Abstract
The first record of an entomopathogenic nematode (EPN) (belonging to the order Rhabditidae, and to the families Steinernematidae and Heterorhabditidae) in South Africa was that of Harington (1953), who reported nematodes from the larval, pupal, and adult stages of the black maize beetle, Heteronychus arator Fabricius (Coleoptera: Scarabaeoidea), which were collected from a maize field near Grahamstown in the Eastern Cape province. After an elapse of 35 years, the first attempt was made to use EPN for the control of the sugarcane stalk borer, Eldana saccharina Walker (Lepidoptera: Pyralidae), and three local EPN isolates were evaluated in laboratory and field trials by the South African Sugarcane Research Institute (SASRI) in KwaZulu-Natal (Spaull, 1988, 1990, 1991). From 1993 to 1994, soil samples were collected from deciduous fruit orchards in the Western Cape province. Heterorhabditis were then isolated from the soil samples, and used for the control of the banded fruit weevil, Phlyctinus callosus (Schönerr) (Coleoptera: Curculionidae) (Basson, 1993). The specimens were sent to France, where they were the first to be identified as Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae), using species–specific satellite DNA as diagnostic probes (Grenier, Bonifassi, Abad, & Laumond, 1996; Grenier, Laumond, & Abad, 1996). Ten years later, the first new species to be described for South Africa was Steinernema khoisanae Nguyen, Malan & Gozel (Rhabditida: Steinernematidae) (Nguyen, Malan, & Gozel, 2006). A revived interest in applied research on EPN ensued during early 2000 (Hatting & Kaya, 2001) with research starting in earnest in 2003 at the South African Agricultural Research Council–Small Grain Institute (ARC–SGI) near Bethlehem, Free State province, continuing a year later at Stellenbosch University, in the Western Cape province (Table 20.1).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Integrate Pest Management
- Entomopathogenic Nematode
- Sterile Insect Technique
- Citrus Orchard
- Western Cape Province
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The first record of an entomopathogenic nematode (EPN) (belonging to the order Rhabditidae, and to the families Steinernematidae and Heterorhabditidae) in South Africa was that of Harington (1953), who reported nematodes from the larval, pupal, and adult stages of the black maize beetle, Heteronychus arator Fabricius (Coleoptera: Scarabaeoidea), which were collected from a maize field near Grahamstown in the Eastern Cape province. After an elapse of 35 years, the first attempt was made to use EPN for the control of the sugarcane stalk borer, Eldana saccharina Walker (Lepidoptera: Pyralidae), and three local EPN isolates were evaluated in laboratory and field trials by the South African Sugarcane Research Institute (SASRI) in KwaZulu-Natal (Spaull, 1988, 1990, 1991). From 1993 to 1994, soil samples were collected from deciduous fruit orchards in the Western Cape province. Heterorhabditis were then isolated from the soil samples, and used for the control of the banded fruit weevil, Phlyctinus callosus (Schönerr) (Coleoptera: Curculionidae) (Basson, 1993). The specimens were sent to France, where they were the first to be identified as Heterorhabditis bacteriophora Poinar (Rhabditida: Heterorhabditidae), using species–specific satellite DNA as diagnostic probes (Grenier, Bonifassi, Abad, & Laumond, 1996; Grenier, Laumond, & Abad, 1996). Ten years later, the first new species to be described for South Africa was Steinernema khoisanae Nguyen, Malan & Gozel (Rhabditida: Steinernematidae) (Nguyen, Malan, & Gozel, 2006). A revived interest in applied research on EPN ensued during early 2000 (Hatting & Kaya, 2001) with research starting in earnest in 2003 at the South African Agricultural Research Council–Small Grain Institute (ARC–SGI) near Bethlehem, Free State province, continuing a year later at Stellenbosch University, in the Western Cape province (Table 20.1).
2 Occurrence and Distribution of Entomopathogenic Nematodes in Africa
South Africa has a diverse climate where summer rainfall, mostly in the form of thundershowers, dominates with a gradient of increasing rainfall towards the east, reaching a maximum along the eastern escarpment and south eastern coastal areas. Much of the interior is classified as semi–arid, but arid to hyper–arid towards the western interior and west coast while dry sub–humid and humid over the eastern high lying areas and coastal regions. Total annual rainfall ranges from less than 150 mm in the west to more than 500 mm over much of the eastern parts, exceeding 1,000 mm over parts of the escarpment and along the south eastern coastal belt. Maximum temperatures during summer can occasionally exceed 40 °C especially over the north western and north eastern low–lying interior, while winter night–time temperatures can drop below freezing over much of the plateau. The extreme south western part of the country has a Mediterranean climate, where precipitation is mainly associated with cold fronts during winter and summers are warm to hot and dry. Total rainfall is closely related to topography, ranging between 200 mm in low–lying areas to more than 1,000 mm in the mountainous terrain in the southwest. Towards the east of this, the coastal belt in the south has a dry sub–humid to humid climate and receives rainfall of between 350 and 1,000 mm throughout the year, also associated strongly with topography. These climatic extremes are likely to impact the distribution of EPN in South Africa, underscoring the need for country–wide surveys across the nine provinces.
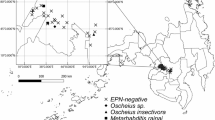
Only in three previous surveys that were conducted in South Africa have EPN been identified to species level. The identification included that of two non–targeted surveys, in an effort to establish the occurrence, and the distribution, of EPN in South Africa (Hatting, Stock, & Hazir, 2009; Malan, Nguyen, & Addison, 2006). From 2009 to 2010, surveys targeting citrus orchards were conducted, to determine the diversity, and frequency, of native EPN in the Western and Eastern Cape, and Mpumalanga, provinces of South Africa (Malan et al., 2011). The main aim of the surveys was to obtain nematodes to use as outdoor biological control agents in subsequent research against key South African insect pests. From the results of the surveys undertaken, it can be concluded that H. bacteriophora was the most frequently found species. The occurrence of EPN species in the different provinces of South Africa is indicated in Fig. 20.1.
In the previous century, only two species, namely Heterorhabditis taysearae Shamseldean, El-Sooud, Abd-Elgawad & Saleh (Rhabditida: Heterorhabditidae), in 1996 from Egypt (Shamseldean, Abou-El-Sooud, Abd-Elgawad, & Saleh, 1996), and Steinernema karii Waturu, Hunt & Reid (Rhabditida: Steinernematidae) in 1997 from Kenya (Waturu, Hunt, & Reid, 1997), were described as being from the African continent. Other reports of EPN from Africa before the twentieth century include those of H. bacteriophora from both South Africa (Grenier, Bonifassi et al., 1996) and Kenya (Waturu, 1998), and of Heterorhabditis indica Poinar, Karunakar & David (Rhabditida: Heterorhabditidae) in 1992, from Egypt (Shamseldean, Adb-Elgawad, & Atwa, 1998) and Kenya (Shamseldean et al., 1996; Waturu, 1998).
