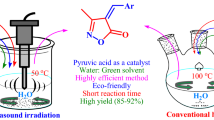
Abstract
An efficient, green and facile route for the one-pot four-component synthesis of pyranopyrazole derivatives through the condensation of the aromatic aldehydes, ethyl acetoacetate, malononitrile and hydrazine hydrate using baker’s yeast as biocatalyst is presented. The important aspects of the present methodology are the use of an environmentally friendly catalyst, high yield of products and mild reaction conditions, i.e. at neutral pH and ambient temperature.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pyranopyrazole derivatives are fused heterocyclic compounds, having various biological properties such as antifungal, antibacterial, antimicrobial, analgesic, antipyretic, anti-inflammatory, vasodilator, antioxidant and anticancer [1,2,3,4,5,6,7,8]. They are also very useful as agrochemical and molluscicidal agents, and some derivatives are also used as precursors of cosmetics and pigments [9,10,11,12].
The first pyranopyrazole was reported in 1973 by the reaction between 3-methyl-1-phenyl-pyrazole-5-one and tetracynomethylene [13]. The 2-amino-4-substituted pyrano-[2,3-c]pyrazol-3-carbonitriles derivatives were obtained in 1974 by the addition of malononitrile on 4-arylidene-2-pyrazolin-5-one [14]. Afterwards, many other synthetic methods to synthesize these compounds were documented [15]. Those schemes include a one-pot four-component cyclocondensation reaction of aromatic aldehydes, ethyl acetoacetate, malononitrile and hydrazine hydrate [16,17,18] which is more convenient than multistep reactions. Later on, multicomponent reactions (MCR) came into the limelight over usual multistep synthesis because of atom economy, energy and cost efficiency and less reaction time. Therefore, they became a more facile route for organic synthesis, and nowadays, heterocyclic compounds are synthesized by MCR methods [19,20,21].
In the recent past, the pyranopyrazoles are synthesized using different catalysts including d, l-proline [22], bleaching earth clay (pH 12.5) [23], cetyltrimethylammonium chloride (CTACl) [24], aspirin [25], sulphonic acid-functionalized ionic liquid [26], glycine [27], [bmim]OH [28], meglumine [29], boric acid [30], Ph3CCl [31], choline chloride/thiourea [32], l-proline [33], piperidine [34], maltose [35] and choline chloride–urea deep eutectic solvent-modified magnetic nanoparticles [36]. Since most of these catalysts are having one or other kinds of drawbacks such as the requirement of high energy, generation of waste and tedious work set-up and some catalysts are costly and hazardous too, the development of greener method for the synthesis of pyranopyrazoles is still desired. The organic transformation which is directed towards “Greenery” arouses always attention from many years [37].
Baker’s yeast is easily available, is inexpensive biocatalyst and can be used without any special kind of expertise in microbiology [38, 39]. This is the reason why baker’s yeast got a lot of attention among synthetic organic chemists. Later on, it is considered as a microbial reagent for organic synthesis [40, 41]. The synthesis of alcohols by the reduction of a variety of ketone using baker’s yeast is a very well-established technology for organic transformation [42]. It has been also used for the production of bioethanol by immobilising it over sodium alginate gel, commercially [43]. Besides redox reactions, it is also found to catalyse useful cyclocondensation, leading to value-added heterocycles [44,45,46]. Enantioselective synthesis of various organic molecules using baker’s yeast is a well-established method in synthetic organic chemistry [47,48,49].
Most of the baker’s yeast-catalysed organic reactions have been operated mainly in an aqueous medium to protect its catalytic activity. An aqueous medium is not practicable for many organic reactions due to the solubility issue of the substrates; therefore, biocatalysis using an organic solvent is gaining much more attention. So, the main advantages of using biocatalyst in an organic medium are higher solubility of organic compounds and easy recovery of the product [50, 51].
