Abstract
Large-scale use of entomopathogenic nematodes (EPNs) (Rhabditida: Steinernematidae and Heterorhabditidae) as biological control agents is impaired by their short shelf life. Three local South African EPN species, including Steinernema yirgalemense, S. jeffreyense and Heterorhabditis bacteriophora, were investigated for their role in formulations. Encapsulation of the infective juveniles (IJs) in alginate beads and diatomaceous earth (DE) was investigated. Survival of the IJs in the formulations was determined at 6 °C, 14 °C and 25 °C for four weeks. Of the IJs, 10-20% were observed to escape from the beads, depending on temperature, and readily survived the encapsulation process. DE did not cause the desiccation of the nematodes, with there still being a lower mortality rate by the 4th week of the study. In both formulations, the survival and virulence rates differed significantly at 6 °C, as compared to at 14 °C and 25 °C, with a drastic decrease over time for S. yirgalemense. The EPN species revealed poor survival and loss of virulence at low temperatures in both formulations. The alginate beads successfully retained most of the IJs and can be stored for a longer time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic nematodes (EPN) of the genera Heterorhabditis Poinar (1975) and Steinernema Travassos (1927) and their associated symbiotic bacteria Xenorhabdus Thomas and Poinar (1983) (Enterobacteriales: Enterobacteriaceae) and Photorhabdus Boemare et al. (1993) (Enterobacteriales: Enterobacteriaceae) (Boemare et al. 1993; Akhurst et al. 1996; Ehlers 2007) are biocontrol agents used for the management of insect pests occurring in soil and cryptic environments worldwide. They have many advantages and for this reason many countries, with a few exceptions like South Africa (Hatting et al. 2019), have exempted EPNs from registration obligations (Akhurst and Smith 2002).
Even though EPNs are competent biological control agents against insect pests, their commercial use is limited by their predetermined shelf life, which is a major drawback in terms of field use (Grewal 2000a, b). Their poor survival rate in storage at room temperature is a major hindrance to producers in their potential use as bioinsecticides (Grewal 2002). In addition, their ability to survive under such conditions is also highly compromised in terms of desiccation (Womersley 1990; Surrey and Wharton 1995).
The successful commercialisation of EPNs after mass production requires the development of storage and formulation techniques that reduce nematode mortality, loss of virulence, and pathogenicity. In many commercial EPN-based biopesticide companies, the formulations that are manufactured result from the use of a variety of methods, ranging from simply saturating EPNs on artificial sponge, all the way through to highly advanced granular formulations. Major challenges have included obtaining room temperature shelf stability, ease of use, and contamination control.
Kagimu et al. (2017) recently reported that diatomaceous earth (DE) is readily used for formulations, due to its many advantages, including its ease of application using available farm implements and irrigation systems, among others. The authors, further, draw attention to the shift in research trends regarding formulations devised, ranging from a focus on the soil to a focus on above-ground applications using adjuvants, and alginate beads and capsules (Kagimu et al. 2017).
In South Africa, investigations to determine the commercial prospects of using a local isolate of Steinernema yirgalemense Nguyen et al. (2004) for the control of codling moth (Cydia pomonella L.) (Lepidoptera, Tortricidae), vine mealybug (Planococcus ficus) (Hemiptera, Pseudococcidae), citrus mealybug (Planococcus citri), and maize stalk borer (Busseola fusca) (Lepidoptera, Noctuidae) have been made (Malan and Hatting 2015; Odendaal et al. 2015, 2016). Similar investigations have been undertaken in considering the commercial possibilities of H. bacteriophora for the control of bollworm (Helicoverpa armigera) (Lepidoptera, Noctuidae), fruit fly (Ceratitis capitata, Ceratitis rosa), (Diptera: Tephritidae), maize stalk borer (B. fusca), and sugarcane borer (Eldana saccharina) (Lepidoptera: Pyralidae) (Malan and Hatting 2015).
Nematodes encapsulated in sodium and calcium alginate gels by means of internal or external techniques (Kaya and Nelsen 1985; Kaya et al. 1987), and in other hydrophilic colloids (Patel and Vorlop 1994), have been used to protect the IJs from both desiccation and ultraviolet light (Navon et al. 1998, 2002). The beads and capsules are produced by forming droplets from liquids, and by solidifying the liquid droplets to form particles. The process of gelation, or of membrane formation, is categorised by the way in which droplet formation (dripping and emulsification) occurs (Hiltpold et al. 2012; Vemmer and Patel 2013). Increasingly, beads and capsules are coated using such polyelectrolytes as xanthan gum, with altering charges (Hiltpold et al. 2012; Vemmer and Patel 2013; Kim et al. 2015) being used to strengthen them, and a few drops of colouring being added to distinguish the solution during gelation. Presently, such EPN formulation is still undergoing development, with the process encountering many diverse challenges. For example, Hiltpold et al. (2012) and Kim et al. (2015) report that EPNs readily escaped from soft capsules within a few days, especially when they were unrefrigerated, and that the capsules did not retain EPNs over an extended period, therefore limiting the long-term storage of such a medium. Adjusting the capsule properties, like the formation of alginate capsules at 4 °C, resulted in thinner shelled, yet harder, capsules than when the polymerisation was performed at 24 °C. Post-treatment of the capsules with additional Ca2+ markedly improved the hardness of the capsules concerned. Although the hardened capsules retained EPNs significantly better than did the unhardened ones, surprisingly post-treatment with Ca2+ exerted an adverse effect on EPN retention, with very few IJs being able to escape from the capsules concerned. Ideally, EPN beads should retain their EPNs inside the bead, until they are required, and they should maintain the EPN viability for a few months, at room temperature (Kim et al. 2015).