A total of24 species are currently described as being from Africa, of which eight represent Heterorhabditis, and 16 Steinernema (Table 20.2). Of these, five Steinernema and two Heterorhabditis were described from South Africa, indicating the strong potential for new EPN species and isolates from the African continent, and highlighting the necessity of bioprospecting. New isolates reported from this century include H. indica and H. bacteriophora from Kenya and Egypt (Hominick, 2002; Stack et al., 2000). New isolates of Steinernema yirgalemense Nguyen, Tesmafariam, Gozel, Gaugler & Adams (Rhabditida: Steinernematidae) have also been reported from South Africa (Malan et al., 2011) and Ethiopia (Mekete et al., 2005). Steinernema karii and Steinernema weiseri Mráček, Sturhan & Reid (Rhabditida: Steinernematidae) have been reported from the Central Rift Valley Region of Kenya (Mwaniki et al., 2008). Tarasco et al. (2009) reported 13 isolates of Steinernema feltiae (Filipjev) Wouts, Mráček, Gerdin & Bedding (Rhabditida: Steinernematidae) and two of H. bacteriophora, during a survey that was undertaken of EPN in Algeria. This report is the first record of S. feltiae on the African continent. In Ethiopia, the dominant species was found to be S. yirgalemense, which was reported, together with two isolates of H. bacteriophora (Mekete et al., 2005). Kanga, Waeyenberge, Hauser, and Moens (2012) reported Heterorhabditis baujardi Phan, Subbotin, Nguyen & Moens (Rhabditida: Heterorhabditidae) from Cameroon, which is a species that was originally described from Vietnam, and which was later also recorded from Brazil (Dolinski, Del Valle, Burla, & Machado, 2007). Surveys in the Guinean zone of Southern Benin reported two species, Heterorhabditis sonorensis Stock, Rivera-Orduño & Flores-Lara (Rhabditida: Heterorhabditidae) (Stock, Rivera-Orduño, & Flores-Lara, 2009), and H. indica (Zadji et al., 2013), described from the Sonoran Desert in Mexico. Heterorhabditis amazonensis Andaló, Nguyen & Moinohas (Rhabditida: Heterorhabditidae) has been described from the Amazonas in Brazil, and it was also recently found during a survey in Cameroon (Kanga, Waeyenberge et al., 2012).
A total of five species described from Africa belong to Clade V (Spiridonov, Reid, Podrucka, Subbotin, & Moens, 2004), and, morphologically, to the glaseri-group (Nguyen, Hunt, & Mráček, 2007). A new group, called the Cameroonian Clade VI (Nthenga, Knoetze, Berry, Tiedt, & Malan 2014), is formed by Steinernema cameroonense Kanga, Trinh, Waeyenberge, Spiridonov, Hauser & Moens (Rhabditida. Steinernematidae), Steinernema nyetense Kanga, Trinh, Waeyenberge, Spiridonov, Hauser & Moens (Rhabditida: Steinernematidae), and Steinernema sacchari Nthenga, Knoetze, Berry, Tiedt & Malan (Rhabditida: Steinernematidae) (Fig. 20.2a). The Heterorhabditis spp. described from Africa belong to both of the two broad clades, the indica group, with Heterorhabditis noenieputensis Malan, Knoetze & Tiedt (Rhabditida: Heterorhabditidae) and Heterorhabditis baujardi Phan, Subbotin, Nguyen & Moens (Rhabditida: Heterorhabditidae), and the megidis group, with Heterorhabditis safricana Malan, Nguyen, De Waal & Tiedt (Rhabditida: Heterorhabditidae), Heterorhabditis zealandica Poinar (Rhabditida: Heterorhabditidae), and H. bacteriophora (Nguyen et al., 2007) (Table 20.1).
The evolutionary analysis of Steinernema (a) and Heterorhabditis (b) (including the associated bacteria) reported from South Africa, as inferred using the maximum parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA6. Species in bold = present in South Africa
Four symbiotic bacteria (Fig. 20.2), of which two were Xenorhabdus, and two Photorhabdus, were identified from endemic South African EPN, with three being described as new species, including Xenorhabdus khoisanae Ferreira, Van Reenen, Gozel, Malan & Dicks (Enterobacteriales: Enterobacteriaceae), associated with S. khoisanae (Ferreira et al., 2013b), Photorhabdus zealandica Ferreira, Van Reenen, Endo, Tailiez, Pagès, Sprӧer, Malan & Dicks (Enterobacteriales: Enterobacteriaceae) associated with H. zealandica (Ferreira et al., 2014a), and Photorhabdus luminescence subsp. noenieputensis Ferreira, Van Reenen, Pagès, Tailiez, Malan & Dicks (Enterobacteriales: Enterobacteriaceae), associated with H. noenieputensis (Ferreira et al., 2013). The bacteria associated with S. yirgalemense was identified as being Xenorhabdus indica (Ferreira et al., 2014b), previously described from Steinernema abbasi (syn. S. termophylum). The bacteria of H. zealandica found in South Africa differed from those of H. zealandica that were originally found in New Zealand and Florida. The associated bacterium from H. zealandica from New Zealand was identified as being Photorhabdus temperata Fischer-Le Saux, Viallard, Brunel, Normand & Boemare (Enterobacteriales: Enterobacteriaceae), while those that were from the South African H. zealandica were identified as being P. zealandica (Ferreira et al., 2014a).
3 Entomopathogenic Nematode Biological Control of Major Insect Pests in South Africa
3.1 The Codling Moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) in Apples and Pears
The codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae), is the key pest of apples and pears in South Africa (Barnes, 1991). Apples are mainly produced in the Western and Eastern Cape provinces. In South Africa, infestation rates in certain areas can be as high as 80 %, if no control measures are taken (Pringle, Eyles, & Brown, 2003). A key factor in the biology of codling moth is that the total population is represented as a diapausing overwintering population during the winter months of June to August. During early spring, when the temperature increases, the larvae again turn into pupae, from which the moths emerge in late spring. They lay their eggs on the young fruit, and on the adjacent leaves, with feeding larvae creating frass–filled tunnels that are equipped with an exit hole (with, usually, one codling moth larva per fruit), rendering the fruit unmarketable (Welter, 2008). In early, and late, autumn, when the fruit are ready for harvest, the last instar of the codling moth larvae move from the fruit to such cryptic habitats as pruning wounds, main spurs, and the trunk of the tree, close to the soil, as well as to debris around the tree, especially when the tree in question is a smooth–barked young apple tree (Cossentine, Sholberg, Jensen, Bedford, & Sheperd, 2004; Riedl, Blomefield, & Giliomee, 1998).
