Abstract
The performance of A. virlai on six Cicadellidae and three Delphacidae (Hemiptera) species was assessed under laboratory conditions to clarify its host specificity. In addition, the influence of host egg size on the body size and egg load of the emerging parasitoids was investigated. The Deltocephalinae (Cicadellidae) Amplicephalus marginellanus (Metcalf), Amplicephalus dubius Linnavuori and Dalbulus maidis (DeLong & Wolcott) were the most parasitised species. Wasps were unable to parasitise the eggs of the Cicadellinae (Cicadellidae) Hortensia similis (Walker), Plesiommata mollicella (Fowler) and Scopogonalia subolivacea (Stål) or successfully develop in the eggs of A. marginellanus. The parasitism and emergence rates recorded in Delphacodes kuscheli Fennah, Metadelphax propinqua (Fieber) and Peregrinus maidis (Ashmead) (Delphacidae) were lower than in the other parasitised species. Of all the Cicadellidae tested, S. subolivacea laid the largest eggs and D. maidis the smallest. The Delphacidae deposited the smallest eggs of all hopper species evaluated as hosts. Parasitoids emerged from the eggs of A. dubius were larger and carried higher egg loads than the other wasps reared in A. marginellanus and D. maidis. There was no correlation between most measured morphometric variables and the egg load of wasps. Our results provide valuable insights into the host specificity of this egg parasitoid, but further studies are desirable to fully understand how target and non-target hosts affect the population dynamics of A. virlai in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural enemies provide a valuable agroecosystem service by controlling pest population densities through predation and parasitism. Most parasitoid hosts are herbivorous insects that can use more than one plant species as hosts of different taxonomical groups, constituting an intricate trophic network (Volf et al. 2017). As a result, parasitoids have evolved the ability to exploit different host species, resulting in a diverse host range determined by behavioural responses to hosts and their environment (Antolin et al. 2006).
Egg parasitoids are the most important natural enemies of leafhoppers (Cicadellidae) and planthoppers (Delphacidae) in rice and maize agroecosystems (Liu et al. 2010; Torres Moreno and Moya Raygoza 2020). Most are considered generalist species moving towards host patches driven by volatile signals released by their hosts during feeding and egg laying (Lou et al. 2005; Krugner et al. 2008a). Furthermore, leafhopper and planthopper species can migrate from overwintering places to the crop when conditions become favourable, resulting in a bottom-up influence on mobile organisms like parasitoids (Corbett and Rosenheim 1996; Hu et al. 2017).
The presence of wild vegetation in and around the crops provides parasitoids with refuge and alternative hosts, potentially assisting in pest control. This is particularly desirable from a conservation biological control (CBC) perspective, where crops with high connectivity with uncultivated areas can facilitate parasitoid movement and recolonization over large areas (Tscharntke et al. 2008). In addition, the use of alternative hosts can be advantageous for CBC because parasitoids with broader host ranges are more likely to successfully survive in agroecosystems in the temporal absence of the target pest (Raymond et al. 2016). However, host specificity may play an ambivalent role for biological control. For moderately generalist species, broad host plasticity might guarantee long-term control, whereas specialist parasitoids would instead display higher parasitism rates, beneficial for short-term control programs (Raymond et al. 2016).
Egg parasitoids of the genus Anagrus Haliday (Hymenoptera: Mymaridae) are frequently classified as generalists. Most of their hosts are Hemiptera Auchenorrhyncha, specifically species belonging to the Cicadellidae and Delphacidae families (Triapitsyn 2015). Nevertheless, the host range of several species remains unknown. The New World egg parasitoid Anagrus virlai Triapitsyn is a common and generalist species whose host range includes nine species of Cicadellidae: Agalliana ensigera Oman (Magophtalminae); Ciminius platensis (Berg), Dechacona missionum (Berg), and Hortensia similis (Walker) (Cicadellinae); Chlorotettix fraterculus (Berg), Dalbulus elimatus (Ball), Dalbulus maidis (DeLong & Wolcott), and Exitianus obscurinervis (Stål) (Deltocephalinae); and Xerophloea viridis (Fabricius) (Ledrinae); and two species of Delphacidae: Delphacodes kuscheli Fennah and Peregrinus maidis (Ashmead) (Delphacinae) (Luft Albarracin et al. 2009; Triapitsyn et al. 2019). All of them, except for D. elimatus, are native species inhabiting maize agroecosystems in subtropical Argentina (Marino de Remes Lenicov et al. 2004; Luft Albarracin et al. 2009).
Crop seasonality plays a key role in the population dynamics of pests and their natural enemies. In the case of maize, in its centre of origin (Mexico) and other tropical regions, this crop is cultivated either year-round during both the wet season and the dry season or only during the wet season (Moya Raygoza et al. 2005). In contrast, in more temperate zones such as subtropical regions, maize is only cultivated during the wet season (spring and summer) (Virla et al. 2013). Consequently, herbivorous species that are present in maize in the limit areas (i.e., southernmost areas in the Southern Hemisphere) exhibit a wide variety of strategies to increase their tolerance to cold and to overwinter on either non-crop habitats (weeds and edge grasses) or winter crops (Pinedo Escatel and Moya Raygoza 2015; Brentassi et al. 2019; Rodríguez Juárez et al. 2020). These changes in maize availability and its herbivorous hosts might affect parasitoid’s communities (Prischmann et al. 2007). In the subtropics of Argentina, the Auchenorrhyncha (Hemiptera) community that inhabits maize crops and the vegetation that surrounds them is made up of at least 21 species of leafhoppers (Cicadellidae) (Luft Albarracin et al. 2009) and 13 planthoppers (Delphacidae) (Marino de Remes Lenicov et al. 2004).