In contrast, DE, consisting of unicellular, or colonial silicified skeletons of algae (Bacillariophyceae) (Buchholz et al. 2009), and composed of 89% amorphous SiO2, 4%, Al2O3 1.7% Fe2O3, 1.4% CaO, > 1% MgO + K2O and 3% H2O, was tested equally (Wakil et al. 2011). Inert dusts have been reported to be effective for the control of various pests (Golob 1997). Although several DE formulations have been tested and evaluated against the insect pests of stored products, they vary in their effectiveness. Thus, the use of a proper tested grade of DE is encouraged for formulation (Wakil et al. 2011).
DE desiccates the IJs by causing partial anhydrobiosis, whereby the nematode enters a physiological state of quiescence, in which its metabolic activity and energy reserve consumption diminish, so that it can retain its survival capacities and infectivity until field application (Silver et al. 1995). Upon being reactivated by the moisture in the soil, it is liberated, and, if it locates a susceptible host, the effective biological control of the pest infestation may be achieved (Matadamas-Ortiz et al. 2014). The efficacy of EPN formulation can be influenced by some characteristics such as homogeneity (shape, size, quantity of nematodes, and weight), structure, mechanical resistance, and the properties of the inert granular materials of DE formulation (Silver et al. 1995; Hiltpold et al. 2012).
The objectives of the study were to formulate three South African EPN species and to test them for suitability and survival enhancement, using two different types of formulation. The types of formulation used consisted of alginate beads and DE. With the beads, the number of nematodes escaping from them was determined, after formulation and storage at different temperatures, as was the pathogenicity of the IJ. In the case of DE, the viability of the nematodes was determined after storage at different temperatures, up until a period of four weeks had elapsed.
Materials and methods
Source of nematodes and host insects
The three nematode species used in the study were Steinernema jeffreyense Malan et al. (2016), S. yirgalemense and Heterorhabditis bacteriophora Poinar (1975), all endemic to South Africa. The species, which were collected from previous surveys, were maintained in the EPN collection of the Nematology laboratory, at the Department of Conservation Ecology and Entomology, Stellenbosch University (Malan and Hatting 2015; Malan et al. 2016).
Galleria mellonella (Lepidoptera: Pyralidae) larvae were cultured according to van Zyl and Malan (2015), on an artificial diet kept at 25 °C in a growth chamber. The Galleria larvae were inoculated with IJs of the three nematode species in 9-mm-diameter Petri dishes, supplied with moist filter paper. Freshly harvested IJs were cultured in vivo, using last-instar larvae of G. mellonella, at 25 °C in growth chambers. Modified white traps (Kaya and Stock 1997) were used to harvest the emerged EPNs. The harvested IJs were stored in distilled water at 14 °C, collected in 5 l Erlenmeyer flasks that were constantly stirred (for aeration) using a 70 × 10 mm cylindrical magnetic stirring bar on an AGE magnetic stirrer (VELP® Scientifica) for approximately three weeks, until the desired concentration of IJs for each batch was achieved.
Formulation in alginate beads
The encapsulation of IJs in sodium alginate beads was done by means of modification of the methods of Chen and Glazer (2005) and of Kim et al. (2015) at room temperature (24 °C), with two batches per species being studied. Two solutions of different composition were utilised. Ninety-eight thousand infective juveniles (IJs) of each nematode species were suspended in 20 ml of solution containing 10% glycerol, 2% sodium alginate (FMC Biopolymer, Cape Town, South Africa), and 0.075% of formaldehyde and Moir’s crimson red or apple green food colour dye in distilled water. A droplet of 10-µl alginate solution was dripped from a 1-ml disposable syringe into 20 ml of Ca2+-solution, containing 0.5% CaCl2 H2O [Merck SA (Pty) Ltd], 10% glycerol and 0.075% formaldehyde in distilled water. The formation of the alginate beads was immediate. The Ca2+ solution was shaken at 1200 rpm, using an orbital shaker (Benchmark’s ORBI-SHAKER™ JR), which prevented the beads from sticking together. After 20 min of bead formation, the beads were removed from the reaction beaker with a spatula and dried on paper towels (SCOTT® KIMDRI*, Bedfordview, South Africa).
