive or negative association with the dependent variable, respectively (Granadeiro et al. 2004). To illustrate univariate differences between sites with and without each species, mean values (±standard error, SE) were calculated for each of the variables. Differences were tested using a nonparametric Mann-Whitney U test (Sokal and Rohlf 1994). Pearson correlation coefficients (r) were calculated to test if the number of redhorse species at each sampling site was positively correlated with river fragment length and upstream drainage area.
A variance ratio test (Schluter 1984; Taylor 1996) was used to determine if the occurrences of redhorse species covaried among sites sampled. Values that deviate from 1 indicate that species tend to covary positively (greater than 1) or negatively (less than 1). To determine statistical significance, a statistic, W, was calculated and compared to the chi-square distribution. The G-test of Independence was applied to test whether the occurrence of each species was independent of physiographic region (Sokal and Rohlf 1994).
Results
Habitat condition
The first three principal components explained 66.7% of the total variance in habitat condition among all Grand River sites (Table 3). The first axis (PC1) reflected QHEI habitat scores with strong positive loadings for Channel, Pool/Current, Riffle/Run, and Substrate (Table 3). PC2 reflected Gradient and Drainage Area and PC3 had positive loadings for Cover. PC scores were significantly different among the four physiographic regions (MANOVA: Wilk’s Lambda = 0.64, P < 0.0001). Lower Grand sites had significantly lower PC1 scores than Upper Grand and Speed River basin sites (Table 4). Lower Grand sites had significantly higher PC2 scores (lower gradient and larger drainage area) than the other areas, while Speed River basin sites were significantly lower (higher gradient and smaller drainage area) than the other regions.
General patterns in redhorse distribution
Redhorse occurrence and species richness were greatest in the lower Grand River, and declined with increasing distance upstream of Lake Erie (Table 5). The number of redhorse species captured at individual sampling sites was positively correlated to drainage area (r = 0.67; P < 0.0001). The number of redhorse species captured from individual river fragments was positively correlated to river fragment length (r = 0.68, P < 0.0001). River fragment length and drainage area were not correlated (r = 0.27, P = 0.11). Redhorse were absent from the Speed River sub-watershed and the upper reaches of the Conestogo River and Grand River. The Speed River sub-watershed has the highest number of dams in the watershed (n = 42) and poor habitat quality (low PC1 scores). Upper limits on redhorse distribution along the Conestogo and Grand rivers coincided with either a large upstream barrier (Conestogo River: Conestogo Lake dam) or a series of successive upstream barriers (Grand River: the Elora Gorge, multiple dams in the Town of Fergus and the Belwood Lake dam).
Individual redhorse species were captured from 3 to 32% of the sites sampled and from 6 to 26.5% of the 34 river fragments sampled. The most widespread species were golden redhorse and greater redhorse, while river redhorse were only collected downstream of the Caledonia weir along the lower Grand River (Table 5). The distribution of each redhorse species was consistent with recent surveys (since mid-1990s) of the Grand River and its tributaries (Holm and Boehm 1997; Fitzgerald et al. 1998; Wichert and Rapport 1998). The variance ratio test indicated significant positive covariation of species across sites sampled (VR = 3.01; W = 449; P < 0.001).
Black redhorse
Black redhorse were captured at 26% of the sites sampled. Distribution was not independent of physiographic region (G = 34.4, P < 0.0001) and black redhorse were more likely to be captured from sites in the middle of the Grand River watershed (80% of capture sites; Table 5). Sites with black redhorse had significantly higher Pool/Current and Riffle/Run scores, lower gradient, larger drainages, longer fragment lengths and fewer dams between populations (Table 6). The GAM indicates only UTM-E and PC2 scores were important in predicting site occupancy (Table 7). Site occupancy was positively associated with lower gradients and larger drainages (Fig. 2). The concentration of black redhorse sites in the middle watershed is also indicated by the hump-shaped response curve for UTM-E (Fig. 2).
