Abstract
A MADS-box gene, designated PtAP3, was isolated from a floral bud cDNA library derived from Populus tomentosa. Analysis by multiple alignments of both nucleotide and amino acid sequences, together with phylogenetic analysis, revealed that PtAP3 is an ortholog of Arabidopsis AP3. Analysis of RNA extracts from vegetative and reproductive tissues of P. tomentosa by RT-PCR indicated that PtAP3 is expressed in roots, stems, leaves and vegetative and floral buds. Notably, the expression of PtAP3 fluctuated during floral bud development between September and February with differences between male and female buds. In the former, a gradual down-regulation during this period, interrupted by a slight up-regulation in December, was followed by a sharper up-regulation on February. In developing female floral buds, expression was stable from September to November, sharply up-regulated in December, and then gradually down-regulated until February. The functional role of PtAP3 was investigated in transgenic tobacco plants. Of 25 transformants, nine displayed an earlier flowering phenotype compared with the wild type plants. Furthermore, transgenic tobacco had faster growth and more leaves than untransformed controls. The traits proved to be heritable between the T0 and T1 generations. Our results demonstrate a regulatory role of the PtAP3 gene during plant flowering and growth and suggest that the gene may be an interesting target for genetic modification to induce early flowering in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current knowledge concerning the ABCDE gene model comes largely from extensive studies on the model plant Arabidopsis. Within the B-class genes, APETALA3 (AP3) and PISTILLATA (PI) are of interest because they act as identity genes required for petal and stamen morphogenesis (Jack et al. 1992). Both AP3 and PI gene products belong to the MADS-box super family of proteins, which are known as the MIKC-domains (Theißen et al. 2000). The MADS-box domain contains approx. 60 aa and is highly conserved across the entire super family. It is essential for binding at conserved DNA sites containing the sequence CC (A/T)6GG, known as CArG elements, and is considered to play a role in protein dimerization (Riechmann et al. 1996). The gene products of AP3 form heterodimers that recognize and bind to these sites (Hill et al. 1998).
The particular importance of understanding the flowering mechanism in poplar is due to the economic and ecological importance of the species for forest production, reclamation and biomass (Polle and Douglas 2010). Populus differs from Arabidopsis in many aspects. It is a woody perennial tree with a long juvenile phase and a long life span (Hsu et al. 2006). It flowers annually or seasonally during the reproductive developmental phase (Yuceer et al. 2003) and each seasonal flowering period is interrupted by a vegetative period. Furthermore, the structures of poplar flowers are distinct from those of Arabidopsis (Rottmann et al. 2000). At present, the functional role of AP3 and/or its orthologs in the development of woody plant flowers is unclear. In this study, we report the isolation of PtAP3, an AP3-like gene in Populus tomentosa, and the analysis of its function both in situ and in heterogeneous transformed tobacco plants. Our data provides new insights into the molecular mechanism underlying the development of floral buds in P. tomentosa and, additionally, one possible approach to accelerating early flowering in plants via genetic modification.
Materials and methods
Plant materials
Vegetative and floral buds of P. tomentosa were rapidly frozen in liquid N2 after collection from the adult trees and stored at −80°C until use. Additionally, root, stem and leaf samples were taken from 30-day-old tissue-cultured plantlets of P. tomentosa, followed by immediate RNA extraction. Sterilized tobacco (Nicotiana tabacum) leaf discs were used as transgenic acceptor materials. The young leaves of transgenic tobacco plants were used for extraction of DNA and RNA.
Cloning and characterization of PtAP3
The primers P1F and P1R (Table 1) used for the screening of PtAP3 were designed according to PTD (accession AF057708), an AP3 homolog from Populus trichocarpa. PtAP3 was obtained by PCR screening of the P. tomentosa male floral bud cDNA library. SM buffer containing recombinant bacteriophages was used as DNA template. Thermal cycling was performed at 94°C for 5 min, 94°C for 30 s, 60°C for 20 s, 72°C for 30 s, for 35 cycles, then at 72°C for 5 min. The positive recombinant bacteriophage clones were isolated by PCR.