Keeping the above facts in mind, we have designed a one-pot four-component synthesis of pyranopyrazole derivatives using aromatic aldehydes, ethyl acetoacetate, malononitrile, hydrazine hydrate and easily available, cheaper biocatalyst, i.e. baker’s yeast. According to the best of our knowledge, this is the first endeavour to use baker’s yeast as a biocatalyst for this multicomponent reaction.
Experimental section
General information
All required chemicals were purchased from commercial suppliers and used as such without any further purification. The dry baker’s yeast was purchased from A. B. Mauri India Pvt. Ltd, India. The 1H NMR and 13C NMR spectra of compounds were determined by using a Bruker Avance II spectrometer at 400 MHz and 100 MHz, respectively, using DMSO-d6 as a solvent. Thin-layer chromatography (TLC) was carried out on silica gel-precoated aluminium-backed plates and visualized under UV light.
General procedure for the synthesis of pyranopyrazoles derivatives (5a)
A mixture of various aromatic aldehydes (5 mmol), malononitrile (5 mmol), hydrazine hydrate (5 mmol) and ethyl acetoacetate (5 mmol) was taken with ethanol (20 mL) as a solvent in a round-bottom flask (50 mL), and then, baker’s yeast (2 g) was added to the reaction mixture. The resulting reaction mass was stirred at room temperature on a magnetic stirrer, and the procession of the reaction is monitored using thin-layer chromatography (TLC) in a solvent system having n-hexane/ethyl acetate (3:1). After 34 h of constant stirring at room temperature, the reaction mass was filtered under reduced pressure using silica bed to remove the catalyst and washed it with ethanol (50 mL). The crude products were purified by recrystallization in ethanol and by column chromatography using n-hexane and ethyl acetate.
Spectral data of some representative compounds
6-Amino-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5a)
M.P. 210–212 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.00 (1H, s), 7.30 (2H, t, J = 7.56), 7.22 (3H, m, J = 7.28 and 11.24), 6.71 (2H, s), 4.50 (1H, s), 1.79 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.35, 155.26, 144.93, 136.04, 128.91, 127.94, 127.20, 121.24, 98.13, 57.71, 36.73, 10.20. MS (EI):m/z Calcd. for C14H12N4O = 252.10. Found = 253.15 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-(4-nitrophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (5b)
M.P. 249–250 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.19 (1H, s), 8.21 (2H, m, J = 8.8 Hz), 7.47 (2H, m, J = 8 Hz), 7.10 (2H, s), 4.82 (1H, s), 1.79 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.06, 154.58, 152.01, 146.30, 135.86, 129.52, 128.77, 124.07, 120.45, 96.48, 55.80, 35.79, 9.66. MS:m/z Calcd. for C14H11N5O3 = 297.09. Found = 298.14 [M+ + 1].
6-Amino-4-(4-chlorophenyl)-1,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (5c)
M.P. 228–230 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.12 (1H, s), 7.38 (2H, m, J = 11.2 Hz), 7.21 (2H, m, J = 11.2 Hz), 6.90 (2H, s), 4.63 (1H, s), 1.79 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 160.84, 154.61, 143.38, 135.68, 132.49, 131.19, 129.96, 128.39, 120.62, 97.12, 56.68, 18.45, 9.66. MS:m/z Calcd. for C14H11ClN4O = 286.06. Found = 287.10 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-p-tolylpyrano[2,3-c]pyrazole-5-carbonitrile (5d)
M.P. 207–208 °C . 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.19 (1H, s), 8.21 (2H, m, J = 8.8 Hz), 7.47 (2H, m, J = 8.8 Hz), 7.03 (2H, s), 4.82 (1H, s), 3.32 (3H, s), 1.79 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.06, 154.58, 152.02, 146.30, 135.84, 128.51, 128.77, 123.83, 120.45, 96.48, 55.80, 35.80, 9.66. MS:m/z Calcd. for C15H14N4O = 266.12. Found = 267.14 [M+ + 1].
