Abstract
5-Aminolevulinic acid (ALA) is a non-protein amino acid with a significant potential for cancer treatment and plant stress resistance. Microbial fermentation has gradually replaced the traditional chemical-based method for ALA production, thus increasing the need for high-ALA-producing strains. In this study, we engineered the glutamate producing strain, Corynebacterium glutamicum S9114, for ALA production. To efficiently convert l-glutamate to ALA, hemA and hemL from Salmonella typhimurium and Escherichia coli were tandemly overexpressed. In addition, ncgl1221 encoding a glutamate transporter was deleted to block glutamate secretion and thus improve ALA production. Furthermore, the intrinsic ribosome-binding site (RBS) of hemB was replaced by a relatively weak RBS to reduce the conversion of ALA to porphyrin. Transcriptional and fermentation data confirmed that inactivation of lysE and putP reduced the conversion of glutamate to arginine and proline, which also contribute to ALA production. The final SA14 strain produced 895 mg/L concentration of ALA after 72 h incubation in a shake flask. This amount was 58-fold higher than that obtained by the parent strain C. glutamicum S9114. The results demonstrate the potential of C. glutamicum S9114 for efficient ALA production and provide new targets for the development of ALA-producing strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corynebacterium glutamicum is an aerobic gram-positive actinomycete that secretes a large amount of glutamate in the absence of biotin or the presence of penicillin (Becker and Wittmann 2016). It is used for the long-term production of various amino acids such as glutamate, arginine, lysine, ornithine, and alanine (Wieschalka et al. 2013), diamines such as putrescine (Nguyen et al. 2015) and cadaverine (Mimitsuka et al. 2007), dicarboxylic acids such as succinate (Chung et al. 2017; Jo et al. 2017), diols such as 1,3-propanediol (Huang et al. 2017) and 1,2-propanediol (Siebert and Wendisch 2015), and terpenes such as pinene and carotenoid (Heider et al. 2012; Kang et al. 2014). In China, C. glutamicum S9114, a subspecies of C. glutamicum derived from Brevibacterium tianjinese T6–13, has been widely used for glutamate fermentation (Mei et al. 2016). To emphasize the importance of the microbe in glutamate production, its complete genome sequence was published in 2011 (Lv et al. 2011). Compared with other C. glutamicum strains, C. glutamicum S9114 secretes a large amount of glutamate, is resistant to high sugar content, and shows rapid growth, indicating that it is an ideal host for the production of glutamate-related compounds (Zhang et al. 2005).
5-Aminolevulinic acid (ALA), an intermediate metabolite produced during tetrapyrrole biosynthesis, has become noteworthy because of its efficient application in photodynamic therapy (Tetard et al. 2016) and as a photosensitizer in the photodynamic diagnosis for cancer patients (Ishikawa et al. 2015; Kennedy et al. 1990). Low ALA concentrations are used in agriculture to increase the tolerance of plants to low temperature and high salt concentrations (Akram and Ashraf 2013). However, the industrial scale production of ALA depends mainly on chemical synthesis methods that are unsustainable.