Codling moth is mostly chemically controlled throughout the growing season; however, integrated pest management (IPM) options are currently being employed in commercial orchards (Addison, 2005). Some of the tactics that are currently being used include mating disruption and ‘attract and kill’, as standard practice. The sterile insect technique (SIT) is being employed on a semi–commercial basis in specific regions (Pringle et al., 2003), with it being low density dependent (Judd & Gardiner, 2005). To use EPN successfully for the control of codling moth, the nematode isolate used should be: highly virulent; able to infect codling moth at low temperatures; and effective at low–water activity levels. The window of opportunity for aerial applications of EPN in a low humidity, water–scarce region is only a period of approximately 24 h, during which it is essential to maintain humidity of above 80 %, with a few hours of temperatures exceeding 20 °C. The nematode isolate used should also be able to locate cocooned larvae hiding on the tree. Different aspects involved in efficacy have been investigated in studies that have been undertaken into the potential of using EPN for the control of codling moth in South Africa (De Waal, Malan, & Addison, 2011a, 2011b; De Waal, Addison, & Malan, 2013; De Waal, Malan, Levings, & Addison, 2010).
IPM measures are currently hampered by infested wooden fruit bins, acting as a potential source of re–infestation. The investigation evaluated mini wooden fruit bins (built from the planks taken from old bins) that were artificially infested with last–instar diapausing codling moth larvae, which were inoculated with 25 infective juveniles (IJ)/mL (De Waal et al., 2010). Maximum mortality was achieved when the bins were pre–wet for at least 1 min, and then maintained at maximum humidity post–treatment for at least 3 days (De Waal et al., 2010). Tarping of the bin was the method used to obtain the desired high level of humidity that was required for effective insect control. By adding an adjuvant, increased mortality of the codling moth larvae was obtained. Moreover, the study revealed that, by using the correct concentrations of H. zealandica and high humidity, the addition of adjuvants to the nematode suspension has the potential to disinfest wooden fruit bins of codling moth successfully (De Waal et al., 2010). More research is required to evaluate the logistics of handling the wooden fruit bins, and their successful treatment with nematodes, in terms of commercial orchards.
The concept of mulching in orchards has been investigated in a further study, especially in the case of smooth–barked apple trees, where codling moth can be tempted to hide, and to overwinter, in the mulch. De Waal et al. (2011b) evaluated the potential of using H. zealandica in combination with mulches (pine chips, wheat straw, pine wood shavings, blackwood and apple wood chips) to control diapausing codling moth. Mesh cages filled with the different mulches, used as a larval confinement method, showed high levels of codling moth mortality (88 %), with pine wood shavings as mulch. Again, it was imperative that a high humidity of above 95 % was maintained for at least 3 days, to ensure nematode efficacy. A noteworthy point in this regard was that nematodes were found to have the ability to move 10 cm upwards into moist mulch, so as to infect codling moth larvae. Low temperatures (<15 °C) recorded during the first field trial resulted in low levels of control (<48 %), as opposed to the higher mortality recorded during the second field trial, with temperatures between 20 and 25 °C (De Waal et al., 2011b).
The biocontrol potential of six isolates, namely H. zealandica, S. citrae, S. khoisanae (J96, SF87), S. yirgalemense, and Steinernema sp., was evaluated (De Waal et al., 2011a). At optimum conditions in the laboratory, codling moth was found to be highly susceptible to all nematode isolates, at a low concentration of 50 IJ/insect, with mortalities between 78 and 100 %. A laboratory study at a suboptimal low temperature cycle, starting with 10 h at 17 °C, and 14 h at 12 °C, negatively affected the efficacy of all isolates to below 3 % codling moth mortality. The levels of free water in which a nematode is able to move, in a form of movement that is called water activity (aw), were investigated for the above–mentioned nematode isolates, with the average aw50–values for all isolates tested found to be 0.94, except for S. khoisanae, which had a higher aw level of 0.97 (De Waal et al., 2011b).
Laboratory conditions, and the containment method used for evaluating the field mortality levels of codling moth, were found to be not necessarily representative of the related field performance. In most of the previous studies with codling moth in field trials, cardboard strips (Lacey & Unruh, 1998) were used as a containment method, with high codling moth mortality. Three isolates, H. zealandica, S. khoisanae, and Steinernema sp., were used for field testing, with the latter being proven to be more effective, with a mortality of 70 %, compared to H. zealandica, with 59 % mortality. Insect containment methods used during field trials were shown to influence efficacy against codling moth, as different levels of mortality were obtained with the use of various containment methods (wooden planks vs. pear tree logs versus mesh cages) (De Waal et al., 2011a). Predictive equations were subsequently developed, enabling future trials to be conducted using either planks or cages (with pear tree logs proving impractical), and enabling the prediction of the expected level of control on the tree logs. All tested isolates showed a certain degree of biological control potential, although none of the experiments showed clear efficacy differences among the isolates. As the study showed that higher levels of control were obtained using the containment methods mentioned, the factor in question should be taken into consideration, when reporting the actual level of control during normal field applications (De Waal et al.).
All laboratory and field trials indicated that the main problem with the control of codling moth by means of EPN is the maintenance of adequate moisture levels that are required for nematode survival, and for their efficacy as biocontrol agents. De Waal et al. (2013) investigated the addition of a superabsorbent polymer, Zeba®, on the performance of H. zealandica, which was able to infect codling moth larvae only at aw ≥0.92, with aw50 = 0.94 and aw90 = 0.96. Laboratory experiments showed the highest level of mortality recorded to take place at 80 IJs/codling moth larva, which required at least 4 h of optimum conditions to ensure infectivity, and subsequent efficacy. Further studies showed that the addition of Zeba® to nematode suspensions improved the level of control obtained at 60 and 80 % RH in the laboratory, as well as enhancing the survival, and the infection ability, of the nematodes in the field.