Number of IJs escaping from alginate beads
The 22 beads used for storage and the eight beads used for crushing at each temperature were placed individually in 2.0 ml microcentrifuge tubes (QSP®, USA). The tubes were stored in cardboard microtube freezer boxes (TrueNorth®) in the dark, at 6 °C, 14 °C and 25 °C. The number of IJs that escaped from each bead, after being retrieved from each tube with 500 µl of distilled water, were counted using a stereomicroscope (× 40 magnification) weekly up to four weeks, to determine the survival percentage. Each bead was transferred to a new microcentrifuge tube. Two representative beads per sample (as a control) were crushed in a 1.5 ml microcentrifuge tube, using a disposable tissue grinder pestle (Axygen®, Axygen Biosciences, Union City, USA) and observed, using a stereomicroscope, in an embryo dish, to determine the survival of the IJs that remained inside the beads. The process was repeated on a different test date, using a fresh batch of nematodes.
Pathogenicity of IJs stored in alginate beads
The three EPN species that were encapsulated in sodium alginate beads, as was previously described, and which were stored for four weeks at 6 °C, 14 °C and 25 °C, were crushed in an embryo dish, following which dilutions of 100 IJs in 50 µl were made. The live IJs in the dilutions were counted and tested for pathogenicity against the last-instar larvae of G. mellonella, using 24-well bioassay plates. Twelve alternate wells of a 24-well bioassay plate were used, fitted with a piece of filter paper, to which one Galleria larva was added. Each well was inoculated with 100 IJs in 50 µl of distilled water, while water only was used for the controls. The lid of each well was fitted with a piece of glass of the same shape as the lid, to prevent the G. mellonella larvae from escaping. Five 24-well plates, each consisting of 12 wells (n = 60), were used for each treatment (nematode species), with the control receiving water only. The plates were placed in a plastic container, lined with wet paper towels to maintain high RH, and kept in a growth chamber at 25 °C for a period of 48 h. Mortality was confirmed by means of the visual observation of the colour of the cadavers of the wax moth larvae, which turned yellow, brick-red, and black, for S. yirgalemense, H. bacteriophora, and S. jeffreyense, respectively. The experiment was conducted twice with each nematode species on different test dates, using fresh batches of nematodes encapsulated in sodium alginate beads, and stored in the same conditions described above.
Survival of nematodes in diatomaceous earth
Nematodes were concentrated into a paste using a 32 µm sieve (Clear Edge Filtration SA (Pty) Ltd, South Africa). Each of the three EPN species were formulated in DE (Celite 209—Imerys Refractory Minerals SA (Pty) Ltd). The proportions of the ingredients used in the formulation were followed according to Grewal and Jagdale (2002). The relative free water activity of the formulation was 0.970, which induced partial anhydrobiosis and slow desiccation in the IJs (Grewal 2000a, b) the final density of the formulation was 50,000 IJs g−1.
The formulated nematodes were weighed, with 10 g being added to the STC2P plastic containers with lids (Mambo’s plastics) (n = 30). The ten containers were placed in larger plastic containers, lined with moistened paper towels (SCOTT® KIMDRI*, Bedfordview, South Africa), and covered with a lid to maintain high RH. The containers were stored for four weeks at 6 °C, 14 °C and 25 °C. The experiment was repeated for each nematode species on a different test date, using another batch of nematodes, and stored in similar conditions to those described above.
Formulated nematodes were counted according to the modification method of Peters (2004). One gram from a container of 10 g containing 500,000 IJs was dissolved in 10 ml of distilled water in a 50-ml beaker. Air was bubbled in from an aquarium air pump (Second Nature Whisper™ 1000), with its tube leading to the bottom of the beaker. One 100 µl sample was pipetted into 5 ml distilled water in a clean beaker. Mixing was undertaken by shaking, and 1 ml was further diluted with 2 ml of distilled water, with the number of IJs being counted, using a binocular microscope, weekly for four weeks, to determine the survival percentage in each of the ten containers, at the respective temperatures of 6 °C, 14 °C and 25 °C.
Statistical analysis
Statistical analyses were conducted using STATISTICA 13.2 software (StatSoft. Inc). The two-way ANOVA and repeated-measures ANOVAs were calculated accordingly. Where the results were not normally distributed, bootstraps were performed on the data for least significance difference (LSD) multiple comparisons. In other instances, the means were accordingly separated by means of the Fisher’s least significant difference or the Games-Howell post-hoc test.