Golden redhorse
Golden redhorse were captured at 30% of the sites sampled. Although its distribution was not independent of physiographic region (G = 19.86, P < 0.001), it was found at similar frequencies (38–47% of sites sampled) in all regions except for the Speed River (Table 5). Sites with golden redhorse had larger drainages, longer fragment lengths, fewer dams between populations and significantly higher scores for all habitat variables except Cover and Riparian (Table 6). The GAM indicates that only PC1 scores were important in predicting site occupancy (Table 7). Occurrence was positively associated with higher Channel, Pool/Current, Riffle/Run and Substrate scores (Fig. 3).
Greater redhorse
Greater redhorse were detected at 32% of the sites sampled and had similar occurrence characteristics as golden redhorse. It was found at 33–46% of sites sampled but was absent from the Speed River (G = 22.56, P < 0.0001). The GAM indicates that only PC2 scores were important in predicting site occupancy (Table 7). Occurrence of greater redhorse was positively associated with lower gradients and higher drainage areas (Fig. 3).
Shorthead redhorse
Shorthead redhorse were captured at 21% of the sites sampled. Its distribution was not independent of physiographic region (G = 23.2, P < 0.0001) as it was more likely to be captured from lower and upper (Nith River only) Grand River sites. Sites with shorthead redhorse had lower gradients, larger drainage areas, larger fragment lengths, fewer dams between populations and significantly higher Pool/Current scores (Table 6). The GAM indicates that river fragment length (FRAG) and PC3 scores were important in predicting site occupancy (Table 7). Occurrence was positively associated with larger river fragments and low Cover scores (Fig. 4).
River redhorse and silver redhorse
River redhorse and silver redhorse were captured at only 3 and 9% of the sites sampled, respectively. For both species, collection sites were characterized by low gradients, large drainage areas and large fragment lengths (Table 6). Due to the low number of capture sites, associations with physiographic regions were not tested, and no GAMs were developed.
Discussion
Blockage of migratory routes by dams and the conversion of free-flowing rivers into reservoirs with poor tailwater habitat have been implicated in the decline of riverine fishes, including redhorse (Patriarche and Campbell 1958; Santucci et al. 2005). In the Grand River watershed, redhorse were absent upstream of major barriers along the upper reaches of the Conestogo River and Grand River and completely absent from the highly fragmented Speed River sub-watershed. Redhorse species richness was also positively correlated to fragment size. However, for individual redhorse species, GAMs only provided support for a fragment size effect on the distribution of shorthead redhorse. For other redhorse species, habitat condition, watercourse size and gradient were more important in determining site occupancy in the Grand River.
Vulnerability to habitat fragmentation can vary among species (Davies et al. 2000). For example, after reservoir creation in Missouri and Arkansas, the size of golden redhorse populations increased, while black redhorse populations either declined or were unaffected (Patriarche and Campbell 1958; Rainwater and Hauser 1982). The lack of effect on all species may also be explained by the type of dams found throughout the Grand River. Past studies, identifying a negative effect on redhorse species, generally investigated large hydro-electric and flood control dams which, in addition to having large upstream impoundments, created downstream tailwater habitats characterized by releases of cold hypolimnetic water and variable flows (Patriarche and Campbell 1958; Eley et al. 1981; Swink and Jacobs 1983; Quinn and Kwak 2003). Peaking based hydro-power generation does not occur in the Grand River watershed and most dams are low head weirs.