Recently, research attention has turned to microbial fermentation, which is an environmentally safe, economical, and sustainable method. Microbes such as Rhodobacter sphaeroides (Sasaki et al. 2002), Escherichia coli (Kang et al. 2011), and C. glutamicum (Yu et al. 2016) have been engineered to produce ALA. The biosynthesis of ALA occurs via the C4 and C5 pathways. In the C4 pathway, ALA is produced from succinyl-CoA and glycine, in a reaction that is catalyzed by the gene products of hemA from R. sphaeroides. Recently, a recombinant C. glutamicum strain was constructed by overexpressing codon-optimized hemA from R. sphaeroides, disrupting all known genes involved in acetate and lactate synthesis, overexpressing endogenous genes encoding phosphoenolpyruvate carboxylase and RhtA from E. coli, and by inactivating high-molecular weight penicillin-binding proteins (Feng et al. 2015). This recombinant C. glutamicum strain produced an ALA titer of 7.53 g/L in the presence of appropriate glycine and succinate concentrations in a 5-L bioreactor. Another study reported the engineering of a strain derived from C. glutamicum ATCC 13032 by overexpressing a codon-optimized hemA from R. capsulatus SB1003, inactivating sucCD genes, and overexpressing rhtA from E. coli. The engineered strain produced 14.7 g/L concentration of ALA in a fed-batch fermentation culture through the C4 pathway (Yang et al. 2016). However, the requirements for glycine and succinate as precursors in the fermentation medium in the C4 pathway have prompted researchers to shift attention to the C5 pathway, which is considered a potential pathway for the direct production of ALA from glucose. In the C5 pathway, ALA is produced from glutamate through two reactions catalyzed by enzymes that are the gene products of endogenous hemA and hemL. A mutant C. glutamicum strain was developed by constitutively coexpressing hemA and hemL, in which the addition of penicillin and baffled flask fermentation under optimized conditions resulted in an ALA titer of 2.2 g/L through the C5 pathway (Ramzi et al. 2015). At the same time, other researchers reported the construction of a recombinant C. glutamicum strain derived from the model strain C. glutamicum ATCC 13032 by the inducible coexpression of hemA and hemL (Yu et al. 2015). By optimizing dissolved oxygen and Fe2+ concentrations, the researchers attained an ALA yield of 1.79 g/L after 144 h of incubation in a shake flask.
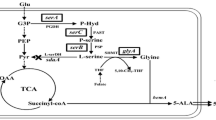
In this study, we developed a new metabolically engineered C. glutamicum S9114 strain by overexpressing hemA with different synthetic ribosome-binding sites (RBSs) by replacing the original RBS of hemB to inhibit the conversion of ALA to porphyrin, and by disrupting arginine and proline transport systems to inhibit a competing metabolic pathway, which improved ALA production (Fig. 1). The present results indicate an efficient strategy for the development of ALA-producing industrial strains.
Metabolic pathways associated with 5-ALA biosynthesis in C. glutamicum and the metabolic engineering strategies for 5-ALA overproduction. The genes that are involved are: hemA, encoding glutamyl-tRNA reductase; hemL, encoding glutamate-1-semialdehyde aminotransferase; hemB, encoding 5-aminolevulinic acid dehydratase; rhtA, encoding gene encoding inner membrane transporter for L-threonine; ncgl1221, encoding glutamate transporter; lysE, encoding lysine/arginine transporter; and putP, encoding L-proline transporter
Materials and methods
Strains and culture conditions
The strains and plasmids used in this study are listed in Table 1. E. coli DH5α was used for routine cloning procedures. C. glutamicum S9114, origin to C. glutamicum CICC 20935 accessible in China Center of Industrial Culture Collection (CICC), was used as the parent strain to develop the engineered strains. The suicide vector pK18mobsacB containing the sucrose screening marker gene, sacB, from Bacillus subtilis was used for markerless gene deletion, insertion, and RBS replacement through double crossover recombination, as described previously (Xu et al. 2014). E. coli/C. glutamicum shuttle expression vector pXMJ19 was used to overexpress hemA and hemL (Jakoby et al. 1999). The C. glutamicum host strain was transformed with the plasmid using a method we previously described (ZHANG et al. 2015a). Luria Bertani medium containing an appropriate concentration of antibiotic (12.5 µg/mL chloromycetin or 30 µg/mL kanamycin) was used for bacterial growth and plasmid construction. C. glutamicum was cultured in brain heart infusion (BHI) broth and terrific broth (TB) as previously described (Ramzi et al. 2015), with some modification. For shake flask cultivation, each recombinant strain was inoculated in 10 mL BHI broth in a 100-mL unbaffled flask and was cultured for 14 h at 30 °C and 200 rpm. The pre-culture was inoculated in 25 mL TB (containing 12 g tryptone, 24 g yeast extract, 4 mL glycerol, 2.31 g KH2PO4, 12.54 g K2HPO4, and 40 g glucose per liter of water) in a normal 250-mL flask to an optical density at 600 nm (OD600) of 0.3. The pH of the culture medium was adjusted to 7.0. ALA production in shake flasks was performed at 30 °C and 200 rpm for 72 h.