Hoppers have become a serious phytosanitary problem among maize pests for a variety of reasons: they cause direct damages to host plants by feeding from phloem and xylem tissues, by laying their eggs endophytically in different organs of the host plant, and some species by removing the cell contents from mesophyll cells (Virla et al. 2021). Additionally, they cause crop damage by transmitting several phytopathogens such as viruses and bacteria. For example, the corn leafhopper D. maidis is an efficient vector of several maize pathogens, such as “maize rayado fino virus” (Tymovirales: Marafivirus), “corn stunt spiroplasma” (Mollicutes: Spiroplasmataceae: Spiroplasma kunkelii Whitcomb), “maize bushy stunt phytoplasma” (Mollicutes: Acholeplasmataceae: Candidatus Phytoplasma ateris), and the recently described Mastrevirus that causes “maize striate mosaic virus” (Giménez Pecci et al. 2012; Vilanova et al. 2022). Members of the Cicadellinae subfamily are vectors of Xylella fastidiosa Wells et al., a gram-negative bacterium that produces several plant diseases (Alves et al. 2008; Dellapé et al. 2016; EFSA Panel on Plant Health (PLH) et al. 2019). The planthoppers D. kuscheli, Metadelphax (= Toya) propinqua (Fieber) and P. maidis transmit the “Mal de Río Cuarto Virus” (MRCV), one of the most important pathogens affecting maize in Argentina (Mattio et al. 2005; Giménez Pecci et al. 2012; Dumón et al. 2018).
The host specificity of a natural enemy has long been a critical issue in biological control. The spectrum of action of a biological control agent has evolutionary, environmental, and economic implications (van Lenteren et al. 2006). Specificity first establishes the intrinsic potential of a given species to become an efficient natural enemy of a target pest, but empirical evidence showed that parasitoid species may suffer a trade-off between host-range breadth and host-use efficiency (Rossinelli and Bacher 2015). The relevance of A. virlai as a potential biocontrol agent has been largely recognized because it is able to parasitise D. maidis eggs and potentially control its populations in different maize fields in the Americas (Moya Raygoza et al. 2012; Virla et al. 2013; Luft Albarracin et al. 2017; Torres Moreno and Moya Raygoza 2020). Furthermore, A. virlai exhibits a certain degree of specificity for Cicadellidae hosts, attacking only occasionally some Delphacidae species (Hill et al. 2019). Although there are reports on A. virlai biology and its field parasitism, its performance on different host species has been scarcely studied. Furthermore, its proposed broad host range was mistakenly based on the Palearctic species Anagrus incarnatus Haliday or its synonym Anagrus breviphragma Soyka (Triapitsyn et al. 2019), providing unclear data that needs revision. Clarification on this matter is especially important for biological control assessment, as in the case that A. virlai is too specialized and could not exploit alternative hosts when the target pest is absent, it might result in slower recolonization of the crop. In this work, we evaluated the performance (parasitism and emergence rates) of A. virlai on six Cicadellidae species [Amplicephalus dubius Linnavuori, A. marginellanus (Metcalf), D. maidis, H. similis, Plesiommata mollicella (Fowler), and Scopogonalia subolivacea (Stål)] and three Delphacidae species (D. kuscheli, M. propinqua and P. maidis) using maize as host plant. Additionally, we analysed if host size and egg clustering may have affected parasitoid host selection. Furthermore, we tested if the host eggs of the three most parasitised species influenced both morphometric characters and the egg load of emerged parasitoids. Finally, we carried out a multivariate analysis to investigate if the host species affected parasitoid size. An integrative approach of A. virlai performance on different Auchenorrhyncha host insects is provided.
Materials and methods
Origin and maintenance of insect colonies
Cicadellidae and Delphacidae insects were collected from August 2019 to May 2021 during the spring, summer and autumn seasons from two fields (26°48′34.4″S 65°14′28.3″W and 26°48′39″S 65°14′24.2″W) in San Miguel de Tucumán, Argentina, using the sweep–net technique. These sites were not treated with pesticides and were characterized by the presence of grasses and weeds such as Brachiaria sp., Bromus catharticus Vahl var. catharticus, Cynodon dactylon (L.), Paspalum notatum Flüggé, Setaria parviflora (Poir.) and Sorghum halepense (L.) Pers. (Poaceae); and Bidens pilosa L. and Eryngium elegans Cham. et Schlecht (Asteraceae); among others. The collected insects were later transferred to voile bags for identification in the laboratory. Taxonomic identification was based on morphological characters and specific taxonomic keys (Linnavuori 1959; Young 1977; Marino de Remes Lenicov et al. 1999; Dietrich 2005; Zahaniser and Dietrich 2013). Five species of Cicadellidae: A. dubius, A. marginellanus, H. similis, P. mollicella and S. subolivacea, and one Delphacidae: M. propinqua, were obtained from the field. The adults of the corn leafhopper and the planthoppers D. kuscheli and P. maidis were obtained from a continuous rearing colony from PROIMI-Biotecnología (CONICET), kept under greenhouse conditions (temperature between 20 and 30 °C, natural photoperiod and no humidity control). Dalbulus maidis and P. maidis were continuously reared on maize plants (landrace sweet white maize “maizón”), whereas for D. kuscheli, oat (Avena sativa L.) and maize plants (“maizón”) were also used as hosts. Greenhouse colonies were maintained for several generations (more than 20) and periodically refreshed with wild insects to avoid inbreeding.
A thelytokous population of A. virlai was continuously reared for over 20 generations in D. maidis eggs laid in maize plants using the method described by Hill et al. (2019). Females of the corn leafhopper were allowed to oviposit on maize plants over the course of 24 h and the eggs obtained were subsequently offered to newly emerged wasps (age ≤ 24 h) during 24 h.