By influencing habitat volume and heterogeneity, watercourse size and gradient can account for much of the variation in riverine fish distribution (Quist et al. 2004; Brunger-Lipsey 2005). Watercourse size has also been found to influence the persistence of isolated populations of stream dwelling salmonids (Rieman and McIntyre 1995; Morita and Yamamoto 2002). As well, Santoul et al. (2005) reported the richness of rare fishes within the River Garonne watershed to be positively correlated to stream order. Black redhorse and greater redhorse occurrences in the Grand River were positively associated with lower channel gradients and larger watercourse sizes, and were only captured from sites that were at least 22 m wide and 7 m wide, respectively. The positive relationship between black redhorse occurrence and watercourse size is consistent with Bowman (1970) who reported black redhorse to be more abundant in medium- and large-sized Ozark Highland streams than small ones, and Jenkins (1970) who reported black redhorse to be typically absent from streams less than 3 m wide. Similarly, Becker (1983) described greater redhorse as occurring in medium- to large-sized rivers with adults occasionally occurring in streams as narrow as 5–9 m wide. Watercourse size has also been found to be an important factor influencing the distribution of golden redhorse, shorthead redhorse and silver redhorse, M. anisurum, in Illinois (Larimore and Smith 1963) and Wisconsin (Newall and Magnuson 1999). Curry and Spacie (1984) suggested that watercourse size thresholds associated with catostomid distributions reflect the minimum amount of required spawning habitat. As many redhorse species are pool-dwelling species (Larimore and Smith 1963), small streams may simply not provide suitably-sized pools for these large-bodied fishes.
Although considered less sensitive to habitat degradation than black redhorse or greater redhorse (Trautman 1981), the occurrence of golden redhorse was positively associated with higher quality habitat conditions (i.e. Riffle/Run and Pool/Current scores). As compared to other river bed material, Nelson and Franzin (2000) found golden redhorse along the Assiniboine River, Manitoba to be strongly associated with areas of gravel-cobble and cobble-boulder. In Ohio, Trautman (1981) found golden redhorse to be most abundant in moderately clear, unpolluted streams with large permanent pools and well defined rocky-riffles. As well, Yoder et al. (2005) found the distribution of golden redhorse in Ohio rivers to have increased since improvements to habitat and water quality. Source-sink dynamics could limit the detection of habitat associations for other redhorse species. If sufficiently large, immigration from productive source habitats will result in individuals occurring or even common at presumably unsuitable sites (Pulliam 2000).
It is not clear whether the absence of redhorse species from the Speed River is due to the large number of dams. Exponential increases in the likelihood of population extinction with increasing river fragmentation have been predicted by modelling simulations for white sturgeon (Jager et al. 2001). In this study, a fragment length relationship was identified for shorthead redhorse. Along the Fox River, Illinois, Santucci et al. (2005) found disruptions in fish species distribution (including black redhorse, river redhorse and shorthead redhorse) to primarily occur along the most heavily fragmented reach (8 dams over 22 km). The mean length of river fragments sampled in the Speed River (1 dam/16.8 km) was more reflective of less fragmented reaches of the Fox River (1 dam/15.3 km) that supported greater species diversity. In other parts of the Grand River, mean river fragment length was 30 km, and over 80% of sites sampled were located in river fragments longer than 15 km.
The absence of redhorse species in the Speed River may be related to the influence of physiography on habitat condition. Landscape variation in surficial geology and topography has been linked to local habitat characteristics and the distributions of benthic invertebrates (Richards et al. 1996), freshwater mussels (McCrae et al. 2004), darters (Wang et al. 2003; Reid et al. 2005) and stream salmonids (Nelson et al. 1992; Hicks and Hall 2003). During this study, redhorse were generally captured from deep runs or pools downstream of riffles. The development of pools and overall channel complexity along the Speed River has been limited by bedrock (OMNR and GRCA 1998). The mean QHEI Pool/Current score for Speed River sites was 5.5 out of 12. By contrast, deep runs and pools were more abundant at sites where the channel flowed through less confining tills and moraines (upper and middle Grand regions) or along the lower Grand River where flows were higher. Mean QHEI pool scores for these physiographic regions were higher, ranging between 7.1 and 7.7.