The amino acid sequences of AP3 homolog genes were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi) and aligned with CLUSTALX1.81 and Bioedit software. Conserved domain sequences and functional domains were analyzed using the Expert Protein Analysis System (ExPASy) proteomics server of the Swiss Institute of Bioinformatics (SIB) (http://cn.expasy.org/). The tertiary structure of the PtAP3 protein was built using software 3D-JIGSAW 2.0 (http://bmm.cancerresearchuk.org/~3djigsaw/).
Phylogenetic relationship analysis
Genetic relationships were analyzed using a number of B-class MADS-box protein sequences of plant species retrieved from GenBank. Multiple sequence alignment was performed with ClustalW, genetic distance matrices were obtained from the alignments and a neighbor-joining tree was constructed with bootstrap sampling of 1,000 replications using MEGA 4.1(Tamura et al. 2007).
RT-PCR analysis
Total RNAs were extracted from vegetative and reproductive tissues of P. tomentosa according to the method of Chang et al. (1993). Total RNA was pre-treated with RQ1 DNase I (Promega) to remove contaminating genomic DNA. The first-strand cDNA was synthesized using 1 μg treated total RNA, Superscript III (Invitrogen) and oligo d(T)20, subsequently diluted 1:10 with water as template. P2F and P2R primers (Table 1) were used for PCR amplification. Conditions of thermal cycling were identical to those described above. No-template controls for each primer pair were included in each run. P. tomentosa actin was used as an internal reference to normalize small differences in template amounts with P3F and P3R primers (Table 1).
Construction of expression vectors and transformation of tobacco
Both the plasmid DNA containing PtAP3 and the binary vector pBI121 DNA were digested with both BamHI and SacI. The corresponding fragments were recycled and purified using QIAquick Gel Extraction Kit (Qiagen) and ligated with T4-DNA ligase (Promega) at 16°C overnight. The ligation product was then transferred into competent cells of E. coli TG1. The pBI121-PtAP3 was verified by PCR (using P4F and P4R primers) (Table 1) and digestion. Tobacco leaf disc transformation was performed using the Agrobacterium-mediated method.
Molecular identification of transgenic tobacco plants
PCR and DNA gel blot analysis
Genomic DNA was extracted from young leaves of transformed plants and used as the template for PCR identification. The PCR reaction system and conditions of thermal cycling were identical to those described in the previous sections. PCR products were separated by electrophoresis on a 0.8% agarose gel and blotted onto positively charged nylon membrane by capillary transfer in the presence of 20 × SSC buffer. A 203 bp fragment used as DNA probe for gel blot analysis was amplified from genomic DNA of P. tomentosa using P4F and P4R primers (Table 1). The blots were hybridized with a DIG-labeled DNA probe at 58°C, and washed at high stringency. Immunological detection was performed with the DIG DNA Labeling and Detection Kit (Roche) according to the manufacturer’s protocol.
Quantitative RT-PCR analysis
Total RNA was extracted from transgenic tobacco leaves using the SV Total RNA Isolation System (Promega). Reverse transcription was performed as described in previous sections. As template, first-strand cDNA was diluted 1:10 with water. P2F and P2R were used for qPCR analysis. The qPCR reaction was performed using PowerSYBR Green PCR Master Mix (Invitrogen) on a DNA Engine Opticon 2 system (MJ Research). The program included a preliminary step of 2 min at 50°C, predenaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 15 s, 60°C for 30 s and 72°C for 30 s, with a final extension of 7 min at 72°C. No-template controls for each primer pair were included in each run. Tobacco actin was used as an internal reference to normalize small differences in template amounts with P5F and P5R primers (Table 1). Three replicates were employed for the qPCR analysis of each sample.
Phenotypic analysis of transgenic tobacco plants
Twenty-five transgenic plantlets were transplanted into individual pots with artificial soil mix as growing support. The plants were moved outside the greenhouse when they had produced four to five leaves (June 15). Plant height and number of leaves of each T0 transgenic line were recorded on August 11 and 21, September 1, 11 and 21, and October 2. The date when flower primordia were first visible was also recorded.
