Abstract
Anagrus epos Girault (Hymenoptera: Mymaridae) is a candidate for a classical biological control program targeting the glassy-winged sharpshooter (GWSS), Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae), in California. Because mass production of GWSS is expensive and labor-intensive, a factitious host that is more economical to produce is desirable to mass produce A. epos for colonization and augmentation efforts. Here, we report the results of host specificity tests and potential rearing techniques for A. epos under laboratory conditions. Females discriminated and oviposited into eggs of seven cicadellid species: H. vitripennis, Circulifer tenellus (Baker), Erythroneura variabilis Beamer, Amblysellus grex (Oman), Graphocephala atropunctata (Signoret), Macrosteles severini Hamilton, and H. liturata Ball, and two cerambycid species: Phoracantha recurva Newman and P. semipunctata (F.). Anagrus epos successfully completed development in the eggs of H. vitripennis, C. tenellus, E. variabilis, A. grex, G. atropunctata, M. severini, and H. liturata. The use of a factitious host and potential nontarget effects of this generalist parasitoid are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The glassy-winged sharpshooter (GWSS), Homalodisca vitripennis (Germar), is a cicadellid native to the southern US that was first detected in California in 1990 (Sorensen and Gill 1996). It probably entered via egg masses upon nursery stock, and rapidly became responsible for spreading the bacterium Xylella fastidiosa Wells among vineyards throughout the Temecula area of southern California (Blua et al. 1999). Xylella fastidiosa is the causal agent of Pierce’s disease (PD) in grapes (Davis et al. 1978), which is one of the most economically important crops in California (US$4.1 billion/year), with over 337,371 ha distributed throughout the state (CDFA 2006). Current management of PD, resulting from X. fastidiosa spread by GWSS, includes removal of diseased grapevines (Hashim and Hill 2003) and the use of insecticides and biological control agents to reduce GWSS populations in citrus groves, urban areas, and vineyards (Wendel et al. 2002; Hix et al. 2003).
The search for effective parasitoids throughout the US and Mexico (Triapitsyn and Phillips 1996, 2000; Triapitsyn et al. 1998, 2003; Hoodle and Triapitsyn 2004; Goolsby et al. 2006) and South America (Logarzo et al. 2004; de Leon et al. 2006) resulted in the collection of several mymarid and trichogrammatid species. Among these egg parasitoids, the mymarids Gonatocerus ashmeadi Girault, G. fasciatus Girault, G. morrilli (Howard), G. triguttatus Girault, and G. walkerjonesi Triapitsyn have been mass produced and released in California to suppress GWSS populations (CDFA 2003; Morse et al. 2006). Anagrus epos Girault (Hymenoptera: Mymaridae) is a newly introduced egg parasitoid brought to California from Minnesota, where it attacks eggs of Cuerna fenestella Hamilton (Hemiptera: Cicadellidae), a native, univoltine proconiine sharpshooter (Triapitsyn and Rakitov 2005; Morse et al. 2006). While in quarantine, A. epos was found to successfully parasitize GWSS eggs. It is now a candidate for mass production and release against GWSS in California. Additionally, A. epos is known to parasitize the eggs of at least five cicadellid species found in New Mexico, New York, and Colorado (Triapitsyn 1998), which suggests that if established, it may potentially use native California species as alternative hosts in areas where GWSS has not yet established or during periods when GWSS egg masses are scarce or unavailable.
Production of GWSS egg masses for insectary production of parasitoids is expensive, laborious, and space-demanding. Given this, an easier and less expensive method was sought to produce A. epos using the eggs of a factitious host. We also sought to define the potential host range of A. epos in California. Here, we report the results of (1) tests to determine levels of A. epos female discrimination and host suitability and (2) a potential laboratory rearing technique for A. epos using beet leafhopper, Circulifer tenellus (Baker) (Hemiptera: Cicadellidae), and the aster leafhopper, Macrosteles severini Hamilton (Hemiptera: Cicadellidae).