Natural barriers (e.g. waterfalls) resulting from past geologic events have influenced the distribution of Great Lakes basin fishes (Bailey and Smith 1981; Mandrak 1994). Along the Genessee and Trent rivers (two Lake Ontario tributaries), the upstream limits of redhorse occurrence are marked by waterfalls (Carlson and Daniels 2004, S. Reid unpublished data). The upstream extent of redhorse along the Grand River mainstem could be the result of the Elora Gorge limiting post-glacial colonization. Considering that power generation and mill dams are constructed at sites with the greatest hydraulic head (i.e. high gradient reaches or sites with large elevation drops), upstream distribution boundaries at dams along the Conestogo River and Speed River may coincide with historical limits that occurred at natural barriers such as waterfalls.
Occurrence data may not be a sensitive indicator for identifying the effects of river fragmentation. While large declines in redhorse population sizes have been measured after the construction and operation of dams, the negative effect on distribution is less common (Patriarche and Campbell 1958; Eley et al. 1981; Swink and Jacobs 1983; Quinn and Kwak 2003). As well, models based on occurrence data rather than abundance can present functionally different relationships between organisms and environmental variables (Barry and Welsh 2002). As different sampling gears were required in this study, comparable among-site abundance data were not available. The demographic consequences of fragmentation may not always be extirpations. Source-sink dynamics can result, where the distribution of a species across the landscape is maintained through source areas where population growth is positive and sink areas where population growth is below replacement and maintained by immigration (Schlosser and Angermeier 1995). Redhorse in the highly fragmented Trent River, Ontario have been collected from river fragments less than 2 km in length, although spawning runs were only present in larger fragments (S. Reid, unpublished data).
Harding et al. (1998) found that historic land-use and riparian conditions were better predictors of current benthic invertebrate and fish diversity in southern Appalachian Mountain watersheds than current conditions. Fifty years ago, the Grand River was described by Dunham (1945, cited in Plummer et al. 2005) as an, “ineffectual malodorous sewer”. Domestic sewage and industrial discharges were so severe that some reaches contained no game fish (Ontario Department of Planning and Development 1953). The negative impacts of pollution on redhorse have been well documented (Trautman 1981; Jenkins and Burkhead 1993). The absence of redhorse from the Speed River and shorthead redhorse and silver redhorse from parts of the middle Grand River may be the result of historical pollution events combined with dams preventing upstream re-colonization. The disruption of extinction-recolonization dynamics by dams has been used to explain declines of speckled chub, Macrhybopsis aestivalis (Lutterell et al. 1999) and Topeka shiner, Notropis topeka (Schrank et al. 2001). As with most Ontario rivers, historical distribution data are not available and, therefore, hypotheses regarding historical factors cannot be evaluated.
Habitat degradation and migration barriers are considered key threats to black redhorse and river redhorse in the Grand River (Portt et al. 2006), and generally for catostomids in mid-western North America (Cooke et al. 2005). Accordingly, habitat protection, dam removal and the provision of fish passage past barriers are frequently identified as important recovery actions. Unequivocal support for these recovery actions was not provided by this study. Lack of a strong and consistent effect of dams on the distribution of individual redhorse species could reflect the relatively large size of river fragments, fish passage opportunities provided by fish ladders at several dams (Bunt et al. 2001; Reid 2006), dam type, historical and contemporary influences of surficial geology and topography on habitat, or simply a lack of pre-impoundment distribution data.
Changes to the Grand River fish assemblage have been attributed to species introductions, impoundments, pollution and physical habitat degradation (Spence and Hynes 1971; Fitzgerald et al. 1998; Wichert and Rapport 1998; Reid 2005). While efforts to improve water quality have been largely successful (Plummer et al. 2005), challenges persist for aquatic species at risk recovery. The Grand River watershed is projected to be one of the fastest growing areas in Ontario and stresses on threatened fishes and freshwater mussels due to changes in land use, water utilization, sewage disposal and recreational activities are expected to increase (Portt et al. 2006). In addition to habitat protection and restoration, recovery of black redhorse and river redhorse would benefit from investigations on critical habitat requirements and the effects of sport-fishing activities in the Grand River (i.e. incidental harvest of redhorse and stocking of non-native piscivores such as brown trout).