Host specificity and performance of Anagrus virlai
To obtain eggs prior to the tests, all Cicadellidae and Delphacidae species were caged during 24 h inside circular cages (8 cm diameter) that were spliced and fastened through metal clips to maize leaves. These rearing procedures were carried out for three consecutive years, from August 2019 to May 2021, in the laboratories of PROIMI-Biotecnología, Argentina. The number of adults enclosed varied from five to 30 female hoppers to obtain a variable density of host eggs. A total of 3366 A. dubius eggs, 84 A. marginellanus eggs, 1860 D. maidis eggs, 327 H. similis eggs, 381 P. mollicella eggs and 144 S. subolivacea eggs, for Cicadellidae; whereas 1218 D. kuscheli eggs, 1238 M. propinqua eggs and 1834 P. maidis eggs, for Delphacidae, were used in the assays (Table 1).
To estimate the performance of A. virlai, we assessed percentages of parasitism (both emerged and non-emerged parasitoids were considered) and wasp emergence in no-choice tests. A maize leaf containing hopper eggs was exposed to a newly emerged wasp in a glass tube (25 cm × 2.5 cm diameter) for 24 h in a controlled environment chamber (25 ± 1 °C, 50 ± 10% RH, and L:D 12:12). This procedure was repeated several times for each hopper species (the total number of exposed eggs and leaves is given in Table 1). A honey supplement placed on a small piece of opaline paper was added to the arena as food for the parasitoids. Leaves were left on the plant for ten to 12 days, and later cut off and transferred to Petri dishes for at least 20 days. Parasitised eggs were then identified by changes in egg coloration (they acquired a reddish coloration due to pupal development) (Jepsen et al. 2007). For unhatched eggs, the presence of undeveloped larvae or fully developed parasitoids that failed to emerge was verified by dissections using a stereoscopic microscope (Zeiss Stemi 2000c).
Effect of host size on the body size and egg load of parasitoids
To determine the influence of host size on parasitism and development success of parasitoids, eggs of all Cicadellidae and Delphacidae species were measured. Eggs were extracted from plants and placed on a concave microscope slide containing physiological saline solution. The formula of Berrigan (1991),\(V= \frac{1}{6} \pi {W}^{2} L\) (W = width and L = length), was used to estimate the volume of host eggs. A total of ten A. dubius, 15 A. marginellanus, 15 D. maidis, 15 H. similis, 16 P. mollicella and 15 S. subolivacea eggs; and 15 D. kuscheli, 15 M. propinqua, and 11 P. maidis eggs, were measured.
After the emergence of parasitoids from the three most parasitised species (A. dubius, A. marginellanus and D. maidis), egg loads of newly emerged females were recorded and the following morphometric parameters, often used to estimate parasitoid body size (Riddick 2005; Santolamazza Carbone et al. 2009), were measured: total body length, forewing maximum width and length, and hind tibia and gaster lengths. We performed these measurements on 22, three (a small number of wasps emerged from this host was obtained; see Results section) and 20 dissected A. virlai females emerged from eggs of A. dubius, A. marginellanus and D. maidis, respectively.
Data analysis
We conducted all statistical analyses and graphics using R software version 4.2.1 (R Core Team 2022). The effect of host species on parasitism and wasp emergence was analysed by fitting generalized linear models (GLM) with a binomial error distribution and a logit link function. For the analysis of emergence, we considered only those species that were successfully parasitised by A. virlai. We then performed likelihood ratio tests (LRT) and all pairwise comparisons (α = 0.05) using estimated marginal means (EMMs) (Lenth 2023) with Holm-Šidák adjustments.
Volumes of host eggs were tested using a GLM with inverse Gaussian family and mu-2 link function. We then conducted a LRT and an all pairwise comparison to compare groups. Comparisons between morphological traits measured on emerged wasps of the three most parasitised species were modelled using a GLM based on the Tweedie family of distributions with a log link function. To contrast differences, we performed a LRT and all pairwise multiple comparisons on least square means using a Holm-Šidák correction method. To determine if there was a relationship between morphometric variables and egg load of emerged parasitoids, we performed Spearman correlation analyses. In addition, we carried out a non-metric multidimensional scaling (NMDS) using Bray–Curtis dissimilarity index and the measured morphometric parameters as variables to visualize the similarity between emerged wasps of the three most parasitised host species. The resulting value of ordination stress (a measure of fit) for NMDS was below the accepted threshold of 0.20 (a stress value of 0.2 or less indicates a good fit) (Dexter et al. 2018). A permutational multivariate analyses of variance (PERMANOVA) with 5000 random starts and 999 permutations was conducted to test the null hypothesis that the centroids and dispersion of the groups as defined by measure space were equivalent for all groups. Finally, we performed a multilevel pairwise comparison using the Holm-Bonferroni method to determine which groups differed from each other. The NMDS and PERMANOVA were carried out using the metaMDS and adonis2 functions (Oksanen et al. 2022), whereas pairwise multilevel comparison using PERMANOVA was performed with the pairwise.adonis function (Martinez Arbizu 2020).
Results
Host specificity and performance of Anagrus virlai
Rates of parasitism and emergence of A. virlai were affected by host species and their taxonomic level (χ2 = 3177.90, df = 8, P < 0.001 and χ2 = 222.59, df = 5, P < 0.001). The three most parasitised species were the Deltocephalinae A. marginellanus, A. dubius and D. maidis, whereas parasitism rates were lower for the Delphacidae D. kuscheli, M. propinqua and P. maidis. The wasps were unable to parasitise the eggs of Cicadellinae H. similis, P. mollicella and S. subolivacea (Table 1). Although A. marginellanus was one of the most parasitised species, wasps did not develop successfully in this species and their emergence rate was nearly 2.6 times lower than those recorded in D. maidis and A. dubius, the most suitable hosts for the development of A. virlai. In Delphacidae, despite the low parasitism rate, some parasitoids developed successfully in the eggs of D. kuscheli, but their emergence rate was 55.3% lower than those recorded in A. dubius and D. maidis. Finally, M. propinqua and P. maidis also appeared to be occasional hosts for A. virlai, as evidenced by the lowest rates of parasitism and emergence observed in our tests. The results are summarized in Table 1.