6-Amino-1,4-dihydro-4-(4-methoxyphenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (5e)
M.P. 209–210 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.08 (1H, s), 7.10 (2H, m, J = 8 Hz), 6.89 (2H, m, J = 4 Hz), 6.82 (2H, s), 4.55 (1H, s), 3.74 (3H, s), 1.80 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.16, 158.45, 155.24, 136.96, 136.02, 128.96, 121.28, 114.25, 98.37, 58.15, 55.48, 35.93, 10.21. MS:m/z Calcd. for C15H14N4O2 = 282.12. Found = 283.16 [M+ + 1].
6-Amino-4-(4-(dimethylamino)phenyl)-1,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (5f)
M.P. 234–235 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 11.13 (1H,s), 7.21-6.96 (4H, m, J = 12 Hz), 6.69 (2H, s), 4.64 (1H, s), 2.52 (6H, s), 1.97 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 160.59, 148.91, 129.44, 128.05, 124.75, 124.08, 121.28, 115.99, 105.45, 55.61, 29.16, 10.36. MS:m/z Calcd. for C16H17N5O = 295.14. Found = 296.15 [M+ + 1].
6-Amino-4-(2-chlorophenyl)-1,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (5g)
M.P. 244–245 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.13 (1H, s), 7.44 (1H, m, J = 12 Hz), 7.33 (1H, m, J = 4 Hz), 7.29 (1H, s), 7.20 (1H, m, J = 4 Hz), 6.95 (2H, s) 5.08 (1H, s), 1.78 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.78, 155.45, 141.41, 135.85, 132.45, 131.19, 129.96, 128.24, 120.85, 97.34, 56.26, 9.99. MS:m/z Calcd. for C14H11ClN4O = 286.06. Found = 287.10 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-(3-nitrophenyl)pyrano[2,3-c]pyrazole-5-carbonitrile (5h)
M.P. 214–215 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.19 (1H, s), 8.13 (1H, m, J = 10.4 Hz), 8.02 (1H, m, J = 1.2 Hz), 7.63 (2H, s), 7.03 (1H, s), 4.87 (1H, s), 1.80 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.07, 154.61, 147.80, 146.74, 135.88, 134.34, 130.19, 121.94, 121.77, 120.49, 96.59, 56.04, 35.55, 9.68. MS:m/z Calcd. for C14H11N5O3 = 297.09. Found = 298.13 [M+ + 1].
6-Amino-4-(2-chloroquinolin-3-yl)-1,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile (5i)
M.P. 216–217 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.19 (1H, s), 8.40 (1H,s), 8.07 (1H, m, J = 8 Hz), 7.98 (1H, m, J = 4 Hz), 7.84 (1H, m, J = 12 Hz), 7.68 (1H, m, J = 12 Hz), 7.08 (2H, s), 5.21(1H, s), 1.79 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.99, 155.57, 149.61, 146.67, 140.00, 136.05, 131.31, 128.43, 127.99, 120.88, 10.16. MS:m/z Calcd. for C17H12ClN5O = 337.07. Found = 338.70 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-(4-trifluoromethyl)pyrano[2,3-c]pyrazole-5-carbonitrile (5j)
M.P. 249–250 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.18 (1H, s), 7.72 (2H, d, J = 4 Hz), 7.43 (2H, d, J = 8 Hz), 7.00 (2H, s), 4.77 (1H, s), 1.81 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.55, 155.21, 149.63, 136.22, 128.83, 128.02, 127.77, 125.97, 123.70, 121.04, 97.37, 56.86, 36.44, 10.21. MS:m/z Calcd. for C15H11F3N4O = 320.09. Found = 321.14 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-(4-fluoro)pyrano[2,3-c]pyrazole-5-carbonitrile (5k)
M.P. 216–218 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 12.12 (1H, s), 7.98 (1H. m, J = 4 Hz), 7.39 (1H, t, J = 8 Hz), 7.23 (1H, t, J = 8 Hz), 7.17 (1H, t, J = 8 Hz), 6.90 (2H, s), 4.65 (1H, s), 1.80 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.31, 160.87, 155.20, 136.10, 131.20, 129.85, 116.64, 116.47, 115.75, 99115.56, 97.98, 57.61, 35.93, 10.19. MS:m/z Calcd. for C14H11FN4O = 270.09. Found = 271.14 [M+ + 1].