Materials and methods
Establishment and maintenance of the A. epos colony
An A. epos colony was established from individuals that emerged in the Entomology Department Quarantine Facility at the University of California at Riverside (UCR) on 8 June 2004 from eggs of C. fenestella Hamilton on Solidago sp. (goldenrod, Compositae) and Zigadenus sp. (death camus, Liliaceae) collected by R. Rakitov from 31 May to 1 June 2004 in Glyndon, Minnesota. Mated females were exposed to fresh GWSS eggs laid on Euonymus japonica L. f. leaves on 9 June 2004. The F1 generation emerged on 29–30 June.
The A. epos colony was maintained weekly by adding 5–10 E. japonica leaves (ca. 8 cm2) infested with GWSS egg masses laid by field-collected adults. Leaves were inserted through foam sheets (9.7 × 9.7 cm2), which were floated on 3 cm of water within the bottom of rectangular, acrylic cages (10 × 10 × 15 cm3) with fine mesh screening on the sides. To refill the water within the cages, the bottom of the cage was perforated and cages were kept in water-filled trays. Honey was streaked on the cage wall(s) and about 50 newly emerged females and 10 males were introduced into each cage. Cages were held under constant room conditions (20–25 °C, 60–80% RH, and 16:8 h L:D). Parasitoid development from egg to adult was completed within 20–30 days, and the rearing process was repeated following adult eclosion. A. epos was reared for over 14 generations on GWSS eggs until use in the experiments described herein. Experiments were conducted from March 2005 to November 2006 using wasps reared from GWSS eggs.
Species used for host preference and suitability testing
Insect species selected for the assays were chosen based on (1) host taxonomy, because they shared similar physiological properties and defense mechanisms; (2) shared ecology, because unrelated hosts may share the same host plant or feeding niche (Shaw 1994; Godfray 1994; Strand and Obrycki 1996), and (3) relatively low cost needed to mass produce the species in a controlled environment throughout the year. Seven cicadellid species [GWSS, H. vitripennis; beet leafhopper, C. tenellus; blue-green sharpshooter, Graphocephala atropunctata (Signoret); aster leafhopper, M. severini; smoketree sharpshooter, H. liturata Ball; variegated leafhopper, Erythroneura variabilis Beamer; and Amblysellus grex (Oman)] and the torpedo bug, Siphanta acuta (Walker) (Hemiptera: Flatidae) met these criteria. In addition, two species of Coleoptera [the eucalyptus long-horned borers, Phoracantha recurva Newman (Cerambycidae) and P. semipunctata (F.)], and three species of Lepidoptera [tobacco budworm, Heliothis virescens (F.) (Noctuidae), oriental fruit moth, Grapholita molesta (Busck) (Tortricidae), and Mediterranean flour moth, Ephestia kuehniella Zeller (Pyralidae)] that were readily available were tested to determine if wasp ovipositional behavior might occur on eggs of a similar size but in species outside the Hemiptera.
GWSS eggs were obtained as described above under colony maintenance. Beet leafhopper eggs were obtained from a colony initiated from field collections from weedy vegetation near Coalinga, CA, in late winter and early spring 2004. The colony was maintained in a greenhouse on potted sugar beet, Beta vulgaris var. saccharifera L. Sugar beet seedlings (5–7 leaves) infested with beet leafhopper eggs were produced by exposing plants to approximately 200 adult leafhopper females in a wooden cage (80 × 40 × 40 cm3) for 4 days.
Adults of the blue-green sharpshooter were obtained from a laboratory colony at UCR maintained on potted basil, Ocimum basilicum L. plants. Basil plants were kept inside rearing cages (60 × 60 × 60 cm3) constructed with a wooden frame, an acrylic top, and screen mesh sides. Rearing cages were kept in a greenhouse maintained at 24–27 °C, 22–25% RH, and 16:8 h L:D. Basil plants infested with blue-green sharpshooter eggs were obtained by caging five adult females and two males per plant for 6 days.
A colony of the aster leafhopper was established in the Department of Entomology at UCR using adults obtained from a culture maintained by R. P. P. Almeida at the University of California, Berkeley. Rearing cages were kept in a room with artificial light maintained at 16:8 h L:D, 25 °C, and 40–60% RH. Barley plants infested with aster leafhopper eggs were obtained by caging 30 adult females and five males per plant for 2 days.