3.2 The False Codling Moth Thaumatotibia leucotreta Meyrick (Lepidoptera: Tortricidae) in Citrus
False codling moth (FCM), Thaumatotibia leucotreta Meyrick (Lepidoptera: Tortricidae), is a key pest of citrus in South Africa. It is indigenous to South Africa, while also occurring elsewhere south of the Sahara, as well as on the Indian Ocean islands (CIBC, 1984), and Israel. The Eastern Cape produces the most citrus in South Africa, followed by Limpopo, Mpumalanga, the Western Cape, and KwaZulu–Natal. Current control of false codling moth in South Africa consists of orchard sanitation, chemical application, mating disruption, ‘attract and kill’, and SIT, combined with other biological control methods (Carpenter, Bloem, & Hofmeyer, 2007; Moore, 2002; Moore & Hattingh, 2012; Moore, Kirkman, & Stephen, 2004). The harvesting season is usually from May to October. Production in South Africa is confined to areas with mild, virtually frost–free winters. The average minimum temperature in the coldest month should not be below 3 °C to achieve ongoing production. Where rainfall is poor, the use of drip, or sprinkler, irrigation should be used to ensure good growth and production (CABI, 2011).
The FCM moth lays eggs on fruit, or leaves, with the larvae burrowing into the fruit, where they develop into the final instar (Daiber, 1979b), which drop, on a silken thread, to the soil, where they burrow a few mm into dry soil, and spin themselves into a cocoon (Daiber, 1980, 1989). After a few days, the prepupae turn into pupae (Daiber, 1979a), remaining, as such, in the soil for 8–10 days, depending on the prevailing temperature, after which they emerge from the soil as adult moths. False codling moth is multivoltine, producing up to six generations per year (Newton, 1998). The soil stages that are targeted by nematodes include the final–instar larvae, the prepupae, and the pupae, and the emerging moth. The soil stage of FCM, spanning approximately 14–18 days (Daiber, 1980, 1989), depending on the prevailing temperature, offers a long window period for the use of EPN.
Laboratory bioassays have shown isolates of six local EPN species to be highly virulent against the last instar of false codling moth larvae (Malan et al., 2011). This was the first research to be undertaken on the potential use of EPN to control the soilborne life stages of false codling moth, including larvae, pupae, and emerging moths. Steinernema yirgalemense, at a concentration as low as 50 IJ/insect, caused 100 % mortality of codling moth larvae, while, in most cases, the pupae concerned were at least half as sensitive to infection as were larvae using higher concentrations of nematodes. An important finding that was made during this study was that the emerging moths were infected with nematodes, thus potentially facilitating control, and their long–distance dispersal (Malan et al.).
Semi–field trials were conducted with contained FCM larvae in soil mesh cages. Six days after field nematode application, no significant differences were found in FCM mortality between three concentrations (5, 10, and 20 IJs/cm2) of H. zealandica applied, which caused >80 % control. In a field trial using three nematode species (H. bacteriophora, H. zealandica and S. khoisanae), treatment with H. zealandica resulted in significant persistence for each evaluation day, up to day 49.
As soil is the natural habitat for nematodes, they are especially suited to control the soil stages of FCM. All life stages, including the prepupae, the pupae, and the emerging moth, were found to be susceptible to nematodes (Malan et al., 2011). Results from these studies showed local EPN isolates to hold major potential for the control of the soil stages of FCM, with the added advantage of good persistence. Currently, large–scale efficacy trials are under way, with imported formulated H. bacteriophora in the different production areas, with promising results for future commercial use. However, more research into the ecology of nematodes, with regard to persistence in citrus orchards in different production areas in South Africa is required.
3.3 Mealybugs (Pseudococcidae) in Deciduous Fruit, Citrus and Grapevine
Mealybugs (Pseudococcidae) are severe agricultural pests that pose major problems for farmers in South Africa. The obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), is one of the most common, and serious, pests of apples and pears in South Africa (Wakgari & Giliomee, 2004), while the citrus mealybug, Planococcus citri (Risso) (Hemiptera: Pseudococcidae), is a highly destructive pest of citrus (Hattingh & Moore, 2003), with both occurring only in the aerial parts of trees. In the case of grapevine, the vine mealybug, Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae), has been shown to be the dominant mealybug species in South African vineyards. Although they remain predominantly above ground, they can also occur up to 30 cm deep, as colonies in the soil, on grapevine roots (Walton, 2003).
Mealybugs are difficult to control with chemicals, due to their cryptic lifestyles of hiding in crevices, under bark, and below ground on roots, where they are protected from insecticidal sprays. Their hydrophobic waxy secretions repel water–based insecticides, and they have the ability to rapidly develop resistance (Walton & Pringle, 2004). In citrus orchards, mealybug populations are usually suppressed by a complex of natural enemies (Hattingh & Moore, 2003), which is disrupted by the application of chemicals. However, there is a need for new and improved, P. ficus control options, potentially including EPN (Le Vieux & Malan, 2013a).
Laboratory bioassays were conducted to identify isolates of EPN that could cause high percentage mortality against P. viburni (Stokwe, 2009). Notable variation was found in the mortality caused by the different nematode isolates, leading to H. zealandica being selected as the most promising isolate for use in further studies. The biological development of a steinernematid and a heterorhabditid in adult P. viburni, P. ficus, and P. citri females was investigated, with H. zealandica and S. yirgalemense both being found to reproduce successfully in P. viburni (Le Vieux & Malan, 2013b; Stokwe, 2009; Van Niekerk & Malan, 2012).
The effect that mealybug size has on EPN infectivity was assessed. Adult and intermediate P. viburni were found to be more susceptible to nematode infection than were crawlers, because of the latter’s small size (Bastidas, Edgar, & San-Blas, 2014; Stokwe, 2009). Nematodes were tested for their ability to locate, and to infect, mealybugs on the surface, and in the ovary and calyx, of P. viburni field–infested apples. Results from the study indicated that the nematodes are capable of locating, and of infecting mealybugs, even when they are in the cores of infested apples. The LC50 and LC90 values were 54 and 330 nematodes per insect, respectively, with the LT50 and LT90 values being 30 h and 62 h, respectively. The study showed good potential for the use of EPN to control P. vibruni.
