Abstract
Objective
To construct a strain of Corynebacterium glutamicum capable of efficiently producing 5-aminolevulinic acid (5-ALA) via the C4 pathway by modification of serine and glycine pathway using glucose as sole carbon source.
Results
The recombinant C. glutamicum strain AP2 harboring a codon-optimized hemA gene from Rhodobacter sphaeroides was used as host strain for 5-ALA production. A plasmid harboring the serine operon, which contained serB, serC and the site-specific mutant serA Δ197, was constructed and introduced into C. glutamicumAP2, leading to an increase of 70% in 5-ALA production. Further overexpression of the glyA gene increased production of 5-ALA by 150% over the control. 5-ALA production was thus significantly enhanced by engineering the glycine biosynthetic pathway. C.glutamicum AG3 produced 3.4 ± 0.2 g 5-ALA/l in shake-flask cultures in CGIIIM medium with the addition of 7.5 g glycine/l.
Conclusion
This is the first report of remodeling the serine and glycine biosynthetic pathway to improve the production of 5-ALA in C. glutamicum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
5-Aminolevulinic acid (5-ALA) is a common precursor in the biosynthetic pathways of porphyrin, heme and vitamin B12, etc. (Kiatpapan and Murooka 2001). 5-ALA has attracted attention as it can be used in many fields, including as a photosensitizer in medicine, a herbicide or insecticide, as well as improving tolerance to salts and cold in plants (Fu et al. 2008; Kiatpapan and Murooka 2001).
Biological synthesis has been developed into a viable alternative to produce 5-ALA (Fu et al. 2008). There are two major biosynthetic pathways of 5-ALA: the Shemin pathway (C4 pathway) and the C5 pathway. In the C4 pathway, 5-ALA is synthesized via the condensation of succinyl-CoA and glycine, which is catalysed by 5-ALA synthase (Kiatpapan and Murooka 2001) (Fig. 1). As one of the precursors of 5-ALA synthesis, glycine plays an important role in 5-ALA accumulation. The effect of glycine addition on 5-ALA production has been investigated using recombinant Escherichia coli (Chung et al. 2005; Fu et al. 2010; Qin et al. 2006). 5-ALA reached its highest yield when glycine was exhausted (Fu et al. 2008). The same conclusion was obtained in Propionibacterium freudenreichii (Kiatpapan and Murooka 2001). Feng et al. (2016) found that 5-ALA production by Corynebacterium glutamicum increased considerably in the presence of 7.5 g glycine/l. Addition of glycine was necessary in almost all of the reported 5-ALA production strategies that utilise the C4 pathway in different microorganisms.
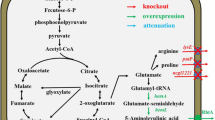
Overview of the relevant pathways and engineering strategies for 5-ALA production via the C4 pathway. Squares indicate genes that were overexpressed and the cross indicates a gene deletion. A codon-optimized hemA was derived from the corresponding Rhodobacter sphaeroides gene. Other non-standard abbreviations: Glu glucose, G3P glyceraldehyde 3-phosphate, PEP phosphoenolpyruvate, Pyr pyruvate, OAA oxaloacetic acid, PGDH 3-phosphoglycerate dehydrogenase, PAST phosphoserine aminotransferase, PSP phosphoserine phosphatase, SHMT serine hydroxymethyltransferase, l-SerDH l-serine dehydratase, P-Hyd phosphohydroxypyruvate, TCA tricarboxylic acid cycle, THF tetrahydrofolate, 5,10-CH 2 -THF 5,10-methylenetetrahydrofolate
In C. glutamicum, 3-phosphoglycerate is oxidized to phosphohydroxypyruvate by phosphoglycerate dehydrogenase (encoded by serA) (Peters-Wendisch et al. 2002). Subsequently, a transamination step catalysed by phosphoserine aminotransferase (encoded by serC) yields phosphoserine, after which phosphoserine is dephosphorylated by phosphoserine phosphatase (encoded by serB) to yield l-serine (Peters-Wendisch et al. 2005). Glycine is then synthesised via the conversion of l-serine by serine hydroxymethyltransferase (encoded by glyA) (Schweitzer et al. 2009; Simic et al. 2002). By contrast, l-serine dehydratase (encoded by sdaA) is involved in l-serine degradation, catalyzing the conversion of l-serine to pyruvate (Hemanshu et al. 2016; Lai et al. 2012; Xu et al. 2015; Zhang et al. 2014) (Fig. 1).