Adults of the torpedo bug, smoketree sharpshooter, variegated leafhopper, and A. grex were collected from citrus near San Juan Capistrano, citrus in Riverside, grapevines near Fresno, and tall fescue in Riverside, respectively. The torpedo bug, variegated leafhopper, and A. grex were confined on potted representatives of the plants from which they were collected for a 1–5-day ovipositional period. The smoketree sharpshooters were confined on E. japonica plants to obtain newly laid eggs. Eggs of the eucalyptus long-horned borers laid on paper sheets were obtained from a laboratory colony maintained at the Citrus Experiment Station, UCR, as described by Hanks et al. (1993). Fresh eggs of the tobacco budworm, oriental fruit moth, and irradiated eggs of Mediterranean flour moth laid on glass slides, wax paper, and paper sheets, respectively, were obtained from laboratory colonies at UCR. Plants, leaves, and rearing substrates containing potential host eggs were taken into quarantine and tested under constant room conditions (20–25 °C, 60–80% RH, and 16:8 h L:D).
Evaluation of A. epos female discrimination
Eggs of all phytophagous species listed above were exposed to 48-h-old, naïve (i.e., with no prior exposure to host eggs) A. epos females under no-choice conditions. Anagrus epos females were kept in the rearing cages with males until they were used in the experiments. Substrates (e.g., leaves, paper, glass slides) infested with or supporting eggs were placed in Petri dishes, were exposed to a group of 10 A. epos females for a 1-h observation period, and were viewed through a video camera mounted on a stereoscope. Because A. epos females are relatively small in size and were tested in groups, it was not possible to simultaneously record the ovipositional behavior and the minimum minutes for egg location by each individual female, except when the event occurred in the same host egg. Among the tested host species, the minimum minutes for egg location were recorded for the first female to locate the egg and initiate the ovipositional behavior. The general host location and ovipositional behaviors were described based on multiple observations. After observation, wasps and leaves or ovipositional substrates were discarded. As a control, two GWSS egg masses were exposed in a separate Petri dish to A. epos females from the same test brood to ensure that females were mated, fertile, and fecund. All observations were conducted between 09:00 and 14:00 h. After observation, host suitability tests were conducted as described below, with all 12 test species, independent of whether or not an ovipositional event was observed.
Host suitability tests
To determine if A. epos larvae could complete development on eggs of the 12 test species, the following methods were used with the listed host plant assemblage and cage setup used for the indicated host species. Beet leafhopper: each of five potted sugar beet plants (5–7 leaves) infested with 1–5-day-old leafhopper eggs were covered with an acrylic cage as described under A. epos colony maintenance (i.e., five replicates were conducted); blue-green sharpshooter: five potted basil plants (10 cm high) infested with 1–6-day-old sharpshooter eggs were covered with an acrylic cage; aster leafhopper: each of five barley bunches (20 seedlings) infested with 1–2-day-old leafhopper eggs were covered with an acrylic cage; A. grex: each of four tall fescue bunches, Festuca arundinacea Schreb (30–50 leaves, 10 cm high), infested with 1–3-day old leafhopper eggs were covered with an acrylic cage; variegated leafhopper: each of five potted Thompson seedless grapevines, Vitis vinifera L., (8–10 leaves) infested with 1–3-day-old leafhopper eggs were covered with a fine mesh screen; torpedo bug: each of six citrus, Citrus volkameriana (Pasq.), leaves infested with a 2-day-old leafhopper egg mass containing approximately 100 eggs each were exposed as described above for A. epos colony maintenance; and smoketree sharpshooter: each of 12 E. japonica leaves infested with a sharpshooter egg mass containing approximately four eggs were exposed as described for torpedo bug. Phoracantha recurva, P. semipunctata, tobacco budworm, oriental fruit moth, and Mediterranean flour moth were tested using their respective rearing substrate placed within the acrylic cages in four, one, three, three, and three replicates, and a total of 82, 28, 19, 42, and >500 eggs, respectively.