References
Bailey RM, Smith GR (1981) Origin and geography of the fish fauna of the Laurentian Great Lakes. Can J Fish Aquat Sci 38:1539–1561
Barry SC, Welsh AH (2002) Generalized additive modeling and zero inflated count data. Ecol Model 157:179–188
Becker GC (1983) Fishes of Wisconsin. University of Wisconsin Press, Madison
Berry WD, Felman S (1985) Multiple regression in practise. Sage Publications, Beverly Hills, 96 pp
Bowman ML (1970) Life history of the black redhorse, Moxostoma duquesnei (Lesueur) in Missouri. Trans Am Fish Soc 99:546–559
Brunger-Lipsey TS, Hubert WA, Rahel FJ (2005) Relationships of elevation, channel slope, and stream width to occurrences of native fishes at the Great Plains-Rocky Mountains interface. J Freshwater Ecol 20:695–705
Bunt CM, van Poorten BT, Wong L (2001) Denil fishway utilization patterns and passage of several warmwater species relative to seasonal, thermal and hydraulic dynamics. Ecol Freshwater Fish 10:212–219
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer-Verlag, New York, 353 pp
Carlson DM, Daniels RA (2004) Status of fishes in New York: increases, declines and homogenization of watersheds. Am Midland Nat 152:104–139
Chang C, Ashenhurst F, Damaia S, Mann W (2002) Ontario flow assessment techniques, version 1. Ontario Ministry of Natural Resources, Northeast Science and Information. South Porcupine. NESI TM-011, 122 pp
Church M (2002) Geomorphic thresholds in riverine landscapes. Freshwater Biol 47:541–557
Cooke SJ, Bunt CM, Hamilton SJ, Jennings CA, Pearson MP, Cooperman MS, Markle DG (2005) Threats, conservation strategies, and prognosis for suckers (Catostomidae) in North America: insights from regional case studies of a diverse family of non-game fishes. Biol Conserv 121:317–331
COSEWIC (2005a) Canadian species at risk. Committee on the Status of Endangered Wildlife in Canada, Ottawa, 64 pp
COSEWIC (2005b) COSEWIC Assessment and update status report on the black redhorse Moxostoma duquesnei in Canada. http://www.cosewic.gc.ca
Curry KD, Spacie A (1984) Differential use of stream habitat by spawning catostomids. Am Midland Nat 11:267–279
Davies KF, Margules CR, Lawrence JF (2000) Which traits of species predict population declines in experimental forest fragments. Ecology 81:1450–1461
Eley R, Randolph J, Carroll J (1981) A comparison of pre- and post-impoundment fish populations in the Mountain Fork River in southwestern Oklahoma. Proc Oklahoma Acad Sci 61:7–14
Fagan WF (2002) Connectivity, fragmentation and extinction risk in dendritic metapopulations. Ecology 83:3243–3249
Fahrig L, Merriam G (1994) Conservation of fragmented populations. Conserv Biol 8:50–59
Fitzgerald DG, Kott E, Lanno RP, Dixon DG (1998) A quarter century of change in the fish communities of three small streams modified by anthropogenic activities. J Aquat Ecosyst Stress Recovery 6:111–127
Frimpong EA, Sutton TM, Engel BA, Simon TP (2005) Spatial-scale effects on relative importance of physical habitat predictors of stream health. Environ Manage 36:899–917
Granadeiro JP, Andrade J, Palmeirim JM (2004) Modelling the distribution of shorebirds in estuarine areas using generalized additive models. J Sea Res 52:227–240
Guisan A, Edwards TC Jr, Hastie T (2002) Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 157:89–100
Harding JS, Benfield EF, Bolstad PV, Helfman GS, Jones EBD III (1998) Stream biodiversity: the ghost of land use past. Proc Nat Acad Sci 95:14843–14847
Harper D, Everard M (1998) Why should the habitat-level approach underpin holistic river survey and management? Aquat Conserv 8:395–413