Construction of plasmids and strains
To construct recombinant expression vectors of pXMJ19-SB-hemA, hemA encoding glutamyl-tRNA reductase from S. typhimurium LT2 was amplified by polymerase chain reaction (PCR). To maintain the stability of glutamyl-tRNA reductase, two lysine residues (KK) were inserted into three and four amino acid residues of hemA using specific primers (Ramzi et al. 2015). Synthetic RBSs with different translation initiation strengths were designed using the RBS Calculator (Tian and Salis 2015) https://www.denovodna.com/software/doLogin and were added upstream of hemA using specific primers. Sequences of the synthetic RBSs are listed in Additional file 1: Table S1. PCR products were purified, digested, and inserted into multiple cloning sites of pXMJ19. For the coexpression of hemA and hemL (the latter encodes glutamate-1-semialdehyde aminotransferase), hemL from E. coli DH5ɑ was amplified, inserted at the end of hemA by overlap PCR, and cloned into pXMJ19 by enzyme digestion and ligation. All the enzymes used for constructing the recombinant plasmid are purchased from TakaRa Bio (Shiga, Japan).
For gene deletion, we used the recombinant plasmids pK18-△ncgl1221, pK18-△lysE, and pK18-△putP, which we previously constructed (Zhang et al. 2017a, b). For the chromosome RBS change of hemB, an RBS sequence was inserted into an overlapping region between the upstream and downstream fragments of hemB using rationally designed primers. Plasmids pK18-hemB100 and pK18hemB500 were constructed by standardized molecular cloning. To integrate rhtA into the ncgl1221 region of C. glutamicum, rhtA was amplified from the genomic DNA of E. coli DH5ɑ and was inserted into plasmid pK18-△ncgl1221 using the Gibson assembly to generate plasmid pK18-△ncgl1221-rhtA. All the recombinant plasmids were transformed by electroporation into engineered C. glutamicum S9114-derived strains. The correct mutants were obtained after two rounds of homologous recombination. Randomly chosen strains from the second round of recombination were confirmed by colony PCR. All the primers are listed in Additional file 1: Table S2.
RT-PCR analysis
For RNA extraction, 500 µL fermentation samples were collected at 12 h. Total RNA was extracted using an RNAprep Pure Cell/Bacteria Kit (Tiangen Biotech Co., Ltd., Beijing, China). RNA integrity was analyzed by 1% agarose gel electrophoresis, and the RNA concentration was determined using a microplate reader (BioTek Instruments, Winooski, VT, USA). Reverse transcription was performed using PrimeScript RT reagent kit with gDNA Eraser (TaKaRa Bio). RT-PCR analysis was performed accordingly as we previously described (Yao and Ye 2015).
Enzyme assay of ALA dehydratase (ALAD)
For ALAD enzyme activity detection, cells were collected after 12 h of cultivation. Disposition of samples and the enzymatic reaction system have been previously described (Sassa 1982; Zhang et al. 2015c).
Analytical procedures
Cell growth was monitored by measuring OD600 with a microplate reader. Fermentation supernatant was filtered a 0.22-µm filter. Glucose, glutamate, and lactate concentrations were analyzed in the filtered sample using a model SBA-40C biosensor (Biology Institute of Shandong Academy of Sciences, Shandong, China). ALA concentration in the culture medium was measured as described previously using a modified Ehrlich reagent (Fu et al. 2007) and using a microplate reader at 554 nm.
