Abstract
Aromatic ketones were reduced using suspension culture of Chlorella sp. MK201 under fluorescent light illumination producing the corresponding chiral alcohols in high yields with excellent enantiomeric excess (ee). For example, 2′,3′,4′,5′,6′-pentafluoroacetophenone at 0.25 mg/ml was converted to the corresponding (S)-alcohol in 80 % yield with >99 % ee by 1 mg dry wt of Chlorella/ml in 12 h illumination (2,000 lux).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorella sp. MK201 that grew under high atmospheric CO2 at a high temperature has been isolated in Japan (Muranaka and Murakami 2001). The Chlorella was registered as an official strain at the Research Institute of Innovative Technology for the Earth (RITE), and is considered useful for adsorbing and removing CO2 in high CO2 concentrations under high temperature such as exhaust gas from thermal power stations.
Microbes such as yeast and fungi are biocatalysts that reduce various ketones to the corresponding optically active alcohols (Matsuda et al. 2009). We reported that acetophenone derivatives and 3-acetylisoxazoles were reduced by photosynthetic microalgae such as Synechococcus elongatus PCC 7942 and Synechocystis sp. PCC 6803 (Nakamura et al. 2000, Nakamura and Yamanaka 2002a, b, Itoh et al. 2005), to the corresponding chiral alcohols in excellent ee. Chiral secondary alcohols are widely used as synthetic intermediates in organic chemistry, for instance, (S)-1-(2-chlorophenyl)ethanol or (S)-1-(2-trifluoromethylphenyl)ethanol are transformed into heteroaryl-linked 5-(1H-benzimidazol-1-yl)-2-thiophenecarboxamides as potent inhibitors of polo-like kinase 1 (PLK1) (Rheault et al. 2010).
Herein, we report an efficient asymmetric reduction of acetophenone derivatives using Chlorella sp. MK201 under fluorescent light illumination. We propose a synthetic method that affords useful chiral secondary alcohols by asymmetric reduction with a biocatalyst that can be prepared only for inorganic materials including CO2.
Materials and methods
Chemicals
The substrates (ketones), ethyl acetate and other chemicals used here were obtained from Aldrich Chemical Co. Inc., Wako Pure Chemical Industries, Ltd., and Tokyo Chemical Industry Co. Inc.
Cultivation
The cultivation of Chlorella sp. MK201 was performed in MC+ liquid medium (3,750 mg KNO3/l, 750 mg KH2PO4/l, 500 mg MgSO4·7H2O/l, 20 mg FeSO4·7H2O/l, 2.86 mg H3BO3/l, 2.5 mg MnSO4·4H2O/l, 0.222 mg ZnSO4·7H2O/l, 0.079 mg CuSO4·5H2O/l and 0.021 mg Na2MoO4/l, see Muranaka and Murakami 2001) under continuous illumination provided by fluorescent lamps (MITSUBISHI/OSRAM FL40SW, 2000 lux) with air-bubbling at 25 °C.
The cultivation of S. elongates PCC 7942 and Synechocystis sp. PCC 6803 were performed in BG-11 liquid medium under continuous illumination provided by fluorescent lamps (MITSUBISHI/OSRAM FL40SW, 2,000 lux) with air-bubbling at 25 °C.
These cultures were being used as growing cells.
Asymmetric reduction of acetophenone derivatives using Chlorella sp. MK201
The cultivated suspension culture of Chlorella sp. MK201 for nine days and MC+ liquid medium were mixed to make the suspension culture of Chlorella (OD730 = 1). In this suspension, dry wt of Chlorella was obtained in 1 mg/ml. Acetophenone derivative in DMSO (50 μl) was added to the suspension culture (6 ml). The mixture was shaken at 120 rpm at 25 °C under fluorescent light illumination (2,000 lux). After the reaction, n-dodecane (10 μl) was added. The resulting mixture was extracted with ethyl acetate (5 ml). The extract was washed with saturated aqueous sodium chloride solution (2 ml). After drying with anhydrous sodium sulfate, the chemical yield was determined by GC analysis using n-dodecane as the internal hydrocarbon standard (GC column: DB-1, 25 m). The enantiomeric purities were determined by GC analysis based on GC area ratio (GC column: CP-cyclodextrin-B-2,3,6-M-19 [CPCD], 25 m). The retention times of ketones, (R)-alcohols and (S)-alcohols in GC analysis were referred to the literature (Nakamura and Matsuda 1998).
Asymmetric reduction of acetophenone derivatives using S. elongates PCC 7942 or Synechocystis sp. PCC 6803
The cultivated suspension culture of S. elongates PCC 7942 or Synechocystis sp. PCC 6803 for nine days and BG-11 liquid medium were mixed to make the suspension culture of S. elongates PCC 7942 or Synechocystis sp. PCC 6803 (OD750 = 2). In these suspensions, dry wt of S. elongates PCC 7942 or Synechocystis sp. PCC 6803 were obtained in 0.6 mg/ml, respectively. After undergoing the same procedures in the Chlorella sp. MK201, the chemical yields and ee were determined by GC analysis.
Results and discussion
The growth and the reductive reactivity of Chlorella sp. MK201 were tested using 2,2,2-trifluoroacetophenone 1a. When 1a at 0.5 mg/ml was added to the suspension culture of Chlorella prepared from the cultivated Chlorella suspension for nine days and shaken for 18 h in the light, the corresponding alcohol (R)-1b was obtained in 84 % yield (97 % ee) (Fig. 1). The reproducibility of this reduction produced in 83.5 ± 2.5 % chemical yields and 96.5 ± 0.5 % ee. In the cultivation for 15 days, the reductive reactivity of Chlorella (reaction time: 24 h) was gradually lowered to 76 % yield (95 % ee) (Supplementary Table 1).