For each plant assemblage described above, 15 A. epos females were introduced into each cage and allowed to oviposit for 72 h. An additional host plant assemblage and cage without A. epos adults was used as a control and two GWSS egg masses were exposed to 15 A. epos as a control. After the exposure period, wasps were removed from the cages and discarded. Plants, leaves with eggs, and cages were thoroughly washed with water to remove remaining wasps. Cages were observed daily for immature host and/or A. epos emergence. We recorded the number and sex ratio of emerged A. epos and the total number of immature hosts that emerged within each cage, except for A. epos adults that emerged from GWSS egg masses (control).
Because it was difficult and prohibitively labor-intensive to simultaneously maintain several insect colonies to provide eggs for such tests, we were forced to conduct experiments on different days, use field-collected adults to provide eggs, and use A. epos females from different broods in our experiments. Moreover, due to the small egg size and the endophytic ovipositional behavior of some hosts (i.e., blue-green sharpshooter, variegated leafhopper, aster leafhopper, beet leafhopper, and A. grex), we were unable to manipulate the total number of host eggs exposed to each A. epos female. Therefore, comparative, statistical analyses among species were considered inappropriate due to the variability and differences among the various host insect/plant assemblages. When possible, means ± SEM were determined for data (e.g., host insect and parasitoid emergence, percentage parasitism) recorded for individual hosts.
Results and discussion
Evaluation of A. epos female discrimination
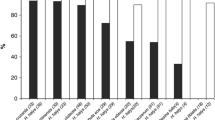
Of the 13 species studied, A. epos showed interest in and inserted its ovipositor into the eggs of eight species (Table 1). None of the eggs of the three lepidopteran species, torpedo bug, or the blue-green sharpshooter were observed being attacked. Anagrus epos females noticeably reduced their walking speed and increased their frequency of turning and drumming the substrate with their antenna after approaching the area on the leaf where the eggs of GWSS, beet leafhopper, variegated leafhopper, A. grex, aster leafhopper, and smoketree sharpshooter were located. Two of the 10 females exposed to beet leafhopper eggs located the eggs within the leaf in less than 40 s after exposure. After 10–12 s of drumming the eggs with their antennae, these females initiated an ovipositor behavioral response (i.e., drilling the leaf epidermis and egg shell followed by insertion of the ovipositor, abdominal contractions, and withdrawal of the ovipositor) that lasted from 20 s to 3 min. Other tested females initially spent some time resting and grooming their antennae, but eventually located host eggs during the observation period. Similar behaviors were observed for GWSS, variegated leafhopper, A. grex, aster leafhopper, and smoketree sharpshooter, except that the entire host location and recognition process took approximately 10, 56, 20, 21, and 10 min, respectively. Interestingly, A. epos females promptly perceived P. recurva and P. semipunctata eggs as potential hosts and performed the ovipositor behavioral response, which lasted from 1 to 3 min. Females performed the ovipositor behavioral response 2–3 times in each individual beetle egg, and sometimes returned to the same egg after visiting other nearby eggs. The cerambycid beetle eggs are relatively large (4 mm in length) compared to A. epos female adults (0.5 mm). This suggests that A. epos might have laid several eggs in each host egg. No ovipositor behavioral response was observed for eggs of tobacco budworm, oriental fruit moth, Mediterranean flour moth, torpedo bug, or blue-green sharpshooter, which suggests that these species were not perceived as potential hosts. However, we were unable to determine to what degree basil cuttings hosted blue-green sharpshooter eggs during the observation period and failed to observe ovipositor insertion or ovipositional behavior with this leafhopper. Based on Anagrus emergence from infested basil plants (below), obviously such behavior occurred. Females exposed to lepidopteran and torpedo bug eggs spent most of their time resting or walking around the Petri dish and never stopped to inspect any eggs. Overall, females in the act of oviposition permitted a second Anagrus female to simultaneously oviposit in the same egg, and only briefly lifted their wings when the second female approached.