Effect of host size on the body size and egg load of parasitoids
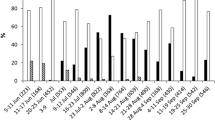
Hopper species had different egg sizes (χ2 = 1132.4, df = 8, P < 0.001). In the case of H. similis and P. mollicella, females laid eggs in groups (egg masses) of up to 12 eggs, whereas S. subolivacea laid them singly. Unlike the other Cicadellinae, H. similis had smaller eggs, whereas S. subolivacea had the largest eggs of all Cicadellidae tested. The smallest eggs within the Cicadellidae were those laid singly by D. maidis. The egg sizes of the two Amplicephalus species were similar. On the other hand, all Delphacidae deposited their eggs grouped, usually in the midrib and in close proximity to each other. These species had the smallest eggs of all hopper species measured, with the exception of D. kuscheli that oviposited eggs similar in size to D. maidis (Fig. 1).
The wasps emerged from the three most parasitised host species had varying body sizes and egg loads (Table 2). The largest wasps were those emerged from the eggs of A. dubius. In addition, the highest egg load was found in wasps reared in this Deltocephalinae. Although A. marginellanus laid larger eggs than D. maidis, wasps emerged from A. marginellanus did not exhibit higher egg loads. In fact, no correlation was found between morphometric variables and egg load for all emerged wasps, except for total body length and hind tibia length in parasitoids reared from D. maidis eggs (Table S1). In both cases, length was positively correlated with the egg load (Fig. 2).
Relationship between egg load and both total body length (a) and forewing length (b) of Anagrus virlai females emerged from Dalbulus maidis eggs. ρ shows the Spearman’s rank correlation coefficient and P-value indicates if the correlation coefficient was significantly different from zero (P ≤ 0.05). n = 20 wasps
The NMDS ordination differentiated between wasps that emerged from the three most parasitised species (PERMANOVA: R2 = 0.34, pseudo-F2 = 10.68, P = 0.001; Fig. 3). Parasitoids reared from the eggs of D. maidis, which were smaller than the rest of the Deltocephalinae, were clustered separately from those emerged from the eggs of A. dubius (R2 = 0.35, pseudo-F1 = 21.67, P = 0.003). Wasps emerged from A. marginellanus eggs did not segregate from those reared from the eggs of A. dubius or D. maidis (R2 = 0.10, pseudo-F1 = 2.44, P = 0.17; and R2 = 0.11, pseudo-F1 = 2.66, P = 0.17).
Discussion
Host specificity of a generalist egg parasitoid that has the potential to control economically important pest species may be a good predictor of its performance in the field, especially in complex agroecosystems where crops and surrounding vegetation are present. From the perspective of conservation biological control, the survival of parasitoids in agroecosystems is not always guaranteed by the provision of food, but also by the presence of alternative hosts and overwintering habitats where they are protected from adverse biotic and abiotic conditions (Gillespie et al. 2016). In our laboratory experiments, A. virlai parasitised several species of hoppers and exhibited different degrees of specificity, with its performance varying depending on the hosts. This wasp showed acceptable parasitic potential against A. dubius, A. marginellanus and D. maidis, all species belonging to the subfamily Deltocephalinae. The former two species, as well as the Delphacidae M. propinqua, are new factitious host records for A. virlai. On the contrary, Cicadellinae sharpshooters that laid larger eggs were not successfully parasitised by this parasitoid. A potential threshold limited by taxonomic, physiological and behavioural characteristics may be present. Our findings suggest that planthoppers may be occasional hosts for A. virlai. Thus, alternative hosts and non-crop habitats could alter the population dynamics of this parasitoid, favouring its persistence in the agroecosystem and its role as a biological control agent of Auchenorrhyncha pests in maize.
Although several egg parasitoids are considered generalists, many exhibit some degree of specialization (Bai et al. 1995). Insects in the family Mymaridae, for instance, are opportunistic wasps that parasitise host species, mainly Hemiptera Auchenorrhyncha, that oviposit hidden and endophytic eggs (Huber 2006). However, because it is difficult to observe and capture individuals in the field, the hosts of Mymaridae are mostly unknown, as well as their biological and ecological traits. Regardless, biological control programs have been developed in the past against Cicadellidae and Delphacidae using Mymaridae, including Anagrus wasps (Williams III and Martinson 2000; Krugner et al. 2009). Therefore, evaluating potential effects on alternative non-target hosts is critical to knowing their potential efficiency in the field (Mansfield and Mills 2004). Our findings are consistent with previous assumptions that Anagrus species have a relatively broad host range but still exhibit host preferences (Williams III and Martinson 2000) that are likely guided by host specificity. Thus, although A. virlai shows remarkable plasticity in development in target species and occasional hosts, this wasp manifests a potential host preference for Deltocephalinae eggs. Host preference between Cicadellidae and Delphacidae species has also been noted in other Anagrus species. The egg parasitoid A. incarnatus, classified as a generalist, appears to have a preference for parasitising Delphacidae eggs (Chantarasa-Ard et al. 1984). For A. epos, only Cicadellidae species are known to be hosts (Krugner et al. 2008b). In addition, interspecific competition between A. virlai and other Anagrus species may have led to a niche differentiation. The egg parasitoid Anagrus flaveolus Waterhouse, which co-occurs with A. virlai, parasitises three species of Cicadellidae, including D. maidis (with a low parasitism rate), and eight Delphacidae, including D. kuscheli, M. propinqua and P. maidis (Triapitsyn 2015), with a parasitism rate of about 40% in the eggs of D. kuscheli (Liljesthröm and Virla 2004). Thus, A. virlai might actively forage for suitable hosts, but would use other species as alternative hosts in their absence. Nevertheless, this hypothesis should be tested further.