In our previous work, we engineered C. glutamicum AP2 harboring a codon-optimized hemA gene from Rhodobacter sphaeroides for 5-ALA production, in which all known genes responsible for the formation of acetate and lactate were deleted, ppc gene was overexpressed, pbp1b was deleted (Feng et al. 2016). In the present work, we reconstructed a 5-ALA-producing C. glutamicum strain by metabolic engineering of the glycine pathway. The deregulated serA Δ197, serB and serC genes were firstly co-overexpressed in a single operon to increase the flux from serine towards glycine. Secondly, the sdaA gene was deleted to explore its role in 5-ALA accumulation. Thirdly, the glyA gene was overexpressed to validate its effect on 5-ALA production. The final engineered strain AG3 produced 3.4 ± 0.2 g 5-ALA/l in shake-flask cultures with the addition of 7.5 g glycine/l.
Materials and methods
Strains, media and culture conditions
The strains and plasmids used in this study are listed in Supplementary Table 1. Corynebacterium glutamicum ATCC 13,032 was the parent strain and C. glutamicum AP2 (Feng et al. 2016) was used as the chassis for 5-ALA production. E. coli DH 5α was used to propagate vector DNA, and E. coli cells were cultured at 37 °C in lysogeny broth (LB) comprising 10 g tryptone/l, 5 g yeast extract/l and 10 g NaCl/l. Physiological characterizations were tested in defined CGXII medium (Feng et al. 2016; Unthan et al. 2014) supplemented with 10 g glucose/l. The CGXIIIM (CGXII with 7.5 g yeast extract/l) and CGIIIM media, as described by Feng et al. (2016), were used for 5-ALA production in shake-flasks. Where appropriate, 0.5 mM IPTG, 10 μg chloramphenicol/l and/or 25 μg kanamycin sulfate/l was added to the media.
Construction of gene overexpression and deletion vectors
Fragments encodingserA Δ197 , serB and serC were amplified by PCR from C. glutamicum ATCC 13,032 genomic DNA using the primers serA Δ197-F1 and serA Δ197-R1, serB-F1 and seB-R1, serC-F1 and seC-R1, respectively. The amplified fragments were purified and subcloned into the constructed plasmid pXA, creating plasmid pXAAMBC. Sequences upstream and downstream of sdaA were amplified from C. glutamicum genomic DNA using the primers sdaA-F1 and sdaA-R1, sdaA-F2 and sdaA-R2, respectively, and the two fragments were fused, purified and subcloned into pD-SacB, creating the plasmid pD-sdaA. The sdaA gene deletion was carried out as described previously (Zhu et al. 2013). The fragments encoding glyA were amplified from C. glutamicum genomic DNA using the primers glyA-F1 and glyA-R1, PglyA-F1 and PglyA-R1, TglyA-F1 and TglyA-R1, respectively. These amplified fragments were purified and subcloned into the plasmids pEC-XK99E, pEP2 and pEP2tuf, generating the plasmids pEC-glyA, pEP-PglyA and pEP-TglyA. Primers used are listed in Supplementary Table 2. The restriction endonuclease enzyme sites are underlined.
Cultivation conditions
The fermentation conditions were identical to the ones described by Feng et al. (2016). The fermentation experiments were carried out in 500 ml shake-flasks. The pre-seed culture was aseptically transferred into 50 ml of CGIIIM medium with 10 g glucose/l in 500 ml shake-flasks and incubated for 10-12 h at 30 °C under constant orbital shaking at 220 rpm. An initial OD600 value of 0.1 was used to inoculate the fermentation flasks.
Analytical methods
Supernatants were used directly for the determination of extracellular 5-ALA concentrations using modified Ehrlich’s reagent (Feng et al. 2016; Kang et al. 2011). Cell concentrations were determined from the OD600 values: 0.25 g cell dry weight (CDW)/l corresponded to an OD600 value of 1. Glucose concentrations were measured using an SBA sensor machine (SBA-40E, Institute of Microbiology, Shandong, China) (Feng et al. 2016).