Host suitability tests
Anagrus epos successfully completed development in GWSS, beet leafhopper, variegated leafhopper, A. grex, aster leafhopper, smoketree sharpshooter, and blue-green sharpshooter eggs. None of the exposed eggs of the torpedo bug, tobacco budworm, oriental fruit moth, or Mediterranean flour moth produced A. epos offspring, thereby confirming our female discrimination observations (Table 1). Table 2 shows a summary of A. epos female developmental time, sex ratio, and parasitism rate observed in host suitability tests from which progeny emerged. Mean female developmental time in beet leafhopper eggs was 25.7 days (range 18–53 days). Among the five tested plants, a mean of 219.0 females were reared. The sex ratio of the progeny that emerged from each cage was relatively constant throughout the emergence period and, most importantly, at least one male emerged each day, which under natural conditions, could reduce the chance that females leave the natal site unmated. The estimated mean number of unparasitized eggs (i.e., leafhoppers that emerged) was 106.2 ± 21.92, which yields a mean parasitism rate of 71.4%.
Anagrus epos female mean developmental time in variegated leafhopper eggs was 23.9 days (range 23–25 days). A mean of 343.8 ± 63.99 leafhopper nymphs emerged per plant, which suggested relatively low parasitism rates. However, microscopic examination of leaf material revealed the presence of numerous A. epos pupae that were unable to emerge from the eggs deposited along the leaf veins. Part of the grape leaves were dry during the A. epos emergence period because of laboratory conditions and intense feeding by variegated leafhopper adults during the infestation period, which suggests that A. epos adults may require fresh leaf tissue to improve their ability to chew through the leaf epidermis and emerge. Nevertheless, our results agreed with previous records of A. epos parasitism on eggs of Erythroneura spp. including E. variabilis in Sonora (Mexico); E. bistrata McAtee, E. aclys McAtee, and E. comes (Say) in New York; and E. vulnerata Fitch in Colorado (Triapitsyn 1998).
Anagrus epos successfully developed in A. grex eggs, a cicadellid species collected in tall fescue, F. arundinacea, a Graminea commonly found in landscapes and parks throughout southern California. Mean female developmental time from egg to adult was 25.7 days (range 19–35 days). A mean of 44.6 ± 17.05 leafhopper nymphs emerged per plant, which resulted in an estimated mean parasitism rate of 21.7%.
Anagrus epos mean female developmental time in aster leafhopper eggs was 26.8 days (range 18–43 days). Among the five tested barley bunches, a mean of 25.8 ± 3.05 leafhopper nymphs emerged per plant. Parasitism rate was estimated to be 50.4%.
A total of seven of the 48 smoketree sharpshooter eggs were parasitized and yielded a total of 19 female and 10 male A. epos adults. Among the remaining sharpshooter eggs, nine produced sharpshooter nymphs and 32 died of fungal infection. It was not possible to determine if dead sharpshooter eggs were parasitized. Mean A. epos female developmental time from egg to adult was 26.1 days (range 22–30 days).
Although the ovipositional behavior of A. epos on blue-green sharpshooter eggs was not observed in the female discrimination study, a mean of 12.0 and 23.4 male and female A. epos adults, respectively, emerged from the five basil plants tested in the host suitability study. Seven sharpshooter nymphs emerged from the control plant and no nymphs emerged from the tested plants. Mean female developmental time from egg to adult was 38.1 days (range 35–58 days).
Although A. epos females appeared to parasitize eggs of P. recurva and P. semipunctata, none of the attacked eggs produced A. epos offspring. Small black scars on the egg surfaces accompanied by browning of internal tissues were visible on the beetle eggs attacked by A. epos. A total of 8 and 15 eggs of P. recurva and P. semipunctata, respectively, were attacked six or seven times by A. epos females and failed to produce first instar beetle larvae, while eggs attacked one to three times developed normally and produced first instar beetle larvae. It is unknown whether physiological interactions with the host egg constrained A. epos oviposition or if host defenses caused an impediment to egg development (e.g., encapsulation). However, because A. epos attacked the eggs of distantly related hosts, this may pose implications to mass releases against GWSS, as eucalyptus is a GWSS oviposition host. Tested beetles oviposited under loose bark and in bark fissures of eucalyptus trees in batches of up to 40 eggs (Hanks et al. 1993). Anagrus epos females will probably behave differently under natural conditions and perhaps never search and attack these beetle eggs, and additional, field-scale evaluations are warranted. However, if proven to the contrary, the beetle eggs may function as a sink to released wasps because they would fail to yield A. epos progeny.