To determine the potential of local isolates of EPN to control P. citri, various laboratory bioassays were conducted (Van Niekerk & Malan, 2012). Adult female P. citri were found to be most susceptible to S. yirgalemense and H. zealandica, causing >90 % mortality. Further bioassays illustrated a linear relationship between mealybug mortality, and the concentration of nematodes applied. If nematodes are to be used as an above–ground application to control P. citri in citrus orchards, the amount of water that is available can be a major limiting factor. Insecticidal activity proved to be dependent on the available surface moisture after nematode application. An aw–bioassay indicated S. yirgalemense to be twice as tolerant to relatively low levels of free water. After application, nematodes have a limited time frame in which to locate, and infect, hosts, as the level of available free water gradually decreases, as trees dry out. Steinernema yirgalemense proved able to locate, and to infect, P. citri more quickly than were H. zealandica. An interesting result in this study was that S. yirgalemense were able to infect P. citri after an exposure time as short as 30 min. The results also showed the first 2–4 h post–application to be the most decisive time for establishing successful infection of mealybugs. The report was the first on the potential use of nematodes for the control of P. citri (Van Niekerk & Malan).
Humidity is one of the key factors to consider when using EPN as biological control agents. The addition of adjuvants to suspensions of EPN, to improve control in a foliar application, was investigated (Van Niekerk & Malan, 2013). An aqueous suspension, containing H. zealandica and 0.3 % Zeba®, significantly increased P. citri mortality at 80 % relative humidity (RH), with a temperature cycle starting at 22 °C for 14 h, and continuing at 11 °C for 11 h. The same polymer formulation was tested for S. yirgalemense, with the mortality of P. citri increasing by 21 % at 60 % RH, and by 27 % at 80 % RH. The addition of Nu–Film–P® and Zeba® to H. zealandica suspensions did not significantly retard application runoff from citrus leaves. The combination of Nu–Film–P® and Zeba®, however, was able to retard sedimentation significantly, increasing the average number of nematodes deposited on 2–cm2 leaf discs by 10 nematodes.
The compatibility of two endemic EPN with biological control agents and agrochemicals, which were likely to be used in an IPM programme for citrus in South Africa, was investigated (Van Niekerk & Malan, 2014a). This is the first report to have been produced on the possible negative effect of EPN against Cryptolaemus montrouzieri Mulsant (Coleoptera: Coccinellidae), a commercially produced biocontrol predatory insect, which is used against mealybugs. Results from bioassays in the laboratory showed the beetle larvae to be highly susceptible to H. zealandica and S. yirgalemense. Adult beetles were found to be twice as susceptible to S. yirgalemense as they were to H. zealandica. Tolerance of both species of IJ to aqueous solutions of Cyperfos 500 EC® (Chlorpyrifos and cypermethrin), Cryptogran™ (Cryptophlebia leucotreta granulovirus), Helicovir™ (Nucleopolyhedrovirus), Nu–Film–P® (Poly–1–P–menthene), and Zeba® (starch–g–polypotassium salt) for infectivity, and survival, was evaluated. Heterorhabditis zealandica proved to be highly compatible with all products tested, with no significant increase occurring in terms of nematode mortality. The products concerned also did not affect the ability of H. zealandica to infect mealworm larvae after exposure to products over a 24–h period.
Laboratory bioassays were conducted to establish the potential of EPN as biocontrol agents of P. ficus (Le Vieux & Malan, 2013b). Screening of local EPN isolates showed promising results for H. zealandica and S. yirgalemense. Bioassays indicated a concentration–dependent susceptibility of P. ficus to H. zealandica, to S. yirgalemense, and to commercially produced H. bacteriophora, with LC50 and LC90 values of 19, 82; 13, 80; and 36, 555, respectively. In soil column bioassays, both H. zealandica and S. yirgalemense were able to move 15 cm vertically downwards, so as to infect P. ficus, with respective mortalities of 82 and 95 %.
EPN can potentially be used within an IPM scheme to control P. ficus, which also occurs on grapevine roots. When S. yirgalemense was applied to the soil of two vineyards together with P. ficus, contained in pierced Eppendorf tubes, and buried at a depth of 15 cm in the soil, mortalities of up to 50 % were obtained after 48 h (Le Vieux & Malan, 2014). The persistence of S. yirgalemense, measured using codling moth larval mortality, was found to be zero in one vineyard, whereas, in another vineyard, it was 70 %, 12 weeks after application. Tests were conducted to establish the production of scavenger–deterrent factors (Le Vieux & Malan, 2015) by H. zealandica and S. yirgalemense. Of the cadavers that were presented 6 days after nematode infection, 49 % of the H. zealandica, and 60 % of the S. yirgalemense infected cadavers were left intact. Olfactometry tests indicated a significant difference concerning the number of S. yirgalemense IJ that were attracted to damaged Vitis vinifera L. (Vitales: Vitaceae) roots, and to P. ficus, indicating the active movement of the IJ, and the attractive ability of organic compounds produced by the roots. These studies showed that EPN, and specifically S. yirgalemense, have promising potential as biological control agents for the control of P. ficus soil populations (Le Vieux & Malan, 2015).
3.4 The Banded Fruit Weevil Phlyctinus callosus Schönherr (Coleoptera: Curculionidae)
Phlyctinus callosus Schönherr (Coleoptera: Curculionidae) was first reported from New Zealand in 1899, from where it spread to Australia (Kuschel, 1972). Phlyctinus callosus is indigenous to South Africa and is described for the first time in 1834 (Barnes, 1987). In deciduous fruit orchards, it is the main weevil pest, amongst others, as well as being a serious pest in grapevine (Allsopp, Barnes, Blomefield, & Pringle, 2015; Annecke & Moran, 1982; Myburgh, 1980), and in blueberries (Bredenhand, Van Hoorn, May, Ferreira, & Johnson, 2010). In South Africa, most damage occurs during November and December, when grape bunches are actively developing. In apple and plum orchards, most of the damage is inflicted on the lower parts of trees. In the Western Cape province, with a Mediterranean climate, it is estimated that P. callosus has the ability to cause up to 40 % damage on apples (Witt, Little, & Crowe, 1995).
In South Africa, P. callosus has one, or two, generations per year. The eggs are laid either just below the soil surface, or in organic matter, with the first–instar larvae then feeding on the roots of the host plant (Barnes, 1987, 1989; Barnes & Pringle, 1989). The majority of the larval stages of P. callosus tend to stay in the top 10 cm of soil during the winter months (Barnes, 1989). Phlyctinus callosus passed through up to 11 instars, with pupation lasting approximately 14 days. Emerging during late spring and early summer, they migrate, as flightless adults, up the tree trunks to reach the available fruits (Barnes; Barnes & Giliomee, 1992).
Since P. callosus has developed a high tolerance to pyrethroids, with an indication of cross–tolerance to acephate, chemical control is not successful against this pest (Barnes, Knipe, & Calitz, 1994, 1996). Trunk barriers are only used for monitoring purposes, as such use is very labour–intensive. The larvae, pupae, and emerging adults remain in the soil throughout the winter months, offering a window of opportunity for the use of EPN.