Results and discussion
A positive phage plaque was isolated from the P. tomentosa male floral bud cDNA library by PCR. Sequencing revealed the length of PtAP3 cDNA to be 717 bp, encoding 238 amino acids. The sequence alignments suggest that PtAP3 belongs to the MADS-box gene family. It contains M-, I-, K- and C-domains, which are typical characteristics of MIKC-type genes (Fig. 1a). The predicted tertiary structure of the MADS-box domain of PtAP3 (Fig. 1b) consists of one helix and two sheet structures. MADS-box genes play crucial roles in plant growth and development (Causier et al. 2002). The M-domain itself is the most highly conserved of the four major MADS-box protein domains and has been widely studied across the taxonomic kingdoms (Leseberg et al. 2006). Proteins incorporating the M-domain are involved in DNA binding and dimerization with other MADS-box proteins (Diaz-Riquelme et al. 2009). The protein PtAP3 reported here shares 82% amino acid similarity with PTD, an AP3 homolog from P. trichocarpa. The data described in the present report will add to existing knowledge in this field (Sheppard et al. 2000).
Sequence alignment and predicted tertiary structure of PtAP3 protein. a Sequence alignment of MADS-box domain proteins from several plant species. The M- and K- and the I- and C-domains are highlighted using red bold frames and blue bold lines respectively. b The tertiary structures of M-domain in PtAP3. The helix was formed by “PTNRQVTYSKRRNGIFKKAQELTVLC”
To understand the relationships between PtAP3 and the B-class MADS-box genes of other species, a neighbor-joining tree was constructed from PtAP3 together with 23 previously reported B-class MADS-box genes from Arabidopsis, gymnosperm, eudicot and monocot species. Similarly to the previous studies (Winter et al. 2002), the B-class MADS-box genes were classified into AP3, PI and GGM2 subclasses (the AP3 and PI subclasses associated with the angiosperm group and the GGM2 subclass with the gymnosperm group). The analysis shows that PtAP3 falls into the eudicot and monocot AP3 subclass of B-class MADS-box proteins (Fig. 2). The result suggests that PtAP3 shares similar functions with those of other genes in the same clade. In other words, PtAP3 is likely to fulfil a B-function role during the development of floral organs in P. tomentosa.
Neighbor-joining tree representing relationships of PtAP3 with gymnosperm and other angiosperm MADS-box gene homologs. Bootstrap support values (%) from 1,000 replications are indicated when over 50%. GenBank accession number for each sequence: PtAP3 (AY210488), AP1 (BT004951), AP3 (AY142590), PI (NM_122031), VvTM6 (BQ979341), PI-like(DQ059750), MASAKO (AB055966), MdTM6 (AB081093), MdPI (AJ291491), Silky1 (AF181479), ZMM16 (NM_001111666), OsMADS2 (L37526), OsMADS4 (L37527), OsMADS16 (AF077760), PeMADS3 (AY378150), LMADS1(AF503913), LRDEF (AB071378), PTD (AF057708), PdPI (EU029172), DAL11-1(AF158539), DAL11-2(AF158540), PrDGL (AF120097), AmDEF (AB516402), NcAP3 (X96428)
The expression profiles of PtAP3, analyzed by RT-PCR, indicate that the gene is expressed in various tissues of P. tomentosa including roots, tender stems and leaves, vegetative buds and male and female floral buds. In floral buds, its relative expression levels fluctuated and differences were apparent between the two sexual forms (Fig. 3a, b, c). In developing male floral buds, a gradual down-regulation between September 13 and January 25 was interrupted by a slight up-regulation, demonstrated by the data point in December and expression was then sharply up-regulated on February 25 (Fig. 3b). In contrast, transcript levels in developing female floral buds were stable from September 13 to November 24, sharply up-regulated in December and then gradually down-regulated until February 25th (Fig. 3c). These results imply that PtAP3 is associated with sexual differentiation of P. tomentosa floral organs.