Results
Rate of nematodes escape from alginate beads
A one-way ANOVA over batches indicated that there were no significant differences (F1,394 = 0.146; p = 0.703) between the total means of the two batches, thus the data were pooled for analysis. A two-way factorial ANOVA was done on the pooled data, on the number of nematodes moving out of the beads, relative to the factors temperature and species. The interaction was significant (F4,387 = 5.194, p = 0.0004). These interactions were investigated with LSD multiple comparisons which indicated temperature to significantly affect the number of nematodes moving out of the beads, but differently for each species. Fewer IJs moved out of the beads at 6 °C, followed by a slight increase at 14 °C and 25 °C, respectively. At 6 °C, the mean numbers of S. jeffreyense differed significantly from those of H. bacteriophora and S. yirgalemense (p < 0.001), which did not differ significantly (p = 1.00) from each other (Fig. 1).
Mean number of infective juveniles (IJs) (95% confidence interval) that moved out of the beads after four weeks in respect of S. jeffreyense, H. bacteriophora and S. yirgalemense, each at three different temperatures. Different letters above the bars indicate significant differences (p < 0.05) in nematode numbers between species and temperature
The highest mean number of 6.50 IJs was recorded for H. bacteriophora, followed by the mean numbers of 3.80 and 0.68 for S. yirgalemense and S. jeffreyense IJs, respectively, at 6 °C. Equally, at 14 °C, the mean number of IJs of S. yirgalemense differed significantly from those of H. bacteriophora (p = 0.005) and S. jeffreyense (p < 0.01), which did not differ significantly (p = 0.13) from each other. At 14 °C, the highest mean number of 62.73 IJs was recorded for S. yirgalemense, followed by those of 13.75 IJs and 9.59 IJs for H. bacteriophora and S. jeffreyense, respectively. Additionally, at 25 °C, the mean number of IJs of S. yirgalemense differed significantly from those of H. bacteriophora (p < 0.01) and S. jeffreyense (p < 0.01), which did not differ significantly (p = 0.99) from each other. The highest mean number of 77.39 IJs was recorded for S. yirgalemense, followed by those of 26.59 IJs and 23.11 IJs for S. jeffreyense and H. bacteriophora, respectively (Fig. 1).
The mean number of IJs moving out of the beads for the nematode species at different temperatures increased, with an increase in temperature. The mean number of Steinernema yirgalemense IJs, at 6 °C, differed significantly from those at 14 °C and 25 °C (p = 0.01). At 14 °C, the mean number did not differ significantly from that at 25 °C (p = 0.99). For S. yirgalemense, the largest mean number of 77.39 IJs moving out of the beads occurred at 25 °C, followed by the mean numbers of 62.73 and 3.80 occurring at 14 °C and 6 °C, respectively (Fig. 1).
For H. bacteriophora, the largest mean number of IJs moving out of the beads was 23.11 at 25 °C, followed by 13.75 and 6.5 at 14 °C and 6 °C, respectively. The S. jeffreyense, at 6 °C, differed significantly from those at 14 °C (p = 0.04) and 25 °C (p = 0.013). At 14 °C, the mean number differed significantly from that at 25 °C (p = 0.01). For the S. jeffreyense IJs, the largest mean number of 26.59 IJs moving out of the beads was observed at 25 °C, followed by the 9.59 and 0.68 moving out at 14 °C and 6 °C, respectively (Fig. 1).
Pathogenicity of IJ stored in alginate beads
A one-way ANOVA over batches indicated that there were no significant differences (F1,118 = 0.878, p = 0.351) between the percentage mortality of the two batches, thus the data from the two batches were pooled before analysis. A two-way factorial ANOVA on the pooled data indicated that the interaction between the treatments and the nematodes species was significant (F6,108 = 22.164, p < 0.001) (Fig. 2). LSD multiple comparisons showed that for treatment T6 no differences were found between the nematode species, for T14 nematode species were again similar, while for T25, S. yirgalemense and S. jeffreyense did not differ, but differed from H. bacteriophora (Fig. 2).
Mean percentage mortality (95% confidence interval) at of G. mellonella larvae at 25 °C, inoculated with the infective juveniles of Steinernema jeffreyense, Heterorhabditis bacteriophora and S. yirgalemense, formulated and stored in alginate beads at three different temperatures after four weeks T6 (6 °C), T14 (14 °C) and T25 (25 °C). Different letters above the bars indicate significant differences (p < 0.05) in insect mortality between nematode species and treatments
For nematodes encapsulated in alginate beads and stored at 6 °C, no significant difference was found in the Galleria mortality between S. jeffreyense and S. yirgalemense (p = 0.38) and H. bacteriophora (p = 0.93), which did not differ significantly (p = 0.22) from each other. The H. bacteriophora IJs caused the highest mean percentage mortality of Galleria larvae, of 27.50% ± 4.34%, followed by S. jeffreyense and S. yirgalemense, with 15.83% ± 4.34% and 4.17% ± 4.34%, respectively (Fig. 2).