Hicks BJ, Hall JD (2003) Rock type and channel gradient structure salmonid populations in the Oregon Coast Range. Trans Am Fish Soc 132:468–482
Hill MF, Hastings A, Botsford LW (2002) The effects of dispersal rates on extinction times in structured metapopulation models. Am Nat 160:389–402
Hinch SG, Somers KM, Collins NC (1994) Spatial autocorrelation and the assessment of habitat-abundance relationships in littoral fish. Can J Fish Aquat Sci 51:701–712
Holm E, Boehm D (1997) Fish sampling in the Grand River 1997. Royal Ontario Museum, Toronto, 24 pp
Hughes MH (1994) The spatial and temporal distribution of fishes in the Holston River system of North Carolina, Tennessee and Virginia. Ph.D Dissertation, The University of Tennessee, Knoxville, 538 pp
Jager HI, Chandler JA, Lepla KB, Van Winkle W (2001) A theoretical study of river fragmentation by dams and its effects on white sturgeon populations. Environ Biol Fish 60:347–361
Jenkins RE (1970) Systematic studies of catostomid fish tribe Moxostomidae. Ph.D Dissertation, Cornell University, Ithaca, 799 pp
Jenkins RE, Burkhead NM (1993) Freshwater fishes of Virginia. American Fisheries Society, Bethesda, 1079 pp
Knapp RA, Preisler HK (1999) Is it possible to predict habitat use by spawning salmonids? A test using California golden trout (Oncorhynchus mykiss aguabonita). Can. J Fish Aquat Sci 56:1576–1584
Larimore RW, Smith PW (1963) The fishes of Champaign County, Illinois, as affected by 60 years of stream changes. Illinois Natural History Survey Bulletin. Volume 28. Article 2. Natural History Survey Division, Urbana, 382 pp
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science BV, Amsterdam, 853 pp
Lutterell GR, Echelle AA, Fischer WL, Eisenhour DJ (1999) Declining status of two species of the Machybopsis aestivalis complex (Teleostei: Cyprinidae) in the Arkansas River basin and related effects of reservoirs as barriers to dispersal. Copeia 1999:981–989
Mandrak NE (1994) Biogeographic patterns in fish species distributions in relation to historical and environmental factors in Ontario lakes and rivers. Ph.D Dissertation, University of Toronto, Toronto, 285 pp
Mandrak NE, Crossman EJ (1992) A checklist of Ontario freshwater fishes. Royal Ontario Museum, Toronto, 176 pp
Manel S, Williams HC, Ormerod SJ (2001) Evaluating presence–absence models in ecology: the need to account for prevalence. J Appl Ecol 38:921–931
Mathsoft (2002) S-Plus 6 for windows user’s guide. Insightful Corporation, Seattle, 987 pp
McCrae SE, Allen JD, Burch JB (2004) Reach- and catchment-scale determinants of the distribution of freshwater mussels (Bivalvia:Unionidae) in south-eastern Michigan USA. Freshwater Biol 48:127–142
Minns CK (2001) Science for freshwater fish habitat management in Canada: current status and future prospects. Aquat Ecosyst Health and Manage 4:423–436
Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv Biol 16:1318–1323
NatureServe (2005) NatureServe Explorer: An online encyclopedia of life. Version 3.0. Available for the Internet URL http:///www.natureserve.org
Nelson PA, Franzin WG (2000) Habitat availability and its utilization by 11 species of fish from the Assiniboine River, Manitoba, with species reference to habitat processes. Canadian Technical Report of Fisheries and Aquatic Sciences, 2313, 55 pp
Nelson RL, Platts WS, Larsen DP, Jansen SE (1992) Trout distribution and habitat in relation to geology and geomorphology in the North Fork Humbolt River drainage, northeastern Nevada. Trans Am Fish Soc 121:405–426
Newall PR, Magnuson JJ (1999) The importance of ecoregion versus drainage area on fish distributions in the St Croix River and its Wisconsin tributaries. Environ Biol Fish 55: 245–254