Expression patterns of PtAP3 in P. tomentosa. a Expression in different tissues. Roots, stems and leaves were collected from one-month-old tissue-cultured plantlets. Vegetative buds were collected from adult trees. b and c The expression patterns of PtAP3 during floral bud development derived from real time RT-PCR analysis in male floral buds (b) and female floral buds (c)
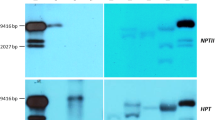
Sixty-three positive transgenic tobacco plants were identified by PCR. The results of both PCR and DNA gel blot analysis of partial transgenic tobacco plants showed that the constructed plasmid 35S::PtAP3 (Fig. 4A) had been integrated into the tobacco genome (Fig. 4B). Relative differences in PtAP3 expression in the transgenic tobacco was also indicated by qRT-PCR (Fig. 4C). Statistical analysis of these results shows that expression was significantly higher in line T62 than in other lines. Significant differences in expression of PtAP3 were also demonstrated between partial lines (T63, T59 and T49) and T5. The difference between T5 and T62 was approx. 400-fold. Investigation of the phenotypic traits of the transgenic tobacco indicated correlations between these and the expression levels of PtAP3. Line T62, with the highest expression level, flowered 25 days earlier than wild type plants, while T63, T59, T49 and T32 flowered 15, 12, 10 and 8 days earlier than wild type, respectively. Faster growth than wild type was observed in lines T62, T63, T59, T49 and T32 while T5 also grew faster but did not flower earlier.
Molecular identification of transgenic tobacco plants carrying the 35S::PtAP3 construct. A Schematic representation of pBI121-PtAP3 for over-expression of PtAP3 in transgenic tobacco. RB (T-DNA right border), LB (T-DNA left border), Nosp (Nos promoter), nptII (Neomycin phosphotransferase II), Nost (Nos terminator), 35S (CaMV 35S promoter), gus (b-glucuronidase gene), PtAP3 (an AP3-like gene from P. tomentosa). B Transgenic tobacco plants identified by PCR amplification and DNA gel blot analysis. T5, T32, T49, T59, T62 and T63 are six transgenic lines, WT is the wild type, P is positive control (the recombinant plasmid pBI121-PtAP3). C The over-expression of PtAP3 in transgenic tobacco plants identified by quantitative RT-PCR (using tobacco ACTIN as reference control). T5, T32, T49, T59, T62 and T63 are six transgenic lines. Significance was assessed using the ANOVA Fisher’s LSD test (*P < 0.05)
Twenty-five independent T0 generation transgenic tobacco plants and ten wild type were transferred to conditions exterior to the greenhouse for further study. The average height of all these transgenic plants was statistically higher than that of wild type during development (Fig. 5A) and the transgenic plants also had more leaves than the control plants (Fig. 5B). Nine flowered earlier than the wild type plants by between 4 and 25 days (Table 2; Fig. 5C). No obvious phenotypic variation of floral organs was observed in the transgenic tobacco compared with the wild type (Fig. 5D). Leaves, however, appeared to be narrower, longer and uneven (Fig. 5E). No difference was observed in fruit shape (Fig. 5Fa, Fb). The T1 generation transgenic plants derived from T0 seeds flowered earlier than wild type (Fig. 5G), indicating that the early flowering trait had been inherited in the T1 generation.
The phenotypic characteristics and growth traits of tobacco plants carrying pBI121-PtAP3 grown in natural conditions. A The height of transgenic and wild type tobacco plants from August 11th to October 2nd. Significance was assessed using the ANOVA Fisher’s LSD test (**P < 0.01) B Change in leaf number during the development of transgenic tobacco plants and wild type. Significance was assessed using the ANOVA Fisher’s LSD test (*P < 0.05, **P < 0.01). C Early flowering phenotype produced in T0 generation of transgenic tobacco. D A flower of transgenic tobacco (T0 generation). E The altered leaf shape of a T0 generation transgenic tobacco plant. Fa and Fb were fruits from wild type and transgenic tobacco plants (T0 generation), respectively. G The early flowering trait was inherited in the T1 generation of transgenic tobacco
Despite varying levels of expression of PtAP3, all transgenic tobacco plants grew faster and produced more leaves, while 9 of the 25 plants flowered earlier. We speculate that the essential genetic factor for such phenotypic traits is the conserved MADS-box domain. The MADS-box domain of the PtAP3 protein bears very close homology to those of the Arabidopsis proteins AtAP3 and AtAP1 (Fig. S1) and it is expected that its function during flower development is similar to that of the Arabidopsis proteins. The effects of over-expression of PtAP3 in transgenic tobacco plants suggest that one of the protein’s major functions concerns the regulation of flowering time. It is possible that faster growth and more leaves also contribute to the transition to flowering.