Results and discussion
Expression levels of hemA and hemL affect ALA production
In previous study, expression of mutant glutamyl-tRNA reductase from S. typhimurium resulted in the highest ALA yield in C. glutamicum ATCC 13032 through the C5 pathway (Ramzi et al. 2015). In the present study, a medium copy number plasmid pXMJ19 with an isopropyl β-D-1-thiogalactopyranoside inducible tac promoter was used to express this enzyme in C. glutamicum S9114 and generate mutant strains having increased ALA production. RBS optimization is an efficient synthetic biological tool that has become widely used recently for pathway engineering, such as the optimization of metabolic flux for shikimic acid production (Zhang et al. 2015b), succinic acid production (Wang et al. 2015), and mevalonate-based farnesyl pyrophosphate biosynthesis (Nowroozi et al. 2014). To improve the ALA yield, four types of synthetic RBSs with gradient translation initiation strength were designed using the RBS calculator and inserted in the upstream of hemA to generate strains SA1, SA2, SA3, and SA4. SA3 produced the most ALA of all the strains (126 mg/L) during shake flask fermentation (Fig. 2a). Mutant SA7 strain coexpressing hemA and hemL produced 509 mg/L of ALA (Fig. 2b), which was approximately 33-fold higher than that obtained by C. glutamicum S9114. The significant improvement of ALA production observed in the present study can be directly attributed to the heterologous expression of S. typhimurium hemA and E. coli hemL. The results are consistent with the previous observation that the overexpression of hemA and hemL lead to high ALA production (Ramzi et al. 2015). Interestingly, the SA7 strain with an RBS having a predicted translation initiation strength of 57705.62 au inserted in front of hemA showed the highest ALA production, which revealed that insertion of disparate RBS in front of hemA greatly affects the biosynthesis of ALA. Compared with the constitutive expression of hemA and hemL in C. glutamicum ATCC 13032 (457 mg/L), a higher concentration of ALA was produced by optimizing RBS of hemA in C. glutamicum S9114.
Influence of different expression of hemA alone and in combination with hemL on ALA production during shake flask cultivations in C. glutamicum S9114. a ALA production by the sole expression of hemA with disparate RBS. SA1, predicted RBS of 5341.55 au; SA2, predicted RBS of 25210.78 au; SA3, predicted RBS of 57705.62 au; SA4, predicted RBS of 580091.25 au. b ALA production by coexpression of hemA and hemL. Results of standard deviations present in three individual experiments
Improvement of precursor accumulation by deleting ncgl1221 for ALA production
Glutamate is a direct precursor of ALA biosynthesis through the C5 pathway, indicating that accumulation of glutamate is a key factor for high ALA production. Ncgl1221 encodes a membrane protein that transports glutamate, and inactivation of ncgl1221 drastically decreases glutamate secretion by damaging the transportation system (Chen et al. 2015). Moreover, deletion of ncgl1221 reportedly increases l-arginine production in C. crenatum and C. glutamicum (Chen et al. 2015; Park et al. 2014). To provide more glutamate for ALA synthesis, ncgl1221 was deleted in the SA7 strain to produce a mutant strain, SA9. Shake flask fermentation was performed to evaluate the effect of ncgl1221 disruption on ALA production. The deletion of ncgl1221 did not affect the growth and glucose consumption by SA9 (Fig. 3a). SA9 produced approximately 616 mg/L of ALA in 72 h (Fig. 3b), which was 9.8% higher than that the ALA yield of 561 mg/L produced by the control strain, SA7. Glutamate concentration in the supernatant was decreased by approximately 43.7% by ncgl1221 deletion, confirming that Ncgl1221 plays a key role in glutamate secretion (Fig. 3c). However, strains SA7 and SA9 produced lactate because of the presence of low oxygen concentration. Therefore, we conclude that inactivation of ncgl1221 contributes slightly to ALA production. The results also demonstrate that in vivo glutamate accumulation affected ALA production. But, the improved titer of ALA was relatively lower than the reduction of glutamate, indicating that glutamate is not the only precursor of ALA in C. glutamicum. In addition to the previous study using penicillin and specific culture (Ramzi et al. 2015), this study provides a genetic strategy in which blocking of the glutamate transport system can improve glutamate accumulation, which in turn improves ALA production.