Results and discussion
Influence of the overexpression of a deregulated l-serine operon on 5-ALA accumulation
Peters-Wendisch et al. (2002) found that a truncation of the serA gene from C. glutamicum encompassing 197 amino acids at its C-terminus (encoded by the serA 197 allele) provided a 3-phosphoglycerate dehydrogenase devoid of feedback inhibition by l-serine. We thus deregulated the feedback inhibition of 3-phosphoglycerate dehydrogenase by l-serine, by truncating the 197 C-terminal amino acids of serA from C. glutamicum, and investigated whether the co-overexpression of serA Δ197, serB and serC was able to increase 5-ALA accumulation in C. glutamicum. Accordingly, serA Δ197, serB and serC were overexpressed to redirect additional carbon flux into the l-serine biosynthesis pathway, since it is the precursor of glycine, and also to decrease the secretion of by-products (Gu et al. 2014).
Corynebacterium glutamicum AG1, which harbors pXAAMBC, was constructed to test the influence of the deregulated l-serine operon on 5-ALA accumulation. C. glutamicum AG1 produced 220 mg 5-ALA/l of with 10 g glucose/l without the addition of glycine, which was 69% higher than in the control strain AP2 (Fig. 2a), indicating that the co-overexpression of serA Δ197, serB and serC was indeed beneficial for 5-ALA accumulation in C. glutamicum. The observed increase was most likely directly caused by an increase of the intracellular l-serine pool due to the co-overexpression of l-serine synthetic genes (Gu et al. 2014; Peters-Wendisch et al. 2005).
Effects of gene regulation on biomass and 5-ALA production. a Overexpression of the serine operon. b Deletion of sdaA. c Overexpression of glyA. The cells were cultivated in CGXII medium supplemented with 10 g glucose/l. IPTG at 0.5 mM was added into the medium when OD600 reached 1. Standard deviations were calculated from the results of three independent experiments
Effect of sdaA deletion on 5-ALA accumulation
Overexpression of the deregulated l-serine operon clearly increased the accumulation of 5-ALA. As shown in Fig. 1, l-serine was converted by serine hydroxymethyltransferase to generate glycine (Schweitzer et al. 2009). At the same time, l-serine dehydratase converted l-serine to pyruvate, and thus depleted the available l-serine pool (Netzer et al. 2004; Peters-Wendisch et al. 2005). In agreement with this, a deletion of the sdaA gene increased l-serine accumulation (Xu et al. 2015).
Thus, in order to increase the supply of glycine for 5-ALA production, the sdaA gene was knocked out in C. glutamicum AG1, yielding strain AG2. The resulting strain, however, did not exhibit any increase in the accumulation of 5-ALA. On the contrary, the production decreased significantly (Fig. 2b). Furthermore, the final biomass of AG2 decreased by 40% compared to the control strain AG1. This may be due to the fact that a knockout of sdaA can slightly increase l-serine production in C.glutamicum (Gu et al. 2014; Netzer et al. 2004). Nevertheless, the deletion of sdaA can markedly decrease the pyruvate concentration (Gu et al. 2014). Thus, a deletion of sdaA showed a negative effect on the growth of AG2 (Fig. 2b), which is in agreement with the literature (Zhu et al. 2013). Therefore, the sdaA gene was not deleted in further C. glutamicum strains engineered for 5-ALA production.
Influence of glyA overexpression on 5-ALA accumulation
Since glycine is one of the precursors of 5-ALA, its supply of glycine is vital for the accumulation of 5-ALA. Schweitzer et al. (2009) concluded that the overexpression of glyA is essential to catalyse sufficient conversion of l-serine to glycine (Schweitzer et al. 2009). We thus overexpressed glyA to investigate whether it can improve 5-ALA production in C. glutamicum.