Our study should contribute to the greater understanding of the genetic factors controlling flower development in poplar. The PtAP3 gene represents a valuable element to further this knowledge not only in poplar but possibly other woody species. Based on these findings, our work will be extended to the production of transgenic poplar expressing altered levels of the endogenous poplar gene to further investigate its involvement in flowering in this species. Control of flowering time is also an important factor in other domains of plant culture. For instance, speed to flowering has an important effect on production costs in floriculture, as it determines the productivity of a defined area of greenhouse space (Chandler and Brugliera 2010). Although some control of flowering can be achieved by manipulation of photoperiod and/or use of plant growth regulators, ectopic expression of PtAP3 might be one approach using genetic modification to achieve early flowering induction in these plants.
References
Causier B, Kieffer M, Davies B (2002) Plant biology. MADS-box genes reach maturity. Science 296:275–276
Chandler SF, Brugliera F (2010) Genetic modification in floriculture. Biotechnol Lett 33(2):207–214
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Reptr 11:113–116
Diaz-Riquelme J, Lijavetzky D, Martinez-Zapater JM, Carmona MJ (2009) Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiol 149:354–369
Hill TA, Day CD, Zondlo SC, Thackeray AG, Irish VF (1998) Discrete spatial and temporal cis-acting elements regulate transcription of the Arabidopsis floral homeotic gene APETALA3. Development 125:1711–1721
Hsu CY, Liu YX, Luthe DS, Yuceer C (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18:1846–1861
Jack T, Brockman LL, Meyerowitz EM (1992) The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68:683–697
Leseberg CH, Li AL, Kang H, Duvall M, Mao L (2006) Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene 378:84–94
Polle A, Douglas C (2010) The molecular physiology of poplars: paving the way for knowledge-based biomass production. Plant Biol (Stuttg) 12:239–241
Riechmann JL, Krizek BA, Meyerowitz EM (1996) Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA, and AGAMOUS. Proc Natl Acad Sci USA 93:4793–4798
Rottmann WH, Meilan R, Sheppard LA, Brunner AM, Skinner JS, Ma C, Cheng S, Jouanin L, Pilate G, Strauss SH (2000) Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant J 22:235–245
Sheppard LA, Brunner AM, Krutovskii KV, Rottmann WH, Skinner JS, Vollmer SS, Strauss SH (2000) A DEFICIENS homolog from the dioecious tree black cottonwood is expressed in female and male floral meristems of the two-whorled, unisexual flowers. Plant Physiol 124:627–640
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Theißen G, Becker A, Di Rosa A, Kanno A, Kim JT, Munster T, Winter KU, Saedler H (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42:115–149
Winter KU, Saedler H, Theißen G (2002) On the origin of class B floral homeotic genes: functional substitution and dominant inhibition in Arabidopsis by expression of an orthologue from the gymnosperm. Gnetum Plant J 31:457–475
Yuceer C, Land SB, Kubiske ME, Harkess RL (2003) Shoot morphogenesis associated with flowering in Populus deltoides (Salicaceae). Am J Bot 90:196–206
Acknowledgments
This work was supported by the Forestry Public Benefit Research Foundation (201004009), the Natural Science Foundation of China (No.30571511) and the National “863” program (2009AA10Z107).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2011_545_MOESM1_ESM.doc
Sequence alignment of PtAP3 (AY210488), AtAP1 (BT004951) and AtAP3 (AY142590) proteins. A. Comparison of PtAP3 and AtAP3 proteins. B. Comparison of MADS-domain proteins from PtAP3 and AtAP1. (DOC 470 kb)
Rights and permissions
About this article
Cite this article
An, X., Ye, M., Wang, D. et al. Ectopic expression of a poplar APETALA3-like gene in tobacco causes early flowering and fast growth. Biotechnol Lett 33, 1239–1247 (2011). https://doi.org/10.1007/s10529-011-0545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0545-4