Research undertaken by Ferreira and Malan (2014a) showed that higher concentrations, and longer exposure times, were required to obtain satisfactory control of P. callosus larvae, in bioassay trials using local EPN. The trials in question involved three isolates, two H. bacteriophora and one H. zealandica, at a concentration of 400 IJ/ insect, with a 4–day exposure time for the adults and larvae. The percentage mortality was found to range between 41 and 73 % for the larvae, and between 13 and 35 % for the adults, with H. zealandica causing the highest mortality.
Optimum control is, however, obtainable by means of applying nematodes during winter and early spring. However, during the mentioned period, the temperature is generally low, with all local South African isolates being inactive at low temperatures. More local isolates still need to be screened, as only three isolates have been tested so far, with the current isolate giving only 43 % control after 2 days. Superior isolates should be selected. The best time for application in South Africa would be when the soil temperature is relatively low, with a low–temperature active nematode being selected.
3.5 The Fruit Flies Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) and Ceratitis rosa Karsch (Diptera: Tephritidae)
In South Africa, two species of fruit flies of economic importance occur in the Western Cape province, namely the Mediterranean fruit fly (Medfly), Ceratitis capitata (Wiedemann); and the Natal fruit fly, Ceratitis rosa Karsch (Diptera: Tephritidae), which are important pests of many fruits (Annecke & Moran, 1982; Prinsloo & Uys, 2015). Not only are the fruit flies responsible for economic crop losses, and for the cost of control, they are also international quarantine pests, causing restrictions on the international trade in fruit. Current control strategies for fruit fly mainly use the application of baits, mixed with insecticides while, in some areas of South Africa, medfly is commercially controlled through the use of the Sterile Insect Technique or SIT (Barnes, Eyles, & Franz, 2002).
Adult fruit fly tend to lay their eggs on the fruit, where the larvae go through several instars before leaving the infested fruit, dropping to the ground, and burrowing a few mm into the soil. After only a few hours, the pre–pupae turn into pupae in the soil (Annecke & Moran, 1982; Prinsloo & Uys, 2015).
The potential of three local isolates of H. bacteriophora, H. zealandica, and S. khoisanae to infect pupariating larvae, pupae, and adults of C. capitata and C. rosa was investigated, using 24–well bioassay plates in the laboratory (Malan & Manrakhan, 2009). Results from the study showed that pupariating larvae and adult flies were susceptible to nematode infection, with no infection being recorded for the pupae. However, some pupariating larvae infected with nematodes still managed to pupate, giving rise to malformed puparia, while trapping the nematodes inside the puparium. Pupariating larvae of C. capitata were generally more susceptible to infection than were those of C. rosa. Significantly, more larvae of C. capitata were infected with H. bacteriophora, and, in the case of C. rosa, the highest infectivity of larvae was obtained with H. zealandica. In contrast, adults of both species were highly susceptible to infection with S. khoisanae.
3.6 Noctuids, the African (Old World) Bollworm
Members of the Noctuidae family (Order: Lepidoptera) are agricultural pests of worldwide significance, of which Helicoverpa armigera (Hübner), Helicoverpa zea (Boddie), and Heliothis virescens (Fabricius) have achieved major pest status (Fitt, 1989). In South Africa, at least 38 commodities have chemical insecticide registrations listed against H. armigera, underscoring the importance of this ubiquitous pest (CropLife South Africa, [s.d.]). Biological control of H. armigera has gained global attention, given the development of resistance against all the major chemical groups, i.e. synthetic pyrethroids, organophosphates, organochlorines and carbamates (Regupathy, Kranthi, Singh, Iqbal, & Russell, 2003). According to the Arthropod Pesticide Resistance Database (http://www.pesticideresistance.com), this insect has shown resistance to at least 48 insecticidal active ingredients, including DDT. The South African scenario raises particular concern, as a large proportion of registered insecticides belong to the synthetic pyrethroid group.
The use of EPN has been attempted against above–ground noctuid pests (Bong & Sikorowski, 1983; Richter & Fuxa, 1990; Vyas, Patel, Yadav, Ghelani, & Patel, 2003), but the application of EPN to plant foliage is challenged by the general intolerance of IJ to desiccation and/or to UV radiation. For this reason, the mixing of EPN with surfactants, gels, polymers, and/or other adjuvants remains an area that is actively explored (see review by Shapiro-Ilan, Han, & Dolinksi, 2012). In contrast, the use of EPN against the soilborne stages of noctuid pests (Bell, 1995; Cabanillas & Raulston, 1995; Feaster & Steinkraus, 1996; Hussain, Ahmad, & Ahmad, 2014) is a more reasonable approach. Rather than applying the IJ directly onto the soil, a more ‘natural’ approach would be to apply the EPN inside their nematode–killed (carrier) hosts (Jansson, Lecrone, & Gaugler, 1993). Doing so has, in the past, demonstrated improved nematode dispersal (Shapiro & Glazer, 1996), infectivity (Shapiro & Lewis, 1999), survival (Perez, Lewis, & Shapiro-Ilan, 2003), and efficacy (Shapiro-Ilan, Lewis, Tedders, & Son, 2003). In an attempt to explore this approach, the Agricultural Research Council–Small Grain Institute evaluated two South African populations of H. bacteriophora (populations SGI 22 and SGI 173) (Hatting et al., 2009), as well as population SGI 148, S. tophus (Çimen, Lee, Hatting, Hazir, & Stock 2014b), against the pre–pupal and pupal stages of H. armigera, using final instar Tenebrio molitor L. (Coleoptera: Tenebrionidae) as carrier host. A glasshouse trial was conducted to measure not only the percentage mortality caused, but also to improve the understanding of the bionomics of the approach in terms of (1) the survival and infective capacity of IJ over a 16–week period post emergence (IJ age); (2) the total number of IJ emerging from the host; (3) the duration of IJ emergence (i.e. the ‘release period’); and (4) the number of IJ emerging from H. armigera, following infection over the above–mentioned period (i.e. in terms of EPN fitness). Briefly, the methodology entailed: the establishment of bean seedlings of Phaseolus vulgaris L. (Fabales: Fabaceae) in pots, the once–off inoculation of soil by means of EPN–infected T. molitor larva, and the release of two H. armigera final instar larvae per pot after 4, 8, 12, and 16 weeks (i.e. approximate IJ ages). Eight days after each release, all insects were removed, mortality noted, and both IJ yield, and day of IJ emergence, recorded (Jankielsohn A & Hatting, J.L. 2005).