The availability (host presence) and quantity of egg resource may change over time (nutritional content of eggs varies among species, and eggs are only available for parasitism for a short period of time until hatching) affecting the development and survival of the egg parasitoid (Barrett and Schmidt 1991). The adaptability of A. virlai is a highly desirable trait when planning biological control strategies through conservation. As previously stated, Anagrus species are able to choose between different host species, which in turn are associated with different plant species (Williams III and Martinson 2000; Zanolli and Pavan 2011). The genus Amplicephalus has been commonly associated with different grasses and crops, including maize, in different countries of North, Centre and South America (Luft Albarracin et al. 2008; De Oliveira et al. 2013; Perilla-Henao et al. 2016; Pérez López et al. 2017; Quiroga et al. 2020; Silva-Castaño et al. 2020; Almendra Paxtian et al. 2021). Amplicephalus has also been documented as vector of phytoplasma strains that infect different host plants (Pérez López et al. 2017) and, specifically in maize, some species may experimentally transmit “Maize Chlorotic Dwarf Virus” (MCDV) (Ammar and Nault 1991). In addition, some Amplicephalus species are commonly found on P. notatum plants, a common grass in maize agroecosystems (Paradell 1995). Anagrus virlai (misidentified as A. breviphragma) has also been recorded attacking eggs of D. kuscheli in oat in Argentina and P. maidis eggs in grasses and cane trash in Guiana (Triapitsyn 1997; Triapitsyn et al. 2019). Consequently, this wasp can use not only alternative host species to develop, but also other host plants where their hosts are available. Moreover, seasonality and availability of maize influence the presence of D. maidis eggs, since the corn leafhopper uses only maize and teosintes as host plants (the corn leafhopper does not oviposit eggs in the winter, and adults are the overwintering stage of the life cycle). Therefore, females of A. virlai like those of other Anagrus species, must exploit other hosts and oviposition sites for overwintering (Wright and James 2007).
The host specificity of an egg parasitoid can be influenced by the size of the host egg (Lytle et al. 2012). Our results showed that parasitoids emerged from larger eggs were larger and had a higher egg load. However, host identity also affected these parameters as females emerged from A. marginellanus eggs (the largest host eggs within the Deltocephalini) did not have the largest size and the highest egg load. In addition, only females reared from D. maidis eggs showed a positive relationship between egg load and total body length and hind tibia length. In other Mymaridae species, some morphometric variables were positively correlated with egg load: larger females of Anaphes nitens Girault (Hymenoptera: Mymaridae) reared from eggs of Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae) had a higher egg load (Santolamazza Carbone and Cordero Rivera 2003). However, some egg parasitoids such as Anaphes iole Girault and Cosmocomidea annulicornis (Ogloblin) (Hymenoptera: Mymaridae) may be exceptions to this rule: the lengths of the hind tibia and forewings of these wasps did not correlate with their egg loads (Riddick 2005; Manzano et al. 2022). Although we were unable to measure all morphometric variables in parasitoids emerged from Delphacidae species (parasitism and emergence rates were too low), Hill et al. (2019) found that the wasps emerged from P. maidis eggs are smaller than those reared from D. maidis eggs.
The relationship between adult size and fitness has received considerable attention in parasitoid behavioural ecology (Petersen and Hardy 1996), and larger females are predicted to carry a higher egg load (Ellers and Jervis 2003). Egg load of pro-ovigenic species (those that mature all or most of their lifetime complement of eggs prior to emergence from the hosts), such as A. virlai, has traditionally been used as a predictor of their potential performance in the field (Riddick 2005), although a trade-off between egg load and other life-history traits such as longevity has been long documented (Ellers et al. 2000). Anagrus virlai is also an autogenous species that parasitises its hosts from emergence. It does not require a preoviposition period and lays the majority of its eggs on the first day after the emergence (Hill et al. 2020). Therefore, a higher egg load may favour it if many host eggs are found in the field.
The tritrophic interaction between plants, herbivorous insects and their egg parasitoids has received much attention because of its key role in biological control. Although we were only able to test the host specificity of A. virlai, our results can be considered in characterizing its ecological host range. Further studies to evaluate the population dynamics of A. virlai and its hosts under field conditions may be necessary to better understand its role as a natural enemy of Auchenorrhyncha pests.