For nematodes encapsulated in alginate beads and stored at 14 °C, no significant differences were found among the mean percentage Galleria mortality for S. jeffreyense, S. yirgalemense (p = 0.99), and H. bacteriophora (p = 0.97), which did not differ significantly (p = 0.99) from each other. The S. jeffreyense IJs had the highest mean percentage Galleria mortality value of 95.00% ± 4.34%, followed by S. yirgalemense and H. bacteriophora, with 90.83% ± 4.34% and 83.33% ± 4.34%, respectively (Fig. 2). For nematodes encapsulated in alginate beads and stored at 25 °C, no significant difference was found between the mean percentage Galleria mortality for S. jeffreyense and S. yirgalemense (p = 0.81), while a significant (p < 0.01) difference was recorded with H. bacteriophora, which also significantly differed from the mean percentage obtained for the S. yirgalemense IJs. The S. yirgalemense IJs had the highest mean percentage Galleria mortality of 98.33% ± 5.27%, followed by S. jeffreyense, and by H. bacteriophora with 90.00% ± 14.05% and 30.00% ± 13.72%, respectively (Fig. 2). No significant differences were found between the mean percentage Galleria mortality values for S. jeffreyense and S. yirgalemense (p = 1.00), and for H. bacteriophora (p = 0.35), which did not differ significantly (p = 0.71) from each other in the control (Fig. 2).
Within the nematode species, there were significant (p < 0.05) and non-significant (p > 0.05) differences per treatment. The S. jeffreyense IJs at 6 °C differed significantly from those at 14 °C (p < 0.001) and at 25 °C (p < 0.001) but did not differ significantly (p = 0.32) from the control. At 14 °C, they did not differ significantly from those at 25 °C (p = 0.99). For the S. jeffreyense IJs, the highest mean percentage mortality value of 95.00% ± 4.34% was observed at 14 °C, followed by 90.00% ± 4.34% and 15.83% ± 4.34%, at 25 °C and 14 °C, respectively (Fig. 2).
The Galleria mortality by the S. yirgalemense IJs, at 6 °C, differed significantly from that at 14 °C (p < 0.01) and 25 °C (p < 0.01). At 14 °C, Galleria mortality differed significantly from that at 25 °C (p = 0.006). At 25 °C, Galleria mortality did not differ significantly from those at 14 °C (p = 0.70). For the S. yirgalemense IJs, the highest mean percentage Galleria mortality value of 98.33% ± 4.34% was observed at 25 °C, followed by 90.83% ± 4.34% at 14 °C, and by 5.00% ± 4.34% at 6 °C, (Fig. 2).
The Galleria mortality caused by H. bacteriophora IJs, at 6 °C, differed significantly from those at 14 °C (p = 0.007), but did not differ significantly from those either at 25 °C (p = 1.00), or from the control (p = 0.89). At 14 °C, Galleria mortality differed significantly from those at 25 °C (p = 0.006), and from the control (p = 0.0002). At 25 °C, Galleria mortality differed significantly from the control (p = 0.002). For H. bacteriophora IJs, the highest mean percentage Galleria mortality of 83.33% was observed at 14 °C, followed by 30.00% ± 4.34% at 27 °C, and the 27.50% ± 4.34% at 6 °C (Fig. 2).
Survival in diatomaceous earth
Combined effect of temperature on the survival of three EPN species
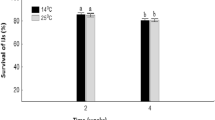
The data for survival of S. yirgalemense, H. bacteriophora, and S. jeffreyense were combined and analysed. The analysis showed higher temperatures (14 °C and 25 °C) having no significant difference between the two batches while the lowered survival trend at 6 °C were generally the same. No significant differences were obtained between batches over survival, (F1,58 = 0.045, p = 0.83), thus the batches were pooled. The repeated-measures analysis of the pooled data over weeks 1 to 4 showed significant interaction (F6,171 = 171.89, p < 0.01) between the temperatures and time regarding the survival of EPNs. Such interaction was interpreted with LSD multiple comparisons. A steep decline occurred in the mean percentage survival of the EPNs over time at 6 °C, whereas a near-stable condition of mean percentage survival was maintained at 14 °C and at 25 °C, respectively (Fig. 3). In week 1, the mean percentage survival of EPNs at 6 °C differed significantly from that at 14 °C and at 25 °C (p < 0.01). In week 1, an almost equal mean percentage survival rate was obtained at 25 °C (93.81% ± 2.36%), and at 14 °C (93.63% ± 2.36%), followed by that which was obtained at 6 °C (71.70% ± `2.36%). The mean percentage survival rate of EPNs remained high between weeks 1 and 4 respectively at 14 °C (93.63% ± 2.36% to 91.01% ± 0.72%), and at 25 °C (93.81% ± 2.36% to 88.31% ± 0.72%) (Fig. 3).