Consequently, the C. glutamicum strain AG3 and the corresponding control strain AG4, which harbor pEC-glyA and pEC-XK99E, respectively, were constructed based on AG1. To our delight, 5-ALA production of AG3 reached 590 mg/l, which was 2.5 times of the control. Importantly, the overexpression of glyA also had no effect on growth, and the biomass of the engineered strain was almost at the same level as the control (Fig. 2c). These results thus clearly demonstrate that the overexpression of glyA is beneficial for 5-ALA accumulation.
In order to investigate whether the synthesis of serine or the further glyA reaction is the limiting step for 5-ALA production in C. glutamicum, glyA was overexpressed with different promoters and different concentrations of serine were added to the medium for 5-ALA production. The results showed that 5-ALA production by strain AG5 was increased by nearly 60% over that of AG6 with overexpression of glyA under control of strong promoter tuf. The accumulation of 5-ALA was decreased when serine was added (data not shown). These results imply that the glyA reaction rather than synthesis of serine maybe the limiting step in glycine synthesizing pathway for 5-ALA production. However, the enzyme activity of SHMT should be controlled in an appropriate rang, not the more the better, and the excess serine addition can lead to cellular toxicity.
Glycine is the key factor of 5-ALA production
5-ALA production was significantly increased by engineering the glycine biosynthesis pathway. Nevertheless, the available glycine pool still seemed insufficient for high-level production of 5-ALA. In our previous work, the effect of glycine addition was investigated, and the 5-ALA production of AP2 reached 2.7 g/l in CGIIIM medium with 7.5 g glycine/l. However, the production of 5-ALA was not as high as expected in C. glutamicum AG3, which contains the engineered glycine biosynthetic pathway. We therefore studied how further, external supplementation of glycine would affect 5-ALA production in the engineered strain C. glutamicum AG3.
As shown in Fig. 3, supplementation of 7.5 g glycine/l was still optimal for the production of 5-ALA (Feng et al. 2016). The engineered strain C. glutamicum AG3 produced 3.4 ± 0.2 g 5-ALA/l with the addition of 7.5 g glycine/l in CGIIIM (Fig. 3a). This, to our knowledge, represents the highest yield obtained in C. glutamicum shake-flask cultures to date. Furthermore, C. glutamicum AG3 produced 2.6 ± 0.1 g 5-ALA/l with 5 g glycine/l, which was almost the same level as was achieved by AP2 with 7.5 g glycine/l (Feng et al. 2016). In contrast to the results in CGIIIM (Fig. 3a), C. glutamicum AG3 produced much more 5-ALA than the control strain when grown in CGXIIIM with different concentrations of glycine (Fig. 3b). These results thus imply that the supply of glycine is critical for 5-ALA production via the C4 pathway.
Relationship between glycine addition and 5-ALA production in CGIIIM (a) and CGXIIIM (b) medium. The media were supplemented with 10 g glucose/l and the indicated amounts of glycine. A final concentration of 0.5 mM IPTG was added to the medium when the OD600 reached 1. Standard deviations were calculated from the results of three independent experiment
Conclusions
A 5-ALA producing C. glutamicum strain was constructed by metabolic engineering of the glycine biosynthesis pathway. The final strain produced 3.4 ± 0.2 g 5-ALA/l in 50 ml flask fermentations with the addition of 7.5 g glycine/l, which to our best knowledge represents the highest yield obtained in C. glutamicum shake-flasks to date. Furthermore, we confirmed that the supply of glycine is the most critical factor for 5-ALA production in C. glutamicum via the C4 pathway. Consequently, further metabolic strategies should be employed to enhance the intracellular glycine pool, in order to enhance 5-ALA production and reduce the necessary supplementation of this relatively expensive precursor.