Effect of ncgl1221 deletion on ALA production and cell growth during shake flask cultivations. a The growth of SA7 and SA9 (SA7 with ncgl1221 deletion); b L-ornithine curves with temporal change. c The residual glucose, glutamate, and lactate concentration at 72 h. Results of standard deviations present in three individual experiments. Compared with the control group, **p < 0.01
ALA production is enhanced by inhibiting its conversion
ALAD, which is encoded by hemB, is the first enzyme involved in the conversion of ALA to porphyrin. ALA production can be improved using ALAD inhibitors such as d-glucose, levulinic acid, phthalic acid, and maleic acid and using genetically engineered HemB, which is obtained by adding a degradation ASV tag at the C terminus of HemB, to reduce the ALAD reaction (Yu et al. 2015). To inhibit ALA conversion to prophyrin, we were initially unsuccessful in our attempts to disrupt hemB, as the deletion of the gene resulted in poor cellular viability. We hypothesize that hemB might play a crucial role in cellular growth. Thus, we lowered the expression level of hemB by replacing its native RBS with a lower one. The translation initiation strength of the original hemB RBS was 5557.35 au, as predicted using the RBS Calculator (https://www.denovodna.com/software/doLogin). Therefore, two designed RBSs with translation initiation intensity of 92.52 and 553.57 au were designed using the RBS Calculator. They were used to replace the native RBS in the SA9 strain to produce strains SA10 and SA11. Shake flask fermentation was performed to evaluate the effect of RBS replacement on ALA production. Compared with the SA9 parent strain, the relative ALAD specific activity of strains SA10 and SA11 were reduced to 47 and 69% (see Table 2). After 72 h of incubation, the recombinant strains SA10 and SA11 produced approximately 827 and 730 mg/L ALA, respectively (Fig. 4b), which was 34.2 and 18.5% higher than that (616 mg/L) obtained using the control SA9 strain. The growth rate of the SA11 strain was similar to that of the parent SA9 strain (Fig. 4a). However, the growth of the SA10 strain was slightly affected, probably due to the low expression level of HemB. These results indicate that attenuation of hemB using a synthetic RBS promotes ALA production. These results also confirmed that ALAD regulation is a restrictive factor for enhancing ALA production, which is consistent with a previous study (Yu et al. 2015). Compared with the inhibition of ALAD achieved by addition of a degradation tag, the attenuated expression of ALAD via RBS replacement provides more strategic options based on the insertion of RBSs with differing strengths of translation initiation.
ALA production and cell growth in engineered strains with attenuation of hemB. a The growth of SA9, SA10 (SA9 with a change in hemB RBS100), and SA11 (SA9 with a change in hemB RBS500). b ALA concentration in fermentation supernatant. Results of standard deviations present in three individual experiments. Compared with the control group, *p < 0.05; **p < 0.01
Effect of transport system modification on ALA production
Since glutamate is also converted to proline and arginine through multistep enzymatic reactions, proline and arginine are considered as competing metabolic by-products and are unfavorable for ALA biosynthesis. We inactivated LysE and PutP, two membrane proteins involved in the transportation of arginine and proline, respectively (Lubitz et al. 2016; Peter et al. 1997), to preserve the glutamate precursor, which promoted intracellular arginine and proline accumulation, and then inhibited its synthesis by feedback regulation to generate mutant strains SA12 and SA13. SA12 and SA13 produced approximately 718 and 734 mg/L of ALA, respectively (Fig. 5b), which was 16.5 and 19.1% higher than the 616 mg/L generated by the control strain, SA9. The growth was not affected by deletion of the two genes, consistent with the previous finding that lysE and putP were not necessary for cell growth (Dong et al. 2016). In addition, the expression levels of argB and argJ involved in arginine synthesis in strain SA12 were decreased to approximately half of the levels in strain SA9 (Fig. 5c). Moreover, the transcription level of proC, which encodes pyrroline-5-carboxylate reductase in the last step of proline synthesis, decreased by approximately 30% in strain SA13 (Fig. 5d). These results indicate that deletion of the transport proteins LysE and PutP promotes ALA production. In combination with the attenuation of hemB, recombinant strain SA14 was constructed. This strain produced 895 mg/L ALA, which was 45% higher than the 616 mg/L obtained by the control strain, SA9. These results confirmed that hemB attenuation and deletion of lysE synergistically enhanced ALA synthesis.