Ontario Department of Planning and Development (1953) Speed valley conservation report. Ontario Department of Planning and Development, Toronto
Ontario Ministry of Natural Resources & Grand River Conservation Authority (1998) Grand River Fisheries Management Plan, Guelph, 105 pp
Patriarche MH, Campbell RS (1958) The development of the fish population in a new flood control reservoir in Missouri, 1948 to 1954. Trans Am Fish Soc 87:240–258
Patton TM, Rahel FJ, Hubert WA (1998) Using historical data to assess changes in Wyoming’s fish fauna. Conserv Biol 12:1120–1128
Plummer R, Spiers A, Fitzgibbon J, Imhof J (2005) The expanding institutional context for water resources management: the case of the Grand River watershed. Can Water Res J 30:227–244
Portt C, Coker G, Barrett K (2006) Recovery strategy for fish species at risk in the Grand River, Ontario. Draft Recovery Strategy submitted to RENEW Secretariat, 95 pp
Preisler HK, Rappaport NG, Wood DL (1997) Regression methods for spatially correlated data: an example using beetle attacks in a seed orchard. Forest Sci 43:71–77
Pulliam HR (2000) On the relationship between niche and distribution. Ecol Lett 3:349–361
Quinn JW, Kwak TJ (2003) Fish assemblage change in an Ozark River after impoundment: a long-term perspective. Trans Amer Fish Soc 132:110–119
Quist MC, Hubert WA, Rahel FJ (2004) Elevation and stream-size thresholds affect distributions of native and exotic warmwater fishes in Wyoming. J Freshwater Ecol 19:227–236
Rahel FJ (2002) Homogenization of freshwater faunas. Ann Rev Ecol Systemat 33:291–315
Rainwater WC, Houser A (1982) Species composition and biomass of fish in selected coves in Beaver Lake, Arkansas, during the first 18 years of impoundment (1963–1980). North Am J Fish Manage 4:316–425
Rankin ET (1989) The qualitative habitat evaluation index (QHEI): rationale, methods, and application. Ohio Environmental Protection Agency, Columbus, 89 pp
Reid SM (2005) Post-impoundment changes to the Speed River fish assemblage. Can Water Res J 28:183–194
Reid SM (2006) Timing and characteristics of Moxostoma spawning runs in three Great Lakes rivers. J Freshwater Ecol 21:249–258
Reid SM, Carl LM, Lean J (2005) Influence of riffle characteristics, surficial geology, and natural barriers on the distribution of the channel darter, Percina copelandi, in the Lake Ontario basin. Environ Biol Fish 72:241–249
Retzer ME, Kowlaik CR (2002) Recent changes in the distribution of river redhorse, Moxostoma carinatum and greater redhorse, Moxostoma valenciennesi (Cypriniformes: Catostomidae) in Illinois and comments on their natural history. Trans IL State Acad Sci 95:327–333
Richards C, Johnson LB, Host GE (1996) Landscape-influences on stream habitats and biota. Can J Fish Aquat Sci 53 (Supplement 1):295–311
Rieman BE, Dunham JB (2000) Metapopulations and salmonids: a synthesis of life history patterns and empirical observations. Ecol Freshwater Fish 9:51–64
Rieman BE, McIntyre JD (1995) Occurrence of bull trout in naturally fragmented habitat patches of variable size. Trans Am Fish Soc 124:285–296
Sanders RE (1992) Day versus night electrofishing catches from nearshore waters of the Ohio and Muskingum rivers. Ohio J Sci 92:51–59
Santoul F, Figuerola J, Mastrorillo S, Cereghino R (2005) Patterns of rare fish and aquatic insects in a southwestern French river catchment in relation to simple physical habitat variables. Ecography 28:307–314
Santucci VJ, Gephard SR, Pescitelli SM (2005) Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the Fox River, Illinois. North Am J Fish Manage 25:975–992