References
Chung S-Y, Seo K-H, Rhee JI (2005) Influence of culture conditions on the production of extra-cellular 5-aminolevulinic acid (ALA) by recombinant E. coli. Proc Biochem 40:385–394
Feng L, Zhang Y, Fu J, Mao Y, Chen T, Zhao X, Wang Z (2016) Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Biotechnol Bioeng 113:1284–1293
Fu W, Lin J, Cen P (2008) Enhancement of 5-aminolevulinate production with recombinant Escherichia coli using batch and fed-batch culture system. Bioresour Technol 99:4864–4870
Fu W, Lin J, Cen P (2010) Expression of a hemA gene from Agrobacterium radiobacter in a rare codon optimizing Escherichia coli for improving 5-aminolevulinate production. Appl Biochem Biotechnol 160:456–466
Gu P, Yang F, Su T, Li F, Li Y, Qi Q (2014) Construction of an l-serine producing Escherichia coli via metabolic engineering. J Ind Microbiol Biotechnol 41:1443–1450
Hemanshu M, Konstantin S, Hanne BC, Alex TN (2016) Engineering of high yield production of l-serine in Escherichia coli. Biotechnol Bioeng 113:807–816
Kang Z, Wang Y, Gu P, Wang Q, Qi Q (2011) Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab Eng 13:492–498
Kiatpapan P, Murooka Y (2001) Construction of an expression vector for propionibacteria and its use in production of 5-aminolevulinic acid by Propionibacterium freudenreichii. Appl Microbiol Biotechnol 56:144–149
Lai S, Zhang Y, Liu S, Liang Y, Shang X, Chai X, Wen T (2012) Metabolic engineering and flux analysis of Corynebacterium glutamicum for l-serine production. Sci China Life Sci 55:283–290
Ma W, Wang X, Mao Y, Wang Z, Chen T, Zhao X (2015) Development of a markerless gene replacement system in Corynebacterium glutamicum using upp as a counter-selection marker. Biotechnol Lett 37:609–617
Netzer R, Peters-Wendisch P, Eggeling L, Sahm H (2004) Cometabolism of a nongrowth substrate: l-serine utilization by Corynebacterium glutamicum. Appl Environ Microbiol 70:7148–7155
Peters-Wendisch P, Netzer R, Eggeling L, Sahm H (2002) 3-Phosphoglycerate dehydrogenase from Corynebacterium glutamicum: the C-terminal domain is not essential for activity but is required for inhibition by l-serine. Appl Microbiol Biotechnol 60:437–441
Peters-Wendisch PS, Etterich H, Kennerknecht N, Sahm H, Eggeling L (2005) Metabolic engineering of Corynebacterium glutamicum for l-serine production. Appl Environ Microbiol 71:7139–7144
Pittard YZJPAHA (1994) Molecular analysis and characterization of a broad-host-range plasmid, pEP2. J Bacteriol 176:5718–5728
Qin G, Lin J, Liu X, Cen P (2006) Effects of medium composition on production of 5-aminolevulinic acid by recombinant Escherichia coli. J Biosci Bioeng 102:316–322
Schweitzer JE, Stolz M, Diesveld R, Etterich H, Eggeling L (2009) The serine hydroxymethyltransferase gene glyA in Corynebacterium glutamicum is controlled by GlyR. J Biotechnol 139:214–221
Simic P, Willuhn J, Sahm H, Eggeling L (2002) Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase l-threonine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68:3321–3327
Unthan S et al (2014) Beyond growth rate 0.6: what drives Corynebacterium glutamicum to higher growth rates in defined medium. Biotechnol Bioeng 111:359–371
Xu G et al (2015) Enhanced production of l-serine by deleting sdaA combined with modifying and overexpressing serA in a mutant of Corynebacterium glutamicum SYPS-062 from sucrose. Biochem Eng J 103:60–67
Zhang X, Xu G, Li H, Dou W, Xu H, Xu Z (2014) Effect of cofactor folate on the growth of Corynebacterium glutamicum SYPS-062 and l-serine accumulation. Appl Biochem Biotechnol 173:1607–1617
Zhu N, Xia H, Wang Z, Zhao X, Chen T (2013) Engineering of acetate recycling and citrate synthase to improve aerobic succinate production in Corynebacterium glutamicum. PLoS ONE 8:e60659
Acknowledgements
This work was supported by the Natural Science Foundation of Tianjin (No. 15JCQNJC06000).
Supporting information
Supplementary Table 1—Strains and plasmids used.
Supplementary Table 2—Primers used.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zou, Y., Chen, T., Feng, L. et al. Enhancement of 5-aminolevulinic acid production by metabolic engineering of the glycine biosynthesis pathway in Corynebacterium glutamicum . Biotechnol Lett 39, 1369–1374 (2017). https://doi.org/10.1007/s10529-017-2362-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2362-x