Mean percentage survival rate (95% confidence interval) of combined results of infective juveniles (IJs) of Steinernema yirgalemense, Heterorhabditis bacteriophora, and S. jeffreyense formulated and stored in diatomaceous earth at three different temperatures after a period of four weeks. Different letters above the bars indicate significant differences (p < 0.05) in IJ survival between temperature and weeks
Combined desiccative effect of diatomaceous earth on the survival of EPNs
No significant differences (F1,178 = 0.0089, p = 0.766) were obtained between the batches and survival using a repeated-measures ANOVA, with one categorical variable namely the batch, thus enabling the data from the two batches to be pooled and analysed. The analysis of the data, using repeated-measures ANOVA, from weeks 1 to 4, showed significant interaction (F6,531 = 8.462, p < 0.0001) between the different nematode species regarding their survival over time. Such interaction was interpreted with LSD multiple comparisons. A general decline in the mean percentage survival rate of the nematodes occurred over time (Fig. 4).
Mean percentage survival (95% confidence interval) infective juveniles of Steinernema yirgalemense, Heterorhabditis bacteriophora, and S. jeffreyense formulated in diatomaceous earth and stored for a period of four weeks (data from different temperatures pooled). Different letters above the bars indicate significant differences (p < 0.05) in IJ survival between species and time
Discussion
The current study reports on suitability of alginate beads formulation of the IJs of S. jeffreyense, H. bacteriophora and S. yirgalemense. Currently, the formulation of IJs in alginate beads is being faced with several challenges, including that of the IJs being able to readily escape from the soft alginate beads (Hiltpold et al. 2012). The result is that the beads cannot retain the EPNs over an extended period, therefore limiting the long-term storage of alginate beads containing nematodes. In addition, the adjustment of the bead properties, as with the post-treatment of the beads with additional Ca2+ to improve their hardness, could exert an adverse effect on EPN retention, thus preventing the IJs from escaping from the beads (Kim et al. 2015). Attaining a month-long room temperature shelf stability in the formulation of the three local EPN species was necessary, given the commercial interest that the EPNs hold in terms of protecting crops within the sphere of South African agricultural production. The mean number of IJs that escaped from the beads at 25 °C during the four weeks was relatively low, indicating that alginate beads can release IJs at a relatively slow rate, and thus improve their long-term room temperature shelf stability.
Temperature had a significant effect on the different EPN species tested, with regard to the number of nematodes moving out of the beads. Kagimu et al. (2017) and Stuart et al. (2015) identified temperature, in terms of heat and cold tolerance, as one of the abiotic factors influencing the survival of IJs. Kagimu et al. (2017) further asserted that the prevailing temperature could add significantly to the survival of IJs. Furthermore, Addis et al. (2016), in their study of the storage temperatures of S. yirgalemense, reported low survival rates, not exceeding 42 days, at a storage temperature of 4 °C. In contrast, they found that, at 15 °C and 25 °C, more than 95% of the IJs of S. yirgalemense survived for a period of up to 66 days. The S. yirgalemense was the same strain used in the current study. All species studied in the present research were local isolates, which are adapted to the relatively warm South African climate (Malan and Hatting 2015), and which are, thus, less well adapted to surviving under conditions of low temperature, further explaining the observations made.
The survival of IJs is often improved in many formulations, by means of storing the nematodes concerned at low temperatures between 4 and 15 °C (Grewal and Peters 2005). The present data do not support the making of such a supposition, however, given the low mean number of IJs observed to be moving out of the beads at 6 °C. Furthermore, the results show loss of virulence after storage at low temperatures. Therefore, the three formulated nematodes should be stored at temperatures ranging between 14 and 25 °C, leading to no refrigeration of the nematodes stored in formulation for the local species. Furthermore, the results clearly show that the temperature had a significant effect on the survival of the three species in DE, with the temperature of 6 °C substantially reducing the survival of IJs over time, especially in the case of S. yirgalemense.
DE is widely used in insect management, with control generally caused by desiccation, due to the hygroscopic nature of some of the grades available. In this study, relatively few of the IJs were expected to survive, due to the low density of IJs in the formulation given the desiccative and hygroscopic nature of DE. Coincidentally, the results showed a stable decrease, or slow lowering, of the shelf life of the IJs stored in DE formulation. The results also showed that the use of DE did not lead to the desiccation of the nematodes, with there being a considerably lower mortality rate by the 4th week of the study. This finding can only be explained by such characteristics as homogeneity (shape, size, quantity of nematodes, and weight), structure, mechanical resistance, and the properties of the inert granular materials of DE formulation that could influence the efficacy of EPNs in the control of pest infestations (Silver et al. 1995; Hiltpold et al. 2012; Matadamas-Ortiz et al. 2014). For example, Ziaee et al. (2016), in their investigation of the possible use of Iranian DEs as grain protectants in stored-product pest management programmes, observed the increased mortality of adults of Oryzaephilus surinamensis (L.), with increasing exposure intervals and concentration levels. The low density of IJs g−1 in the DE, as used in the current study, does not necessarily translate into the reduced shelf life of the IJs, as would be likely to be observed at higher densities. The above is due to the strong desiccative effect of the formulation on the IJs employed. In contrast, the results correspond to those of Matadamas-Ortiz et al. (2014), whose best results were obtained following 100DE:0AC proportions.