References
Almendra Paxtian L, García Martínez O, Robles Hernández VE, Sánchez Peña SR (2021) Cicadomorpha in a vineyard at Parras, Coahuila, Mexico, and vectors of diseases. Southwest Entomol 46:147–152
Alves E, Leite B, Marucci RC, Pascholati SF, Lopes JR, Andersen PC (2008) Retention sites for Xylella fastidiosa in four sharpshooter vectors (Hemiptera: Cicadellidae) analyzed by scanning electron microscopy. Curr Microbiol 56:531–538
Ammar ED, Nault LR (1991) Maize chlorotic dwarf viruslike particles associated with the foregut in vector and nonvector leafhopper species. Phytopathology 81:444–448
Antolin MF, Bjorksten TA, Vaughn TT (2006) Host-related fitness trade-offs in a presumed generalist parasitoid, Diaeretiella rapae (Hymenoptera: Aphidiidae). Ecol Entomol 31:242–254
Bai B, Çobanoĝlu S, Smith SM (1995) Assessment of Trichogramma species for biological control of forest lepidopteran defoliators. Entomol Exp Appl 75:135–143
Barrett M, Schmidt JM (1991) A comparison between the amino acid composition of an egg parasitoid wasp and some of its hosts. Entomol Exp Appl 59:29–41
Berrigan D (1991) The allometry of egg size and number in insects. Oikos 60:313–321
Brentassi ME, Machado-Assefh CR, Alvarez AE (2019) The probing behaviour of the planthopper Delphacodes kuscheli (Hemiptera: Delphacidae) on two alternating hosts, maize and oat. Austral Entomol 58:666–674
Chantarasa-Ard S, Hirashima Y, Hirao J (1984) Host range and host suitability of Anagrus incarnatus Haliday (Hymenoptera: Mymaridae): an egg parasitoid of delphacid planthoppers. Appl Entomol Zool 19:491–497
Corbett A, Rosenheim JA (1996) Impact of a natural enemy overwintering refuge and its interaction with the surrounding landscape. Ecol Entomol 21:155–164
De Oliveira CM, De Oliveira E, De Souza IRP, Alves E, Dolezal W, Paradell S, de Remes M, Lenicov AM, Frizzas MR (2013) Abundance and species richness of leafhoppers and planthoppers (Hemiptera: Cicadellidae and Delphacidae) in Brazilian maize crops. Fla Entomol 96:1470–1481
Dellapé G, Paradell S, Semorile L, Delfederico L (2016) The potential vectors of Xylella fastidiosa from Argentina: a study of leafhoppers and treehoppers (Hemiptera: Auchenorrhyncha) on citrus agroecosystems. Entomol Exp Appl 161:92–103
Dexter E, Rollwagen-Bollens G, Bollens SM (2018) The trouble with stress: a flexible method for the evaluation of nonmetric multidimensional scaling. Limnol Oceanogr Methods 16:434–443
Dietrich CH (2005) Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla Entomol 88:502–517
Dumón AD, Caro EA, Mattio MF, Alemandri V, del Vas M, Truol G (2018) Co-infection with a wheat rhabdovirus causes a reduction in Mal de Río Cuarto virus titer in its planthopper vector. Bull Entomol Res 108:232–240
EFSA Plh Panel, Bragard C, Dehnen-Schmutz K, Gonthier P, Jacques M-A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas-Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H-H, van der Werf W, Vicent Civera A, Yuen J, Zappala L, Candresse T, Lacomme C, Bottex B, Oplaat C, Roenhorst A, Schenk M, Di Serio F (2019) Scientific opinion on the pest categorisation of non-EU Cicadomorpha vectors of Xylella spp. EFSA J 17:5736
Ellers J, Jervis M (2003) Body size and the timing of egg production in parasitoid wasps. Oikos 102:164–172
Ellers J, Sevenster JG, Driessen G (2000) Egg load evolution in parasitoids. Am Nat 156:650–665
Gillespie MA, Gurr GM, Wratten SD (2016) Beyond nectar provision: the other resource requirements of parasitoid biological control agents. Entomol Exp Appl 159:207–221
Giménez Pecci MP, Laguna IG, Lenardón S (2012) Enfermedades del maíz producidas por virus y mollicutes en Argentina. Ediciones INTA, Buenos Aires
Hill JG, Luft Albarracin E, Coll Aráoz MV, Virla EG (2019) Effects of host species and host age on biological parameters of Anagrus virlai (Hymenoptera: Mymaridae), an egg parasitoid of Dalbulus maidis (Hemiptera: Cicadellidae) and Peregrinus maidis (Hemiptera: Delphacidae). Biol Control 131:74–80
Hill JG, Aguirre MB, Bruzzone OA, Virla EG, Luft Albarracin E (2020) Influence of adult diet on fitness and reproductive traits of the egg parasitoid Anagrus virlai (Hymenoptera: Mymaridae), a potential biocontrol agent against the corn leafhopper. J Appl Entomol 144:578–588
Hu G, Lu MH, Tuan HA, Liu WC, Xie MC, McInerney CE, Zhai BP (2017) Population dynamics of rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera, Delphacidae) in Central Vietnam and its effects on their spring migration to China. Bull Entomol Res 107:369–381
Huber JT (2006) Familia Mymaridae. In: Fernández F, Sharkey MJ (eds) Introducción a los Hymenoptera de la región Neotropical. Sociedad Colombiana de Entomología y Universidad de Colombia, Bogotá, pp 765–767
Jepsen SJ, Rosenheim JA, Bench ME (2007) The effect of sulfur on biological control of the grape leafhopper, Erythroneura elegantula, by the egg parasitoid Anagrus erythroneurae. Biocontrol 52:721–732
Krugner R, Johnson MW, Daane KM, Morse JG (2008a) Olfactory responses of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), to host plants infested by Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae). Biol Control 47:8–15
Krugner R, Johnson MW, Groves RL, Morse JG (2008b) Host specificity of Anagrus epos: a potential biological control agent of Homalodisca vitripennis. Biocontrol 53:439–449
Krugner R, Johnson MW, Morgan DJ, Morse JG (2009) Production of Anagrus epos Girault (Hymenoptera: Mymaridae) on Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) eggs. Biol Control 51:122–129