Effect of modified transport system on cell growth and ALA production in engineered C. glutamicum S9114. a Growth of SA9, SA12 (SA9 with deletion of lysE), SA13 (SA12 with deletion of putP), SA14 (SA13 with the replacement of hemB100), and SA15 (SA14 with the insertion of rhtA in the site of ncgl1221) strains. b 5-ALA production by SA9, SA12, SA13, SA14, and SA15 strains. c Comparison of the transcription levels of L-arginine biosynthesis genes in SA9 and SA12 strains. d Comparison of proC transcription level in SA12 and SA13 strains. Samples were collected at 12 h for transcription level analysis. Results of standard deviations present in three individual experiments. Compared with the control group, *p < 0.05; **p < 0.01
The transport protein RhtA from E. coli was demonstrated to be necessary for the transport of ALA, with no other protein having been identified (Feng et al. 2015). To further improve the yield of ALA, rhtA was introduced into the chromosome of strain SA14 to generate strain SA15. During shake flask fermentation, SA15 produced 872 mg/L ALA in the fermentation supernatant, which was lower than the production by SA14. Overexpression of rhtA did not exert a positive effect on the yield of ALA, possibly because the transport system is not a rate-limiting step. ALA production after the genetic modifications is still low compared with the highest level (2.2 g/L) that has been obtained (Ramzi et al. 2015). We anticipate that strategies such as the addition of penicillin or levulinic acid, or optimization of the fermentation conditions will further increase ALA production. This optimism is based on the demonstration of the success of such strategies in promoting ALA accumulation in other C. glutamicum, which is obviously appropriate for strain SA14 (Yu et al. 2015).
Conclusion
We successfully reconstructed the pathway involved in producing ALA from glucose to engineer an industrially-relevant, high-glutamate producing C. glutamicum S9114 strains for improved ALA production. This study provides some efficient strategies such as the optimized synthetic RBS of hemA, deletion of ncgl1221, lysE, and putP, and downregulated expression of hemB by RBS replacement, which could improve the fermentative production of ALA from renewable resources such as glucose. The present study is the first report of the combined application of those targets and their synergistic effect on ALA production, which will inform the development of ALA-producing strains. Additional genetic engineering and optimization of fermentation are needed to further increase ALA yield.