6-Amino-1,4-dihydro-3-methyl-4-(2,3,4-trimethoxy)pyrano[2,3-c]pyrazole-5-carbonitrile (5l)
M.P. 196–198 °C. 1H NMR—(400 MHz, DMSOd6)—δ ppm 11.99 (1H, s), 6.79-6.73 (4H, m), 4.75 (1H, s), 3.78 (3H, s), 3.75 (3H, s), 3.68 (3H, s), 1.80 (3H, s). 13C NMR—(100 MHz, DMSOd6)—δ ppm 161.53, 155.45,152.56, 151.58, 141.93, 135.53, 130.16, 123.84, 121.50, 108.52, 98.49, 61.31, 60.69, 57.39, 56.19, 30.97, 9.92. MS:m/z Calcd. for C17H18N4O4 = 342.13. Found = 343.15 [M+ + 1].
Results and discussion
We have initiated our study by considering the reaction of benzaldehyde, malononitrile, ethyl acetoacetate and hydrazine hydrate in ethanol as our model reaction using baker’s yeast as a catalyst (Scheme 1).
The selection of solvent is the first and foremost step for a multicomponent reaction, especially for biocatalytic reactions. So, at first, we observed the effect of solvent in our model reaction. We carried out our reaction in some protic and aprotic solvents in the presence of baker’s yeast. To our delight, the first glimpse of success was obtained by carrying out model reaction in ethanol as a solvent at ambient temperature and pH under stirring and delivering a target product 5a with 84% yield after 34 h (Table 1, entry 1). We have continuously monitored the model reaction, and it was found that within a few hours, there was formation of the intermediates (Intermediate I and II) and the complete conversion of the intermediates to product 5a needs 34 h of constant stirring.
Invigorated by the above interesting result, other solvents such as methanol (MeOH), acetonitrile (ACN), dichloromethane (DCM), dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF) were also used as media for the model reaction, in which only methanol, acetonitrile and DCM gave desired product in 82, 56 and 50% yield, respectively (Table 1, entries 2–4). Unfortunately, other solvents did not work well for model reaction (Table 1, entries 5–8).
The model reaction proceeds well in the protic solvents as compared to aprotic solvents in which there was no reaction or trace amount of product 5a is formed even after 50 h of the reaction. Therefore, we turned our attention to use an abundant protic solvent which is widely accepted for biocatalysis, i.e. water, but unfortunately, there was the formation of trace amount of product (Table 1, entry 9). That might be due to insolubility of organic substrates; hence, we tried the combination of water/ethanol and water/methanol for the model reaction. This attempt leads to the target product by 75 and 70% yields, respectively (Table 1, entries 10–11). It indicates that adding water to reaction reduces the yield of 5a from 84 to 75% and 82 to 70%. We have also carried out the model reaction in a solvent-free condition using baker’s yeast as well as in the absence of baker’s yeast at room temperature, but in both the cases the expected products did not observe even after 50 h (Table 1, entries 12, 13). After the screening of the solvents, ethanol comes out as a winner among all other solvents. Therefore, ethanol was chosen as the best solvent for further studies.
The control experiment demonstrated that the reaction did not produce the desired product in the absence of baker’s yeast even after 50 h of stirring (Table 1, entry 14). Since the efficiency of the multicomponent reactions is also affected by the catalyst dose and the reaction time apart from the solvent, for getting the best possible results, we have done the condensation of benzaldehyde, malononitrile, ethyl acetoacetate and hydrazine hydrate using a variable amount of baker’s yeast from 0.5 to 3 g. The reaction initially failed to work when we use 0.5 and 1 g of the catalyst and did not get any yield of the product. When we increase the amount of catalyst to 1.5 g, the product formation commences at 30%. It was found that when we increase the amount of the catalyst, we got a better yield of the product. Hence, the amount was further enhanced to 2.5 and 3 g which led to 73 and 65% yield (Table 1, entries 15–19). As indicated in Table 1, entry 1, maximum yield obtained was 84% for the product 5a, when the reaction was carried out with 2 g of the catalyst. It means that further increase of catalyst loading decreases the yield, which might be due to an increase in reaction mass, resulting in slowing down the mixing of reactants that ultimately affects the formation of the product. Therefore, here it has been concluded that this multicomponent cyclocondensation occurs well using 2 g of baker’s yeast as a catalyst and ethanol as a medium.