In conclusion, this study indicates that the properties of alginate beads can be adjusted to enable them to release IJs on a regular basis. Like in other tropical and subtropical EPN species and strains, a loss in virulence and survival is equally reported as occurring at relatively low temperatures for the three EPNs stored at 6 °C. The high survival of the studied species obtained at room temperature in both alginate beads and DE formulations showed potential for a reduction in the existing costs of refrigeration during both storage and transportation. In the present study, we were constrained from obtaining the much-desired number of IJs due to the in vivo culturing of IJs, so that we were only able to formulate IJs at a lower density (IJs g−1) of DE formulation. We recommend, in terms of using in vitro cultures, striving to obtain a much higher density of IJs in formulation, to be able to obtain a more realistic and improved DE formulation.
References
Addis T, Mijušković N, Ehlers R-U, Strauch O (2016) Life history traits, liquid culture production and storage temperatures of Steinernema yirgalemense. Nematology 18:367–376
Akhurst R, Smith K (2002) Regulation and safety. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 311–332
Akhurst RJ, Mourant RG, Baud L, Boemare NE (1996) Phenotypic and DNA relatedness between nematode symbionts and clinical strains of the genus Photorhabdus (Enterobacteriaceae). Int J Syst Bacteriol 46:1034–1041
Boemare NE, Akhurst RJ, Mourant RG (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol 43:249–255
Buchholz S, Merkel K, Spiewok S, Pettis JS, Duncan M, Spooner-Hart R, Ulrichs C, Ritter W, Neumann P (2009) Alternative control of Aethina tumida Murray (Coleoptera: Nitidulidae) with lime and diatomaceous earth. Apidologie 40:535–548
Chen S, Glazer I (2005) A novel method for long-term storage of the entomopathogenic nematode Steinernema feltiae at room temperature. Biol Control 32:104–110
Ehlers R-U (2007) Entomopathogenic nematodes: from science to commercial use. In: Vincent C, Goettel MS, Lazarovits G (eds) Biological control: a global perspective: case studies from around the world. CABI Publishing, Wallingford, pp 136–151
Golob P (1997) Current status and future perspectives for inert dusts for control of stored product insects. J Stored Prod Res 33:69–79
Grewal PS (2000a) Enhanced ambient storage stability of an entomopathogenic nematode through anhydrobiosis. Pest Manag Sci 56:401–406
Grewal PS (2000b) Anhydrobiotic potential and long-term storage of entomopathogenic nematodes (Rhabditida: Steinernematidae). Int J Parasitol 30:995–1000
Grewal PS (2002) Formulation and application technology. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 265–287
Grewal PS, Jagdale GB (2002) Enhanced trehalose accumulation and desiccation survival of entomopathogenic nematodes through cold preacclimation. Biocontrol Sci Tech 12:533–545
Grewal PS, Peters A (2005) Formulation and quality. In: Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) Nematodes as biocontrol agents. CABI Publishing, Wallingford, pp 79–89
Hatting JL, Moore SD, Malan AP (2019) Microbial control of phytophagous invertebrate pests in South Africa: current status and future prospects. J Invertebr Pathol (in press). https://doi.org/10.1016/j.jip.2018.02.004
Hiltpold I, Hibbard BE, French BW, Turlings TCJ (2012) Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant Soil 358:11–25
Kagimu N, Ferreira T, Malan AP (2017) The attributes of survival in the formulation of entomopathogenic nematodes utilised as insect biocontrol agents. African Entomology 25:275–291
Kaya HK, Nelsen CE (1985) Encapsulation of steinernematid and heterorhabditid nematodes with calcium alginate: a new approach for insect control and other applications. Environ Entomol 14:572–574
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey L (ed) Manual of techniques in insect pathology. Academic Press, San Diego, pp 281–324
Kaya HK, Mannion CM, Burlando TM, Nelsen CE (1987) Escape of Steinernema feltiae from alginate capsules containing tomato seeds. Journal of Nematology 19:287–291
Kim J, Jaffuel G, Turlings TCJ (2015) Enhanced alginate capsule properties as a formulation of entomopathogenic nematodes. BioControl 60:527–535
Malan AP, Hatting JL (2015) Entomopathogenic nematode exploitation: case studies in laboratory and field applications from South Africa. In: Campos-Herrera R (ed) Nematode pathogenesis of insects and other pests. Springer International Publishing, Dordrecht, pp 477–508