Abbreviations
- RBS:
-
Ribosome-binding sites
- ALA:
-
5-Aminolevulinic acid
- ALAD:
-
5-Aminolevulinic acid dehydratase
References
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Becker J, Wittmann C (2016) Industrial Biotechnology: Microorganisms. In: Industrial Microorganisms: Corynebacterium glutamicum, chap 6. Wiley‐VCH Verlag GmbH & Co. KGaA, pp 183–220
Chen M, Chen X, Wan F, Zhang B, Chen J, Xiong Y (2015) Effect of Tween 40 and DtsR1 on l-arginine overproduction in Corynebacterium crenatum. Microb Cell Fact 14:119
Chung S-C, Park J-S, Yun J, Park JH (2017) Improvement of succinate production by release of end-product inhibition in Corynebacterium glutamicum. Metab Eng 40:157–164
Dong X, Zhao Y, Hu J, Li Y, Wang X (2016) Attenuating l-lysine production by deletion of ddh and lysE and their effect on l-threonine and l-isoleucine production in Corynebacterium glutamicum. Enzyme Microb Technol 93–94:70–78. https://doi.org/10.1016/j.enzmictec.2016.07.013
Feng L, Zhang Y, Fu J, Mao Y, Chen T, Zhao X, Wang Z (2015) Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Biotechnol Bioeng 113:1284–1293
Fu W, Lin J, Cen P (2007) 5-Aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl Microbiol Biotechnol 75:777–782
Heider SA, Peters-Wendisch P, Wendisch VF (2012) Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol 12:198. https://doi.org/10.1186/1471-2180-12-198
Huang J, Wu Y, Wu W, Zhang Y, Liu D, Chen Z (2017) Cofactor recycling for co-production of 1, 3-propanediol and glutamate by metabolically engineered Corynebacterium glutamicum. Sci Rep 7:42246
Ishikawa T, Kajimoto Y, Inoue Y, Ikegami Y, Kuroiwa T (2015) Chapter seven-critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv Cancer Res 125:197–216
Jakoby M, Ngouoto-Nkili C-E, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Tech 13:437–441
Jo S, Yoon J, Lee S-M, Um Y, Han SO, Woo HM (2017) Modular pathway engineering of Corynebacterium glutamicum to improve xylose utilization and succinate production. J Biotechnol 258:69–78
Kang Z, Wang Y, Gu P, Wang Q, Qi Q (2011) Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab Eng 13:492–498
Kang M-K, Eom J-H, Kim Y, Um Y, Woo HM (2014) Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum. Biotechnol Lett 36:2069–2077
Kennedy JC, Pottier RH, Pross DC (1990) Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B 6:143–148
Lubitz D, Jorge JM, Pérez-García F, Taniguchi H, Wendisch VF (2016) Roles of export genes cgmA and lysE for the production of l-arginine and l-citrulline by Corynebacterium glutamicum. Appl Microbiol Biotechnol 100:8465–8474
Lv Y, Wu Z, Han S, Lin Y, Zheng S (2011) Genome sequence of Corynebacterium glutamicum S9114, a strain for industrial production of glutamate. J Bacteriol 193:6096–6097. https://doi.org/10.1128/JB.06074-11
Mei J, Xu N, Ye C, Liu L, Wu J (2016) Reconstruction and analysis of a genome-scale metabolic network of Corynebacterium glutamicum S9114. Gene 575:615–622
Mimitsuka T, Sawai H, Hatsu M, Yamada K (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci Biotechnol Biochem 71:2130–2135. https://doi.org/10.1271/bbb.60699
Nguyen AQ, Schneider J, Reddy GK, Wendisch VF (2015) Fermentative production of the diamine putrescine: system metabolic engineering of Corynebacterium glutamicum. Metabolites 5:211–231
Nowroozi FF, Baidoo EE, Ermakov S, Redding-Johanson AM, Batth TS, Petzold CJ, Keasling JD (2014) Metabolic pathway optimization using ribosome binding site variants and combinatorial gene assembly. Appl Microbiol Biotechnol 98:1567–1581
Park SH, Kim HU, Kim TY, Park JS, Kim S-S, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for l-arginine production. Nat Commun 5:4618
Peter H, Bader A, Burkovski A, Lambert C, Krämer R (1997) Isolation of the putP gene of Corynebacterium glutamicum and characterization of a low-affinity uptake system for compatible solutes. Arch Microbiol 168:143–151