Having established an efficient condition for the model reaction, we turned our attention to investigate the scope of the aldehydes for this methodology (Scheme 2).
A wide range of various substituted aromatic aldehydes were successfully treated with hydrazine hydrate, malononitrile and ethyl acetoacetate, and results are summarized in Table 2. The attached functional group on the aromatic ring of the aldehyde exerts a slight influence on the product yield. The aldehydes with electron-withdrawing and electron-donating groups could be tolerated under these conditions. The substrate scope is also checked with heterocyclic aldehyde, i.e. 2-chloro-3-formyl quinoline, which surprisingly gave a good yield of the target product (5i).
In order to get some mechanistic details of this transformation, a series of control experiments were carried out. When we separately reacted benzaldehyde and malononitrile in ethanol and ethyl acetoacetate with hydrazine, we got 2-benzylidenemalononitrile and 3-methyl-1H-pyrazol-5(4H)-one, respectively, after 3 h and 1 h of constant stirring with baker’s yeast, respectively. It indicates that the first two intermediates are formed and then condensed to form pyranopyrazoles in the presence of baker’s yeast.
On the basis of the obtained results and the literature, a plausible mechanism for the synthesis of pyranopyrazole 5a can considerately be proposed using benzaldehyde, hydrazine hydrate, malononitrile and ethyl acetoacetate (Fig. 1).
Baker’s yeast contains imidazole residue [56], which can act as a catalyst for this cyclocondensation. Imidazole abstracts the proton from malononitrile and also activates the aldehyde, which leads to the attack of malononitrile on aldehydes to give 2-benzylidenemalononitrile (I) [40].
Simultaneously, cyclocondensation takes place between ethyl acetoacetate and hydrazine hydrate to give five-membered ring, 3-methyl-1H-pyrazol-5(4H)-one II, which is further converted to its corresponding enol form III in the presence of imidazole residue. After that, Michael-type addition of intermediate III to the intermediate I gave intermediate IV, which goes for intramolecular cyclization by the nucleophilic attack of oxygen to nitrile group leading to intermediate V. At the end, the tautomerization of intermediate V gave dihydropyrano[2,3-c]pyrazole 5a.
In order to evaluate the advantages of our protocol for the synthesis of pyranopyrazole derivatives, a comparison of reaction condition and yield with some of the reported catalysts in the literature was done. For the synthesis of pyranopyrazole derivatives, the majority of the reported protocols involves the condensations of aromatic aldehyde, ethyl acetoacetate, malononitrile and hydrazine hydrate. The results obtained after comparing baker’s yeast with other catalysts are given in Table 3, which clearly demonstrates the superiority of the present catalysts with other catalysts. It is comparatively affording a truly green process with higher product yields.
Conclusion
Here, we have successfully demonstrated a green route for the one-pot four-component synthesis of pyranopyrazole under a non-aqueous medium using a biocatalyst, i.e. baker’s yeast, which shows a very good functional group tolerance. The products obtained are with good to moderate yields at room temperature and neutral pH condition without any special workup set-up. The promising points of the given protocol are that the catalyst is completely biodegradable and very cheaper as compared to other chemical catalysts with ease of isolation of product and cleaner reaction profile.