Asymmetric reduction of 2,2,2-trifluoroacetophenone 1a by Chlorella sp. MK201: cultivation time (9 days); the starting concentration of the substrate (0.5 mg/ml); suspension culture (6 ml); dry wt of Chlorella (1 mg/ml); shaking 120 rpm at 25 °C under fluorescent light illumination (2,000 lux) for 18 h; chemical yield and ee were determined by GC analysis
Based on these results, the optimum reaction conditions were investigated. The reaction of 1a at 0.5 mg/ml using Chlorella for 18 h produced (R)-1b in 86 % yield (97 % ee). However, the chemical yield and enantiomeric purities were decreased by increasing the amount of 1 (1.5 mg/ml, reaction time: 48 h) into 31 % yield (82 % ee) (Supplementary Table 2).
The reaction using Chlorella was performed under dark conditions. The reaction of 1a for 24 h in the dark decreased the chemical yield of (R)-1b into 37 % yield (93 % ee). When Chlorella pre-cultured under dark conditions for 24 h was used and the reaction was conducted under darkness, the chemical yield was decreased further into 22 % yield (87 % ee) (Supplementary Table 3). The results confirmed that the light conditions were necessary in the present asymmetric reduction system.
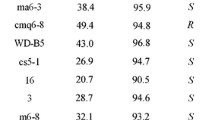
To apply the present proposed asymmetric reduction to other substrates, the reaction was performed with several acetophenone derivatives 2a−14a (Fig. 2), and the results are summarized in Table 1 (Supplementary Table 4). All ketones were transformed into the corresponding alcohols 2b−14b and produced (S)-alcohols in excellent ee. Among them, the reaction of 6a−11a obtained the corresponding alcohols in high yield (Table 1, Runs 6−11). In the case of para-substituted acetophenones, Chlorella effectively reduced acetophenone derivatives including the electron-withdrawing group into the corresponding (S)-alcohol compared with the electron-donating group (Table 1, Runs 3, 4, 7, 9, 10 and 12). In fluoro-substituted acetophenones, ortho-substituted acetophenone was reduced into chiral alcohol in a short time (Table 1, Runs 4−6), and increasing the number of substituents accelerated the reduction (Table 1, Run 11).
More recently, Yang et al reported the comparison of a reduction with cyanobacteria and chlorella (Yang et al. 2012). Therefore, the reactions of 1a, 6a, 10a and 11a using S. elongates PCC 7942 or Synechocystis sp. PCC 6803 were tested (Table 2). The results show that the reductive reactivity of Chlorella sp. MK201 in acetophenone derivatives was higher than S. elongates PCC 7942 or Synechocystis sp. PCC 6803. Therefore, these results show that the biotransformation using Chlorella sp. MK201 was widely and effectively used in the asymmetric reduction of acetophenone derivatives.
In conclusion, the present investigation revealed the potency of Chlorella in the asymmetric reduction of acetophenone derivatives to provide the corresponding alcohols. In particular, since Chlorella sp. MK201 grows under high atmospheric CO2 at a high temperature, this reaction was demonstrated that an efficient material production by the indirect availability of CO2.
References
Itoh K, Sakamaki H, Nakamura K, Horiuchi CA (2005) Biocatalytic asymmetric reduction of 3-acetylisoxazoles. Tetrahedron Asymmetr 16:1403–1408
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetr 20:513–557
Muranaka T, Murakami M (2001) CO2 fixation by a high temperature high CO2 tolerant Chlorella sp. In: Lee YK, Kojima H (eds) Photosynthetic microorganisms in environmental biotechnology. Springer Verlag, New york, pp 77–86
Nakamura K, Matsuda T (1998) Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum. J Org Chem 63:8957–8964
Nakamura K, Yamanaka R (2002a) Light mediated cofactor recycling system in biocatalytic asymmetric reduction of ketones. Chem Commun 2002:1782–1783
Nakamura K, Yamanaka R (2002b) Light-mediated regulation of asymmetric reduction of ketones by a cyanobacterium. Tetrahedron Asymmetr 13:2529–2533
Nakamura K, Yamanaka R, Tohi K, Hamada H (2000) Cyanobacterium-catalyzed asymmetric reduction of ketones. Tetrahedron Lett 41:6799–6802
Rheault TR, Donaldson KH, Badiang-Alberti JG, Davis-Ward RG, Andrew CW, Bambal R, Jachson JR, Cheung M (2010) Heteroaryl-linked 5-(1H-benzimidazol-1-yl)-2-thiophenecarboxamides: potent inhibitors of polo-like kinase (PLK1) with improved drug-like properties. Bioorg Med Chem Lett 20:4587–4592
Yang ZH, Luo L, Chang X, Zhou W, Chen GH, Zhao Y, Wang YJ (2012) Production of chiral alcohols from prochiral ketones by microalgal photo-biocatalytic asymmetric reduction reaction. J Ind Microbiol Biotechnol 39:835–841
Acknowledgments
This work was financially supported by the ‘Nihon University College of Science and Technology Symbolic Project on State-of-the-Art Research Initiatives’.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Itoh, Ki., Nakamura, K., Aoyama, T. et al. Photobiocatalyzed asymmetric reduction of ketones using Chlorella sp. MK201. Biotechnol Lett 34, 2083–2086 (2012). https://doi.org/10.1007/s10529-012-1008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1008-2