Highest mortality (88 %) was noted after 2 and 4 weeks, with populations SGI 173 and SGI 148, respectively. No statistical differences were noted among any of the populations tested over the 16–week period. However, compared to the control, SGI 22 showed insignificant mortality from week 8 onwards. In general, mortalities decreased significantly over time, with an average mortality of only 8 % being recorded among all populations 16 weeks post inoculation. This finding was clearly reflected by the negative correlation coefficient values of −0.957, −0.926, and −0.977 for populations SGI 22, SGI148 and SGI 173, respectively. Compared with week 2, a significant decrease in percentage mortality among all populations was observed from week 8 onwards.
IJ production in H. armigera was generally higher for SGI 173, with a pooled average of 70,564 IJs produced over the entire duration of the trial, compared with the 23,669 and 7,202 produced by SGI 22 and SGI 148, respectively (Table 20.3). Again, measured against IJ age, negative correlation coefficient values of −0.960, −0.855, and −0.777 for SGI 22, SGI 148 and SGI173, respectively, were apparent.
For IJ ≤2 weeks old, significant differences were observed within host species, with the longest duration being 28 days for SGI 148 from T. molitor (Table 20.4). Considering the impact of the time spent in the soil without a host on the duration of IJ emergence, the bollworm data generally showed a negative correlation in this regard, with correlation coefficients of −0.548, −0.742, and −0.366 for SGI populations 22, 148 and 173, respectively. The average durations are presented in Table 20.5.
In vivo production and application of EPN via T. molitor proved successful against H. armigera, with mean pooled mortalities of 80 ± 10 %, and 78 ± 11 %, recorded with 2– and 4–week–old IJ, respectively. A noticeable decline was, however, evident from the eighth week onwards, with only 8 ± 2 % (pooled) mortality being recorded with 16–week–old IJ. Whether this decline was due to a loss of symbiotic bacterial load associated with IJ aging (Flores-Lara, Renneckar, Forst, Goodrich-Blair, & Stock, 2007), to decreasing IJ survival (Kung, Gaugler, & Kaya, 1990; Molyneux, 1985; Perez et al., 2003; Shapiro-Ilan, Stuart, & McCoy, 2006), and/or to a change in IJ foraging behaviour/ability (Grewal, Selvan, & Gaugler, 1994; Lewis, Campbell, & Gaugler, 1997; O’Leary, Stack, Chubb, & Burnell, 1998) affecting the eventual ‘dose’, is unknown. In any event, rapid dispersal/contact between the ‘young’ IJ and its host is critical during the initial stages of emergence, as has been pointed out by Stuart, Lewis, and Gaugler (1996). Coherently, this trait was found to be positively supported by the application of EPN by means of infected host cadavers, compared to aqueous suspension (Shapiro & Glazer, 1996). The ability of EPN to produce offspring was another fitness trait that was found to be negatively correlated with IJ age. In all three isolates, this ability deteriorated, with only SGI 173 producing some offspring (1, 664 IJ/cadaver), following infection with 16–week–old IJ.
An equally important aspect relates to the susceptibility of the pest at the time (life stage) of exposure to the EPN. In a study with S. feltiae, neonate larvae of the noctuid Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) were found to be significantly less susceptible to EPN infection than were 3– or 8–day old larvae (Kaya, 1985). Likewise, the high susceptibility of final instar H. armigera to S. riobrave, S. carpocapse, and Heterorhabditis sp. was reported by Tahir, Otto, and Hague (1995), with a similar trend also being noted with S. glaseri, S. feltiae, and H. indica (Karunakar, Easwaramoorhy, & David, 1999). In contrast, Glazer and Navon (1990) reported a negative relationship between the larval age of H. armigera and susceptibility to a population of S. feltiae, a phenomenon that was also observed with several EPN isolates tested against pecan weevil larvae (Shapiro-Ilan, 2001). These observations seem to support the general notion of employing EPN against latter larval stages of noctuid pests, even under relatively high temperature conditions (Ali, Pervez, Abid Hussain, & Ahmad, 2007; Cabanillas, Poinar, & Raulston, 1994; Grewal et al., 1994), to which species such as H. armigera have shown good adaptability (highest intrinsic rate of increase measured at 27.5 °C [Mironidis & Savopoulou-Soultani, 2008]).
The three EPN isolates tested here originate from the Free State province of South Africa, an area in a climatic zone defined as “humid subtropical with summer rainfall and cool (warmest month <22 °C)” (Hatting et al., 2009), and where the soil is typically expected to harbour H. armigera, given major crops, such as soya beans, wheat, maize, sunflower, apples, and selected vegetables, typically cultivated in the region. Although surveys to quantify the (natural) level of pre–pupal and pupal stage parasitism have not yet been conducted locally, the phenomenon has been explored elsewhere. Surveys over a 5–year period in the Lower Rio Grande Valley, Texas, found infection of 9 and 12 % in fall armyworm and corn armyworm, respectively (Raulston, Pair, Loera, & Cabanillas, 1992). Optimism gained from observing such natural levels of parasitism has led to augmentative attempts against noctuid species such as H. virescens (Bell, 1995; Bell & Hardee, 1994) and H. zea (Cabanillas & Raulston, 1995, 1996; Feaster & Steinkraus, 1996). Recently, a trial under seemingly challenging environmental conditions, and against the soil stages of H. armigera on chickpea, found up to 70 % moth suppression with Steinernema masoodi Alie, Shaheen, Pervez & Hussain (Rhabditida: Steinernematidae) at a rate of 6 × 109 IJ/ha (Hussain et al., 2014). The authors involved proposed that further research should be undertaken to optimise the timing of EPN applications, so as to coincide with irrigation during critical stages of the crop. To further underscore the importance of the correct timing of application, the data presented here suggest time–mediated fitness among IJ, as has been noted for mortality and IJ production (Table 20.2), as well as for the duration of IJ emergence (Table 20.4). Although a similar ‘worst case scenario’ (i.e., no alternative insect host in the soil) is unlikely to occur under natural field conditions, the potential deterioration in IJ fitness over time should be taken into account when considering EPN applications.