Malan AP, Knoetze R, Tiedt LR (2016) Steinernema jeffreyense n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. J Helminthol 90:262–278
Matadamas-Ortiz PT, Ruiz-Vega J, Vazquez-Feijoo JA, Cruz-Martinez H, Cortes-Martinez CI (2014) Mechanical production of pellets for the application of entomopathogenic nematodes: factors that determine survival time of Steinernema glaseri. Biocontrol Sci Technol 24:145–157
Navon A, Keren S, Salame L, Glazer I (1998) An edible-to-insects calcium alginate gel as a carrier for entomopathogenic nematodes. Biocontrol Sci Technol 8:429–437
Navon A, Nagalakshmi VK, Levski S, Salame L, Glazer I (2002) Effectiveness of entomopathogenic nematodes in an alginate gel formulation against lepidopterous pests. Biocontrol Sci Technol 12:737–746
Nguyen, Tesfamariam, Gozel, Gaugler, Adams (2004) Steinernema yirgalemense n. sp. (Rhabditida: Steinernematidae) from Ethiopia. Nematology 6:839–856
Odendaal D, Addison MF, Malan AP (2015) Control of codling moth (Cydia pomonella) (Lepidoptera: Tortricidae) in South Africa with special emphasis on using entomopathogenic nematodes. African Entomol 23:259–274
Odendaal D, Addison MF, Malan AP (2016) Entomopathogenic nematodes for the control of the codling moth (Cydia pomonella L.) in field and laboratory trials. J Helminthol 90:615–623
Patel AV, Vorlop K-D (1994) Entrapment of biological control agents applied to entomopathogenic nematodes. Biotechnol Tech 8:569–574
Peters A (2004) Protocol quality assessment of entomopathogenic nematodes. COST Action 850 meeting programme and mushroom protocols. Wädenswil, Switzerland, pp 4–6
Poinar GO (1975) Description and biology of a new insect parasite rhabditoid, Heterorhabditis bacteriophora n. gen. n. sp. (Rhabditida; Heterorhabditidae. fam.). Nematologica 21:463–470
Silver S, Dunlop D, Grove D (1995) WIPO Patent No. WO 95/0577. World Intellectual Property Organization, Geneva
Stuart RJ, Barbercheck ME, Grewal PS (2015) Entomopathogenic nematodes in the soil environment: distributions, interactions and the influence of biotic and abiotic factors. In: Campos-Herrera R (ed) Nematode pathogenesis of insects and other pests. Springer International Publishing, Dordrecht, pp 97–137
Surrey MR, Wharton DA (1995) Desiccation survival of the infective larvae of the insect parasitic nematode, Heterorhabditis zealandica Poinar. Int J Parasitol 25:749–752
Thomas GM, Poinar GO (1983) Amended description of the genus Xenorhabdus Thomas and Poinar. Int J Syst Bacteriol 33:878–879
Travassos L (1927) Sobre o Genera Oxystomatium. Boletim Biologico, Sao Paula. Brasil 5:20–21
van Zyl C, Malan AP (2015) Cost-effective culturing of Galleria mellonella and Tenebrio molitor and entomopathogenic nematode production in various hosts. African Entomol 23:361–375
Vemmer M, Patel AV (2013) Review of encapsulation methods suitable for microbial biological control agents. Biol Control 67:380–389
Wakil W, Riasat TM, Ghazanfar U, Kwon YJ, Shaheen FA (2011) Aptness of Beauveria bassiana and enhanced diatomaceous earth (DEBBM) for control of Rhyzopertha dominica F. Entomol Res 41:233–241
Womersley CZ (1990) Dehydration survival and anhydrobiotic potential. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press Inc, Boca Raton, pp 117–137
Ziaee M, Atapour M, Marouf A (2016) Insecticidal efficacy of Iranian diatomaceous earths on adults of Oryzaephilus surinamensis. J Agric Sci Technol 18:361–370
Acknowledgements
The authors wish to thank D.G. Nel (Centre for Statistical Consultation, Stellenbosch University) for assistance with the statistical analyses. The financial support of NemaBio (Pty) (Ltd) and National Research Foundation (THRIP-TP14062571871) are greatly appreciated.
Funding
This study was funded by NemaBio (Pty) (Ltd) and National Research Foundation (Grant No. THRIP-TP14062571871).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
The research does not involve human participant or animals.
Additional information
Handling Editor: Ralf Ehlers
Rights and permissions
About this article
Cite this article
Kagimu, N., Malan, A.P. Formulation of South African entomopathogenic nematodes using alginate beads and diatomaceous earth. BioControl 64, 413–422 (2019). https://doi.org/10.1007/s10526-019-09945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09945-1