Lenth, R (2023) Package “emmeans”: estimated marginal means, aka least-squares means. https://cran.r-project.org/package=emmeans. Accessed 15 Feb 2023
Liljesthröm GG, Virla EG (2004) Density-dependent parasitism of Delphacodes kuscheli eggs by Anagrus flaveolus: influence of egg patchiness and density. Biocontrol Sci Technol 14:107–115
Linnavuori R (1959) Revision of the neotropical Deltocephalinae and some related subfamilies (Homoptera). Ann Soc Zool-Bot Fenn Vanamo 20:1–370
Liu F, Bao SW, Song Y, Lu HY, Xu JX (2010) Effects of imidacloprid on the orientation behavior and parasitizing capacity of Anagrus nilaparvatae, an egg parasitoid of Nilaparvata lugens. Biocontrol 55:473–483
Lou YG, Ma B, Cheng JA (2005) Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J Chem Eco 31:2357–2372
Luft Albarracin E, Paradell S, Virla EG (2008) Cicadellidae (Hemiptera: Auchenorrhyncha) associated with maize crops in northwestern Argentina, influence of the sowing date and phenology of their abundance and diversity. Maydica 53:289–296
Luft Albarracin E, Triapitsyn SV, Virla EG (2009) Annotated key to the genera of Mymaridae (Hymenoptera: Chalcidoidea) in Argentina. Zootaxa 2129(1):1–28
Luft Albarracin E, Triapitsyn SV, Virla EG (2017) Egg parasitoid complex of the corn leafhopper, Dalbulus maidis (DeLong) (Hemiptera: Cicadellidae), in Argentina. Neotrop Entomol 46:666–677
Lytle J, Morse JG, Triapitsyn SV (2012) Biology and host specificity of Gonatocerus deleoni (Hymenoptera: Mymaridae), a potential biocontrol agent of Homalodisca vitripennis (Hemiptera: Cicadellidae) in California, USA. Biocontrol 57:61–69
Mansfield S, Mills NJ (2004) A comparison of methodologies for the assessment of host preference of the gregarious egg parasitoid Trichogramma platneri. Biol Control 29:332–340
Manzano C, Virla EG, Coll Aráoz MV, Luft Albarracin E (2022) Ovigeny strategy of the parasitic wasp Cosmocomoidea annulicornis (Hymenoptera: Mymaridae): effect of female age, feeding and host availability on reproductive traits. Bull Entomol Res 112:228–235. https://doi.org/10.1017/S0007485321000766
Marino de Remes Lenicov AM, Virla EG (1999) Delfácidos asociados a cultivos de maíz en la República Argentina (Insecta-Homoptera-Delphacidae). Rev De La Fac De Agron 104:1–15
Marino de Remes Lenicov AM, Paradell S, Virla EG (2004) Homoptera Auchenorrhyncha. In: Cordo H, Logarzo G, Braun K, Diorio O (eds) Catálogo de los insectos fitófagos de la Argentina y sus plantas asociadas. Sociedad Entomológica Argentina Ediciones, Buenos Aires, pp 284–378
Martinez Arbizu P (2020) Pairwiseadonis: pairwise multilevel comparison using adonis. https://github.com/pmartinezarbizu/pairwiseadonis. Accessed 15 Feb 2023
Mattio MF, Velazquez P, Cassol A, Alemandri V, Truol G (2005) Toya propinqua Fieber como vector natural del Mal de Río Cuarto (MRCV). Fitopatologia 40:81
Moya Raygoza G, Larsen KJ, Rauk A (2005) Geographic and seasonal variation in size and color of adult corn leafhoppers (Hemiptera: Cicadellidae) from Mexico. Environ Entomol 34:1388–1394
Moya Raygoza G, Luft Albarracin E, Virla EG (2012) Diversity of egg parasitoids attacking Dalbulus maidis (Hemiptera: Cicadellidae) populations at low and high elevation sites in Mexico and Argentina. Fla Entomol 95:105–112
Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2022) Vegan: community ecology package. https://cran.r-project.org/package=vegan. Accessed 15 Feb 2023
Paradell S (1995) Especies argentinas de homópteros cicadélidos asociados al cultivo de maíz (Zea mays L.). Revista Fac Agron Univ Nac La Plata 71:213–234
Pérez López E, Wei W, Wang J, Davis RE, Luna Rodriguez M, Zhao Y (2017) Novel phytoplasma strains of X-disease group unveil genetic markers that distinguish North American and South American geographic lineages within subgroups 16SrIII-J and 16SrIII-U. Ann Appl Biol 171:405–416
Perilla-Henao L, Wilson MR, Franco-Lara L (2016) Leafhoppers Exitianus atratus and Amplicephalus funzaensis transmit phytoplasmas of groups 16SrI and 16Sr VII in Colombia. Plant Pathol 65:1200–1209
Petersen G, Hardy IC (1996) The importance of being larger: parasitoid intruder–owner contests and their implications for clutch size. Anim Behav 51:1363–1373
Pinedo Escatel JA, Moya Raygoza G (2015) Diversity of leafhoppers during the winter dry season on perennial grasses bordering harvested fields of maize. Southwest Entomol 40:263–272
Prischmann DA, James DG, Storm CP, Wright LC, Snyder WE (2007) Identity, abundance, and phenology of Anagrus spp. (Hymenoptera: Mymaridae) and leafhoppers (Homoptera: Cicadellidae) associated with grape, blackberry, and wild rose in Washington State. Ann Entomol Soc Am 100:41–52
Quiroga N, Gamboa C, Soto D, Pino AM, Zamorano A, Campodonico J, Alma A, Bertaccini A, Fiore N (2020) Update and new epidemiological aspects about grapevine yellows in Chile. Pathogens 9:933
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Raymond L, Plantegenest M, Gagic V, Navasse Y, Lavandero B (2016) Aphid parasitoid generalism: development, assessment, and implications for biocontrol. J Pest Sci 89:7–20
Riddick EW (2005) Egg load of lab-cultured Anaphes iole and effects of mate presence and exposure time on load depletion. Biocontrol 50:53–67
Rodríguez Juárez JG, Bartlett CR, Pinedo Escatel JA, Moya Raygoza G (2020) Diversity of planthoppers (Hemiptera: Delphacidae) on maize crops and edge grasses in Mexico. Environ Entomol 49:1088–1095
Rossinelli S, Bacher S (2015) Higher establishment success in specialized parasitoids: support for the existence of trade-offs in the evolution of specialization. Funct Ecol 29:277–284