Future studies should investigate the relative importance of ecological habitat and host selection process, both of which will likely influence A. epos host range and may help to predict any ecological risks of its introduction. Host range data are difficult to collect because this involves rearing many different insect and plant host species found in California. The observed host range of a parasitoid will be influenced by evolutionary and behavioral aspects (Godfray 1994; Strand and Obrycki 1996). Evolutionary aspects include host taxonomy and shared ecology. Behavioral aspects focus mainly on the host selection process, which involves host quality, host location and recognition, site of emergence, and external and internal host characteristics. A testing procedure to evaluate the relative potential risk to nontarget plant species in weed biological control was summarized by Briese (2005) and logically applies to biological control of arthropods. The risk of attack would increase with increasing phylogenetic relatedness, biogeographical overlap, and ecological similarity among host species. In our studies, A. epos was able to successfully develop in the eggs of seven cicadellid species, each of which occupies different niches in California. Variegated leafhopper, blue-green sharpshooter, and smoketree sharpshooter are the only tested species that have some degree of phylogenetic relatedness, biogeographical overlap, and ecological similarities with GWSS and, therefore, present an increased risk. The beet leafhopper feeds and breeds on an extensive range of plant species (Cook 1967), concomitant with the range of species used by GWSS as feeding and reproductive hosts, but their overlap in time and space needs further investigation. The aster leafhopper and A. grex feed and reproduce in grasses that could be used as cover crops in grape and citrus orchards and may serve as reservoirs or over-wintering sites for A. epos when the availability of GWSS egg masses are dramatically reduced.
In terms of mass production of A. epos, the smoketree sharpshooter, and variegated leafhopper are difficult to rear in controlled environments. Smoketree sharpshooter has similar rearing difficulties as GWSS, and rearing variegated leafhopper requires greenhouse space for potted grapevines and constant removal of nymphs (nonparasitized eggs) to avoid rapid leaf senescence. Sugar beet plants, tall fescue, and barley are relatively easy to grow and can host hundreds of beet leafhopper, A. grex, and aster leafhopper eggs, respectively. In general, there was a 2–3-day overlap between the last emerged leafhopper nymph and the first A. epos adult to emerge, which suggests that plants containing parasitized eggs can be held in the rearing facility until nearly all leafhoppers emerge and later safely placed in the field for mass releases without introducing leafhoppers into the release area. Triapitsyn and Moratorio (1998) developed a rearing technique for A. nigriventris Girault using potted sugar beet plants infested with beet leafhopper eggs. Their method allowed one person to produce 20,000 adult A. nigriventris wasps (males and females) a month. Our rearing system using beet leafhopper and aster leafhopper as factitious hosts for A. epos is still under development.
It is unknown if A. epos reared from beet leafhopper, blue-green sharpshooter, aster leafhopper, or A. grex will search for and parasitize GWSS eggs under natural conditions. However, our findings suggest that A. epos is a generalist egg parasitoid with the potential to establish in California by parasitizing eggs of cicadellid leafhoppers. Our particular interest at the moment is the establishment of A. epos in the northern grape growing regions (e.g., Napa and Sonoma Counties) of California where GWSS has yet to become established, especially as an egg parasitoid of the blue-green sharpshooter, the most important vector of X. fastidiosa in coastal California (Purcell and Feil 2001). To date, there are only two reported egg parasitoids, G. latipennis Girault and Polynema sp. (Hymenoptera: Mymaridae), of the blue-green sharpshooter collected from sentinel plants in southern California (Boyd and Hoddle 2006). Future laboratory studies involving comparative fitness measurements among individuals reared from different hosts followed by small field releases should provide important information allowing comparison of the potential host range observed in laboratory studies with the realized host range in the field, thus helping to determine the efficacy of A. epos as a natural enemy of GWSS.