Ramzi AB, Hyeon JE, Kim SW, Park C, Han SO (2015) 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway. Enzyme Microb Technol 81:1–7. https://doi.org/10.1016/j.enzmictec.2015.07.004
Sasaki K, Watanabe M, Tanaka T (2002) Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl Microbiol Biotechnol 58:23–29
Sassa S (1982) Delta-aminolevulinic acid dehydratase assay. Enzyme 28:133–145
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Siebert D, Wendisch VF (2015) Metabolic pathway engineering for production of 1, 2-propanediol and 1-propanol by Corynebacterium glutamicum. Biotechnol Biofuels 8:91
Tetard M-C et al (2016) Interstitial 5-ALA photodynamic therapy and glioblastoma: preclinical model development and preliminary results. Photodiagn Photodyn Ther 13:218–224
Tian T, Salis HM (2015) A predictive biophysical model of translational coupling to coordinate and control protein expression in bacterial operons. Nucleic Acids Res 43:7137–7151
Wang J et al (2015) Fine-tuning of ecaA and pepc gene expression increases succinic acid production in Escherichia coli. Appl Microbiol Biotechnol 99:8575–8586
Wieschalka S, Blombach B, Bott M, Eikmanns BJ (2013) Bio-based production of organic acids with Corynebacterium glutamicum. Microb Biotechnol 6:87–102
Xu J, Xia X, Zhang J, Guo Y, Qian H, Zhang W (2014) A method for gene amplification and simultaneous deletion in Corynebacterium glutamicum genome without any genetic markers. Plasmid 72:9–17
Yang P, Liu W, Cheng X, Wang J, Wang Q, Qi Q (2016) A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield. Appl Environ Microbiol 82:2709–2717
Yao LL, Ye BC (2015) Reciprocal regulation of GlnR and PhoP in response to nitrogen and phosphate limitations in Saccharopolyspora erythraea. Appl Environ Microbiol 82:409–420. https://doi.org/10.1128/AEM.02960-15
Yu X, Jin H, Liu W, Wang Q, Qi Q (2015) Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb Cell Fact 14:183
Yu X, Jin H, Cheng X, Wang Q, Qi Q (2016) Transcriptomic analysis for elucidating the physiological effects of 5-aminolevulinic acid accumulation on Corynebacterium glutamicum. Microbiol Res 192:292–299
Zhang C, Shi Z, Gao P, Duan Z, Mao Z (2005) On-line prediction of products concentrations in glutamate fermentation using metabolic network model and linear programming. Biochem Eng J 25:99–108
ZHANG B, Fang W, QIU YL, CHEN XL, Li T, CHEN JC, XIONG YH (2015a) Increased l-arginine production by site-directed mutagenesis of N-acetyl-l-glutamate kinase and proB gene deletion in Corynebacterium crenatum. Biomed Environ Sci 28:864–874
Zhang B, Zhou N, Liu Y-M, Liu C, Lou C-B, Jiang C-Y, Liu S-J (2015b) Ribosome binding site libraries and pathway modules for shikimic acid synthesis with Corynebacterium glutamicum. Microb Cell Fact 14:71
Zhang J, Kang Z, Chen J, Du G (2015c) Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci Rep 5:8584. https://doi.org/10.1038/srep08584
Zhang B, Ren L-Q, Yu M, Zhou Y, Ye B-C (2017a) Enhanced l-ornithine production by systematic manipulation of l-ornithine metabolism in engineered Corynebacterium glutamicum S9114. Biores Technol. https://doi.org/10.1016/j.biortech.2017.11.017
Zhang B, Yu M, Zhou Y, Li Y, Ye B (2017b) Systematic pathway engineering of Corynebacterium glutamicum S9114 for l-ornithine production. Microb Cell Fact 16:158
Acknowledgements
We thank Dr. Andreas Burkovski and Dr. Xue-Lan Chen for providing pXMJ19 and pk18mobsacB. Moreover, we thank Dr. Zhong-Gui Mao and Dr. Li-Ming Liu for providing C. glutamicum S9114 strains. This study was supported by China NSF 21575089 and 21335003 and the National Key Technologies R&D Programs (2014AA021502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, B., Ye, BC. Pathway engineering in Corynebacterium glutamicum S9114 for 5-aminolevulinic acid production. 3 Biotech 8, 247 (2018). https://doi.org/10.1007/s13205-018-1267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1267-2