Santolamazza Carbone S, Cordero Rivera A (2003) Egg load and adaptive superparasitism in Anaphes nitens, an egg parasitoid of the Eucalyptus snout-beetle Gonipterus scutellatus. Entomol Exp Appl 106:127–134
Santolamazza Carbone S, Pestaña Nieto M, Pérez Otero R, Mansilla Vázquez P, Cordero Rivera A (2009) Winter and spring ecology of Anaphes nitens, a solitary egg-parasitoid of the Eucalyptus snout-beetle Gonipterus scutellatus. Biocontrol 54:195–209
Silva-Castaño AF, Wilson MR, Brochero HL, Franco-Lara L (2020) Biodiversity, bugs, and barcodes: the Cicadellidae associated with grassland and phytoplasmas in the Sabana de Bogotá, Colombia. Fla Entomol 102:755–762
Torres Moreno R, Moya Raygoza G (2020) Response of egg parasitoids (Hymenoptera: Mymaridae and Trichogrammatidae) to the density of Dalbulus maidis (Hemiptera: Cicadellidae) eggs in maize habitats. Biol Control 150:104344
Triapitsyn SV (1997) The genus Anagrus (Hymenoptera: Mymaridae) in America south of the United States: a review. Ceiba 38:1–12
Triapitsyn SV (2015) Taxonomy of the genus Anagrus Haliday (Hymenoptera: Mymaridae) of the world: an annotated key to the described species, discussion of the remaining problems, and a checklist. Acta Zool Lilloana 59:3–50
Triapitsyn SV, Rugman Jones PF, Tretiakov PS, Luft Albarracin E, Moya Raygoza G, Querino RB (2019) Molecular, morphological, and biological differentiation between Anagrus virlai sp. n., an egg parasitoid of the corn leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) in the New World, and Anagrus incarnatus from the Palaearctic Region (Hymenoptera: Mymaridae). Neotrop Entomol 48:87–97
Tscharntke T, Bommarco R, Clough Y, Crist TO, Kleijn D, Rand TA, Tylianakis JM, van Nouhuys S, Vidal S (2008) Reprint of “Conservation biological control and enemy diversity on a landscape scale” [Biol Control 43 (2007) 294–309]. Biol Control 45:238–253
van Lenteren JC, Cock MJW, Hoffmeister TS, Sands DP (2006) Host specificity in arthropod biological control, methods for testing and interpretation of the data. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. CABI Publishing, Wallingford, pp 38–63
Vilanova ES, Ramos A, de Oliveira MCS, Esteves MB, Gonçalves MC, Lopes JR (2022) First report of a Mastrevirus (Geminiviridae) transmitted by the corn leafhopper. Plant Dis 106:1330–1333
Virla EG, Moya Raygoza G, Luft Albarracin E (2013) Egg parasitoids of the corn leafhopper, Dalbulus maidis, in the southernmost area of its distribution range. J Insect Sci 13:10
Virla EG, Coll Aráoz MV, Luft Albarracin E (2021) Estimation of direct damage to maize seedlings by the corn leafhopper, Dalbulus maidis (Hemiptera: Cicadellidae), under different watering regimes. Bull Entomol Res 111:438–444
Volf M, Pyszko P, Abe T, Libra M, Kotásková N, Šigut M, Kumar R, Kaman O, Butterill PT, Šipoš J, Abe H, Fukushima H, Drozd P, Kamata N, Murakami M, Novotny V (2017) Phylogenetic composition of host plant communities drives plant-herbivore food web structure. J Anim Ecol 86:556–565
Williams L III, Martinson TE (2000) Colonization of New York vineyards by Anagrus spp. (Hymenoptera: Mymaridae): overwintering biology, within-vineyard distribution of wasps, and parasitism of grape leafhopper, Erythroneura spp. (Homoptera: Cicadellidae), eggs. Biol Control 18:136–146
Wright LC, James DG (2007) Anagrus spp. (Hymenoptera: Mymaridae) reared from plants collected during winter in south central Washington and north central Oregon. J Entomol Soc B C 104:17–24
Young DA (1977) Taxonomic study of the Cicadellinae (Homoptera: Cicadellidae). Part 2. New World Cicadellini and the genus Cicadella. Technical Bulletin of the North Carolina Agricultural Experiment Station
Zahaniser J, Dietrich C (2013) A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur J Taxon 4:1–211
Zanolli P, Pavan F (2011) Autumnal emergence of Anagrus wasps, egg parasitoids of Empoasca vitis, from grapevine leaves and their migration towards brambles. Agric for Entomol 13:423
Acknowledgements
This work was partially supported by PICT-FONCyT nº 01309 and PIP- CONICET nº 0521. Jorge G. Hill and Carolina Manzano want to thank CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina) for their scholarships. We are also grateful to the editor and three anonymous reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Contributions
JGH reared and managed both hoppers and parasitoids, conducted experiments, analysed data and wrote the manuscript. EGV conceived and designed research, reviewed and edited the manuscript, and secured funding. SLP contributed to Cicadellidae identification and checking and editing this paper. CM contributed to rearing and managing both hoppers and parasitoids, reviewed and edited the manuscript. ELA contributed with experimental design, manuscript corrections and secured funding. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose and they declare that there are no conflicts of interest.
Research involving human participants and/or animals
No humans and/or animals were used in this work that required informed consent.
Additional information
Handling Editor: Josep Anton Jaques Miret.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hill, J.G., Virla, E.G., Manzano, C. et al. Host specificity and performance on different hopper species of the egg parasitoid Anagrus virlai. BioControl 68, 131–142 (2023). https://doi.org/10.1007/s10526-023-10191-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10191-9