References
Blua MJ, Phillips PA, Redak RA (1999) A new sharpshooter threatens both crops and ornamentals. Calif Agric 53:22–25
Boyd EA, Hoddle MS (2006) Oviposition and flight activity of the blue-green sharpshooter (Hemiptera: Cicadellidae) on southern California wild grape and first report of associated egg parasitoids. Ann Entomol Soc Am 99:1154–1164
Briese DT (2005) Translating host-specificity test results into the real world: the need to harmonize the yin and yang of current testing procedures. Biol Control 35:208–214
Cook WC (1967) Life history, host plants, and migrations of the beet leafhopper in the western United States. USDA Technical Bulletin 1365, 122pp
CDFA (2003) Pierce’s disease program report to the legislature. California Department of Food and Agriculture
CDFA (2006) County agricultural commissioner’s data, calendar year 2005. California Department of Food and Agriculture, Sacramento, CA. http://www.nass.usda.gov/ca/bul/agcom/indexcac.htm. Cited 1 Dec 2006
Davis MJ, Purcell AH, Thompson SV (1978) Pierce’s disease of grapevines: isolation of the causal bacterium. Science 199:75–77
de Leon JH, Logarzo GA, Triapitsyn SV (2006) Genetic characterization of Gonatocerus tuberculifemur from South America uncovers divergent clades: prospective egg parasitoid candidate agent for the glassy-winged sharpshooter in California. In: Esser T, Tariq MA, Medeiros R, Mochel M, Veling S (eds) Proceedings, 2006 Pierce’s disease research symposium, San Diego, CA, 27–29 November 2006. Copeland Printing, Sacramento, CA, pp 40–43
Godfray HCJ (1994) Parasitoids. Behavioral and evolutionary ecology. Princeton University Press, Princeton, NJ
Goolsby J, Bextine B, Skevington J (2006) Exploration for biological control agents in the native range of the glassy-winged sharpshooter. In: Esser T, Tariq MA, Medeiros R, Mochel M, Veling S (eds) Proceedings, 2006 Pierce’s disease research symposium, San Diego, CA, 27–29 November 2006. Copeland Printing, Sacramento, CA, pp 67–69
Hanks LM, McElfresh JS, Millar JG, Paine TD (1993) Phoracantha semipunctata (Coleoptera: Cerambycidae), a serious pest of Eucalyptus in California: biology and laboratory-rearing procedures. Ann Entomol Soc Am 86:96–102
Hashim J, Hill BL (2003) Monitoring and control measures for Pierce’s disease in Kern County, and epidemiological assessments of Pierce’s disease. In: Tariq MA, Oswalt S, Blincoe P, Spencer R, Houser L, Ba A, Esser T (eds) Proceedings, 2003 Pierce’s disease research symposium, Coronado, CA, 8–11 December 2003. Copeland Printing, Sacramento, CA, pp 95–98
Hix R, Toscano N, Gispert C (2003) Area-wide management of the glassy-winged sharpshooter in the Temecula and Coachella Valleys. In: Tariq MA, Oswalt S, Blincoe P, Spencer R, Houser L, Ba A, Esser T (eds) Proceedings, 2003 Pierce’s disease research symposium, Coronado, CA, 8–11 December 2003. Copeland Printing, Sacramento, CA, pp 292–294
Hoodle MS, Triapitsyn SV (2004) Searching for and collecting egg parasitoids of glassy-winged sharpshooter in the central and eastern USA. In: Tariq MA, Oswalt S, Blincoe P, Ba A, Lorick T, Esser T (eds) Proceedings, 2004 Pierce’s disease research symposium, Coronado, CA, 7–10 December 2004. Copeland Printing, Sacramento, CA, pp 342–344
Logarzo GA, Virla EG, Triapitsyn SV, Jones WA (2004) Biology of Zagella delicata (Hymenoptera: Trichogrammatidae), and egg parasitoid of the sharpshooter Tapajosa rubromarginata (Hemiptera: Clypeorryncha: Cicadellidae) in Argentina. Fla Entomol 87:511–516
Morse JG, Morgan DJW, Lytle JM (2006) Seasonal population dynamics of glassy-winged sharpshooter egg parasitoids: variability across sites and host plants. In: Esser T, Tariq MA, Medeiros R, Mochel M, Veling S (eds) Proceedings, 2006 Pierce’s disease research symposium, San Diego, CA, 27–29 November 2006. Copeland Printing, Sacramento, CA, pp 92–94