References
M.A. Zolfigol, M. Tavasoli, A.R. Moosavi-Zare, P. Moosavi, H.G. Kruger, M. Shiri, V. Khakyzadeh, RSC Adv. 3, 25681 (2013)
S. Shahbazi, M.A. Ghasemzadeh, P. Shakib, M.R. Zolfaghari, M. Bahmani, Polyhedron 170, 172 (2019)
S.R. Mandha, S. Siliveri, M. Alla, V.R. Bommena, M.R. Bommineni, S. Balasubramanian, Bioorg. Med. Chem. Lett. 22, 5272 (2012)
G.M. Reddy, J.R. Garcia, G.V. Zyryanova, G. Sravyaa, N.B. Reddy, Bioorg. Chem. 82, 324 (2019)
A. Kumar, P. Lohan, D.K. Aneja, G.K. Gupta, D. Kaushik, O. Prakash, Eur. J. Med. Chem. 50, 81 (2012)
N.R. Mohamed, N.Y. Khaireldin, A.F. Fahmy, A.A. El-Sayed, Der Pharma Chem. 2, 400 (2010)
S.S. Chobe, G.G. Mandawad, O.S. Yemul, S.S. Kinkar, B.S. Dawane, Int. J. Chem. Tech. Res. 3, 938 (2011)
S.A. El-Assiery, G.H. Sayed, A. Fouda, Acta Pharm. 54, 143 (2004)
F.M. Abdelrazek, P. Metz, O. Kataeva, A. Jager, S.F. El-Mahrouky, Arch. Pharm. Chem. Life Sci. 340, 543 (2007)
F.M. Abdelrazek, F.A. Michael, A.E. Mohamed, Arch. Pharm. Chem. Life Sci. 339, 305 (2006)
E.A.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A.A. Ei-Taweel, Heterocycles 26, 903 (1987)
D. Armetso, W.M. Horspool, N. Martin, A. Ramos, C. Seaone, J. Org. Chem. 54, 3069 (1989)
H. Junek, H. Aigner, Chem. Ber. 106, 914 (1973)
H.H. Otto, Arch. Der Pharm. 307, 444 (1974)
Y. Peng, G. Song, R. Dou, Green Chem. 8, 573 (2006)
A.S. Nagarajan, B.S.R. Reddy, Synlett 12, 2002 (2009)
N.M.H. Elnagdi, N.S. Al-Hokbany, Molecules 17, 4300 (2012)
H.M. Al-Matar, K.D. Khalil, A.Y. Adam, M.H. Elnagdi, Molecules 15, 6619 (2010)
M. Bakthadoss, A. Devaraj, D. Kannan, Eur. J. Org. Chem. 7, 1505 (2014)
D.M. D’Souza, T.J.J. Muller, Chem. Soc. Rev. 36, 1095 (2007)
F. Keshavarzipour, H. Tavakol, Catal. Lett. 145, 1062 (2015)
S. Guo, S. Wang, J. Li, Synth. Commun. 37, 2111 (2007)
R.D. Kamble, B.S. Dawane, O.S. Yemul, A.B. Kale, S.D. Patil, Res. Chem. Intermed. 39, 3859 (2013)
M. Wu, Q. Feng, D. Wan, J. Ma, Synth. Commun. 43, 1721 (2013)
M. Fatahpour, F.N. Sadeh, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, J. Iran. Chem. Soc. 14, 1945 (2017)
P. Farokhian, M. Mamaghani, N.O. Mahmoodi, K. Tabatabaeian, J. Iran. Chem. Soc. 15, 11 (2018)
M.B.M. Reddy, V.P. Jayashankara, M.A. Pasha, Synth. Commun. 40, 2930 (2010)
J.M. Khurana, A. Chaudhary, Green Chem. Lett. Rev. 5, 633 (2012)
R. Guo, Z. An, L. Mo, S. Yang, H. Liu, S. Wang, Z. Zhang, Tetrahedron 69, 9931 (2013)
A.R. Moosavi-Zare, H. Afshar-Hezarkhani, M.M. Rezaei, Polycycl. Aromat. Compd. 29, 1 (2017)
A.R. Moosavi-Zare, M.A. Zolfigol, A.M. Tashar, Res. Chem. Intermed. 42, 7305 (2016)
M.G. Dehbalaei, N. Foroughifar, H. Pasdar, A.K. Amiri, N. Foroughifar, M. Alikarami, Res. Chem. Intermed. 43, 3035 (2017)