Schlosser IJ, Angermeier PL (1995) Spatial variation in demographic processes in lotic fishes: conceptual models, empirical evidence, and implications for conservation. In: Neilson JL, Power DM (eds) Evolution and the aquatic ecosystem: defining unique units in population conservation. American Fisheries Society Monograph Series 17, pp 392–401
Schluter D 1984. A variance test for detecting species associations, with some example applications. Ecology 65:998–1005
Schrank SJ, Guy CS, Whiles MR, Brock BL (2001) Influence of instream and landscape-level features on the distribution of Topeka Shiners Notropis topeka in Kansas streams. Copeia 2001:413–421
Scott WB, Crossman EJ (1998) Freshwater fishes of Canada. Galt House Publications Ltd, Oakville, 966 pp
Sokal RR, Rohlf FJ (1994) Biometry, 3rd edn. Freeman WH, and Company, New York, 880 pp
Spence JA, Hynes HBN (1971) Differences in the fish populations upstream and downstream of a mainstem impoundment. J Fish Res Bd Can 28:45–46
Stanfield L (ed) (2005) Ontario stream assessment protocol. Version 7, Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, 256 pp
Swink WD, Jacobs KE (1983) Influence of a Kentucky flood-control reservoir on the tailwater and headwater fish populations. North Am J Fish Manage 3:197–203
Taylor CM (1996) Abundance and distribution within a guild of benthic stream fishes: local processes and regional patterns. Freshwater Biol 36:385–396
Trautman MB (1981) The fishes of Ohio. Ohio State University Press, Columbus, 782 pp
Travnichek VH, Maceina MJ (1994) Comparison of flow regulation effects on fish assemblages in shallow and deep water habitats in the Tallapoosa River, Alabama. J Freshwater Ecol 9:207–216
Wang L, Lyons J, Rasmussen P, Seelbach P, Simon T, Wiley M, Kanehl P, Baker E, Stewart PM (2003) Watershed, reach, and riparian influences on stream fish assemblages in the Northern Lakes and Forest Ecoregion. Can J Fish Aquat Sci 60:491–505
Wichert GA, Rapport DJ (1998) Fish community structure as a measure of degradation and rehabilitation of riparian systems in an agricultural drainage basin. Environ Manage 22:425–443
Winston MR, Taylor CM, Pigg J (1991) Upstream extirpation of four minnow species due to damming of a prairie stream. Trans Am Fish Soc 120:98–105
Yoder CO, Beaumier RA (1986) The occurrence and distribution of river redhorse, Moxostoma carinatum and greater redhorse, Moxostoma valenciennesi in the Sandusky River, Ohio. Ohio J Sci 86:18–21
Yoder CO, Rankin ET, Smith MA, Alsdorf BC, Altfater DJ, Boucher CE, Miltner RJ, Mishne DE, Sanders RE, Thoma RF (2005) Changes in fish assemblage status in Ohio’s nonwadeable rivers and streams over two decades. In: Rinne JN, Hughes RM, Calamusso B (eds) Historical changes in large river fish assemblages of the Americas. American Fisheries Society Monograph Series No 45, pp 399–430
Acknowledgements
The study was supported by the Fisheries and Oceans, Canada, Federal Departmental Recovery Fund, Ontario Ministry of Natural Resources, and Industrial NSERC and Ontario Graduate scholarships awarded to S. Reid. Field sampling was assisted by P. Addison, J. Barnucz, A. Edwards, H. Gignac, N. Koutrilides and J. MacLeod. Comments provided by Al Dextrase and two anonymous reviewers greatly improved earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reid, S.M., Mandrak, N.E., Carl, L.M. et al. Influence of dams and habitat condition on the distribution of redhorse (Moxostoma) species in the Grand River watershed, Ontario. Environ Biol Fish 81, 111–125 (2008). https://doi.org/10.1007/s10641-006-9179-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-006-9179-0