Purcell AH, Feil H (2001) Glassy-winged sharpshooter. Pestic Outlook 12:199–203
Shaw MR (1994) Parasitoid host ranges. In: Hawkins BA, Sheehan W (eds) Parasitoid community ecology. Oxford University Press, New York, pp 111–114
Sorensen JT, Gill RJ (1996) A range extension of Homalodisca coagulata (Say) (Hemiptera: Clypeorrhyncha: Cicadellidae) to southern California. Pan-Pac Entomol 72:160–161
Strand MR, Obrycki JJ (1996) Host specificity of insect parasitoids and predators. Bioscience 46:422–428
Triapitsyn SV (1998) Anagrus (Hymenoptera: Mymaridae) egg parasitoids of Erythroneura spp. and other leafhoppers (Homoptera: Cicadellidae) in North American vineyards and orchards: a taxonomic review. Trans Am Entomol Soc 124:77–112
Triapitsyn SV, Phillips PA (1996) Egg parasitoids of glassy-winged sharpshooter. Citograph 81:10
Triapitsyn SV, Moratorio MS (1998) Host associations of Anagrus nigriventris Girault (Hymenoptera: Mymaridae) and techniques for its rearing under insectary conditions. In: Hassan SA (ed) Egg parasitoids. 5th international symposium, IOBC, Cali, Colombia, March 1998. Mitt. Biol. Bundesanstalt. Land- Forstwirtsch. Berlin-Dahlem, H. 356, pp 185–191
Triapitsyn SV, Mizzell RF III, Bossart JL, Carlton CE (1998) Egg parasitoids of Homalodisca coagulata (Homoptera: Cicadellidae). Fla Entomol 8:241–243
Triapitsyn SV, Phillips PA (2000) First record of Gonatocerus triguttatus (Hymenoptera: Mymaridae) from eggs of Homalodisca coagulata (Homoptera: Cicadellidae) with notes on the distribution of the host. Fla Entomol 83:200–203
Triapitsyn SV, Morgan DJW, Hoddle MS, Berezovskiy VV (2003) Observations on the biology of Gonatocerus fasciatus Girault (Hymenoptera: Mymaridae), egg parasitoid of Homalodisca coagulata (Say) and Oncometopia orbona (Fabricius) (Hemiptera: Clypeorrhyncha: Cicadellidae). Pan-Pac Entomol 79:75–76
Triapitsyn SV, Rakitov RA (2005) Egg parasitoids (Hymenoptera: Mymaridae and Trichogrammatidae) of Cuerna sharpshooters (Hemiptera: Cicadellidae) in the USA, Posters P11–P12. In: Poster abstracts P1–P43. 12th international auchenorrhyncha congress and 6th international workshop on leafhoppers and planthoppers of economic significance, University of California, Berkeley, 8–12 August 2005. http://nature.berkeley.edu/hoppercongress/
Wendel L, Ciomperlik M, Bartels D, Luvisi D, Elms D (2002) The area-wide pest management of glassy-winged sharpshooter in Kern County. In: Tariq MA, Oswalt S, Blincoe P, Esser T (eds) Proceedings, Pierce’s disease research symposium, Coronado, CA, 15–18 December 2002. Copeland Printing, Sacramento, CA, pp 302–307
Acknowledgments
This research was supported in part by the Robert van den Bosch Memorial Scholarship awarded to R. K., a California Department of Food and Agriculture grant awarded to M. W. J. and R. L. G., an USDA ARS Special Cooperative Agreement awarded to M. W. J., and a University of California Pierce’s Disease Research Program grant awarded to J. G. M. and Richard Stouthamer. We thank Timothy D. Paine, Jocelyn G. Millar, Thomas Miller, Mariana C. B. Krugner, Candice Ann Stafford, Elizabeth A. Boyd, Rodrigo P. P. Almeida, and David J. W. Morgan for providing insects for experiments. This research forms part of the Ph.D. dissertation of R. K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krugner, R., Johnson, M.W., Groves, R.L. et al. Host specificity of Anagrus epos: a potential biological control agent of Homalodisca vitripennis . BioControl 53, 439–449 (2008). https://doi.org/10.1007/s10526-007-9080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9080-6