H. Mecadon, M.R. Rohman, I. Kharbangar, B.M. Laloo, I. Kharkongor, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 3228 (2011)
G. Vasuki, K. Kumaravel, Tetrahedron Lett. 49, 5636 (2008)
M. Kangani, N. Hazeri, M.T. Mghsoodlou, S.M.H. Khorasani, S. Salahi, Res. Chem. Intermed. 41, 2513 (2015)
F. Keshavarzipour, H. Tavakol, Appl. Organomet. Chem. 31, e3811 (2017)
F. Keshavarzipour, H. Tavakol, J. Iran, Chem. Soc. 13, 149 (2016)
U.R. Pratap, J.R. Mali, D.V. Jawale, R.A. Mane, Tetrahedron Lett. 50, 1352 (2009)
U.R. Pratap, D.V. Jawale, B.S. Londhe, R.A. Mane, J. Mol. Catal. B Enzym. 68, 94 (2011)
U.R. Pratap, D.V. Jawale, P.D. Netankar, R.A. Mane, Tetrahedron Lett. 52, 5817 (2011)
M. Inal, M. Yigitoglu, J. Chem. Technol. Biotechnol. 86, 1548 (2011)
D. Acetti, E. Brenna, C. Fuganti, F.G. Gatti, S. Serra, Eur. J. Org. Chem. 1, 142 (2010)
A. Wolfson, C. Dlugy, D. Tavor, J. Blumenfeld, Y. Shotland, Tetrahedron Asymmetry 17, 2043 (2006)
N.G. Singh, R. Nongrum, C. Kathing, J.W. Star, R. Nongkhlaw, Green Chem. Lett. Rev. 7, 137 (2014)
P. Shrivas, U. Pratap, Chem. Pap. 73, 1301 (2019)
T. Izumi, T. Hino, A. Kasahara, J. Chem. Tech. Biotechnol. 50, 571 (1991)
T. Izumi, K. Satou, K. Ono, J. Chem. Tech. Biotechnol. 66, 233 (1996)
Y. Shi, Y. Fang, Y. Ren, H. Guana, J. Zhang, J. Chem. Tech. Biotechnol. 84, 681 (2009)
H.N. Borah, D. Prajapati, R.C. Boruah, Synth. Commun. 39, 267 (2008)
E. Brenna, F.G. Gatti, A. Manfredi, D. Monti, F. Parmeggiani, Eur. J. Org. Chem. 20–21, 4015 (2011)
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 81, 431 (2011)
R.S. Aliabadi, N.O. Mahmoodi, RSC Adv. 6, 85877 (2016)
H.V. Chavan, S.B. Babar, R.U. Hoval, B.P. Bandgar, Bull. Korean Chem. Soc. 32, 3963 (2011)
G.M. Reddy, J.R.J. Garcia, J. Heterocyclic Chem. 54, 89 (2017)
K.S. Mani, S.P. Rajendran, Synth. Commun. 47, 2036 (2017)
F.A. Csonka, J. Biol. Chem. 105, xix (1934)
K.G. Patel, N.M. Misra, R.H. Vekariya, R.R. Shettigar, Res. Chem. Intermed. 44, 289 (2018)
S. Sadjadi, M.M. Heravi, V. Zadsirjan, V. Farzaneh, Res. Chem. Intermed. 44, 6765 (2018)
Acknowledgements
We are very thankful to the Science and Engineering Research Board, New Delhi, for providing essential financial support (SERB/EMEQ-279/2013). We are also grateful to SAIF, NEHU, Shillong and CDRI, Lucknow, for providing spectral characterizations of our compounds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shrivas, P., Pandey, R., Zodape, S. et al. Green synthesis of pyranopyrazoles via biocatalytic one-pot Knoevenagel condensation–Michael-type addition–heterocyclization cascade in non-aqueous media. Res Chem Intermed 46, 2805–2816 (2020). https://doi.org/10.1007/s11164-020-04122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04122-x