3.7 The Sugarcane Stalk Borer Eldana saccharina Walker (Lepidoptera: Pyralidae)
The sugarcane stalk borer, Eldana saccharina Walker (Lepidoptera: Pyralidae), is the most injurious insect pest of sugarcane, Saccharum spp., in Southern Africa (Goebel & Sallam, 2011; Leslie, 2004). With its local identification dating back to the 1940s (Dick, 1945), it is today known to infest numerous host plants that are of economic significance throughout Africa (Polaszek & Khan, 1998). Larval feeding is associated with stalk tissue damage, with reduced sucrose levels, and with compromised plant vigour (Goebel & Way, 2003). Control of E. saccharina in South Africa is based on the adoption of an IPM approach encompassing cultural, genetic, chemical, and biological strategies (Carnegie, 1981; Conlong & Rutherford, 2009; Conlong & Way, 2015; Keeping, 2006; Leslie, 2009; Rutherford & Conlong, 2010).
Some of the earliest attempts at pest suppression using EPN in South Africa, have been against the larval stages of E. saccharina (Spaull, 1988, 1990, 1991). During the first trial, EPN were applied at midday to the foliage, at concentrations ranging from 100,000 to 1,000,000 IJ, in 200 ml suspension per infested stalk, with up to 56 % larval mortality being recorded (Spaull, 1988). By reducing both the number of IJ (87,000), and the water volume (57 mL) per stalk, late afternoon applications realised control levels ranging from 40 to 45 % (Spaull, 1990). In a subsequent trial, using a more cold–tolerant population at 100,000 IJ, borer mortality was 6 %, suggesting a typical dose response rather than a temperature–linked response (Spaull, 1991).
In addition to the EPN concentration, another aspect to be considered is the potential impact of sugarcane sap (sucrose), as osmolyte, on the surviving IJ after entry into the borer tunnel. Delivery of IJ directly to the stubble and/or root stool shortly after harvesting might serve as another strategy for targeting E. saccharina, especially in older fields where infestation increases progressively. As follow–up to the earlier research by Spaull, bioassays were conducted by ARC–SGI, in collaboration with the South African Sugar Research Institute (SASRI; Dr Des Conlong), to (1) verify the pathogenicity of several newly collected indigenous EPN (Hatting et al., 2009) against E. saccharina; (2) measure the survival of IJ in sugarcane sap; and (3) investigate the movement and ability of IJ to infect E. saccharina inside infested sugarcane stalks. Briefly, the methodologies entailed the use of a piece of filter paper in a Petri dish assay, exposing final instar E. saccharina to five H. bacteriophora populations (SGI 32, SGI 43, SGI 180, INF 61, SASRI 75), three S. tophus (R 343, SASRI 356, SASRI 426), two S. innovationi (SGI 35, SASRI 198), and one S. khoisanae (R 293); the exposure of five EPN populations (SGI 32, SGI 35, SGI 43, SASRI 75, and SASRI 426) to sugarcane (cultivar N12) sap concentrations of 50 % (diluted with sterile distilled water) and 100 % (undiluted sap), with survival checks being undertaken after 24, 48, and 72 h; and the artificial infestation of sugarcane stalks with mid–instar E. saccharina larvae by way of vertically drilled holes, and topical inoculation with 1 ml EPN (SGI 35) suspension per stalk (Fig. 20.3).
Six EPN populations (SGI 35, SGI 43, R 293, R 343, SASRI 75, SASRI 356) caused 100 % mortality, with positive recycling in the host. Three populations (SGI 32, SGI 180, INF 61) killed only 33 % of larvae, with no recycling being recorded for SGI 32 and INF 61. Population SGI 35 was selected for a concentration–response assay with an LC90 of 44 (fiducial limits: 26–617) IJ per larva. The apparent susceptibility of E. saccharina to EPN infection is also supported by the findings of Pillay, Martin, Rutherford, and Berry (2009). The authors concerned tested ten indigenous EPN, recording 100 % mortality after 48 h with two Steinernema populations, EST3D and GING13G.
Of the five populations tested, only SGI 35 showed >80 % survival after 72 h in 100 % (undiluted) sap (Table 20.6). Survival in 50 % sap after 72 h decreased markedly among all isolates tested, with the highest survival being only 64 % noted with SGI 32. In a study by Glazer and Salame (2000), the effect of different osmolytes on the viability of Steinernema carpocapsae (Weiser) Wouts, Mráček, Gerdin & Bedding (Rhabditida. Steinernematidae) ‘All’ was evaluated. Viability was found not to have been affected by sucrose concentrations ranging from 1.2 to 3.7 mol/L after 24 h, but declined to only 27 %, in the higher concentration after 72 h. Similarly, differences in EPN tolerance towards a 20 % sucrose solution were also noted by Shamseldean, El-Sadawy, and Allam (2004). These authors found S. carpocapsae ‘All’ to be the most tolerant, while H. taysearae was found to survive for only 31 h. Superior osmotic tolerance to a mixture of fortified artificial seawater and glycerol at 15 °C was also noted for S. carpocapsae ‘All’ by Yan et al. (2010). Seemingly, the selection of EPN species/populations, based on their ability to tolerate the sucrose–rich environment within a sugarcane plant, should be considered when targeting E. saccharina and other borer species such as Diatraea saccharalis (Fabricius) (Lepidoptera: Crambidae). The latter species had previously been targeted with H. baujardi LPP7 and S. carpocapsae NCAll (Bellini & Dolinski, 2012).
Three of the 40 treated stalks had missing larvae on day 7, resulting in their omission from further calculations. Of the 37 remaining stalks, 24 (65 %) harboured dead E. saccharina (Fig. 20.4), of which 14 (58 %) larvae showed positive EPN recycling. Control mortality was 5 %. The data concerned support the notion of using EPN to target E. saccharina larvae inside the stubble, and directly after harvest (Fig. 20.4), while the cut wounds are still ‘fresh’ and relatively uncontaminated. Moreover, the low water volume of only 1 ml per stalk supports the practicality of adopting such a strategy under field conditions. Additional research aimed at optimising the dose (IJ/mL), the formulation and the application method, is warranted.
4 Current Legislation with Regard to Entomopathogenic Nematodes in South Africa
In South Africa, EPN–based products constitute an ‘agricultural remedy’, and, as such, are governed by the Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act 36 of 1947. According to this Act, an ‘agricultural remedy’ means any chemical substance, or biological remedy, or any mixture, or combination, of any substance, or remedy, that is intended, or offered, to be used (a) for the destruction, control, repelling, attraction, or prevention of any undesired microbe, alga, nematode, fungus, insect, plant, vertebrate, or invertebrate, or any product thereof. The use of EPNs, however, is still in its infancy in this country, largely impeded by the lack of locally–produced/formulated (indigenous) products. Although many chemical insecticides are available on the local market, the South African government has, through legislation, banned, or limited, the use/sale of several insecticides since the late 1970s. Recent interventions include the withdrawal of monocrotophos (2005), chlorpyrifos (products for home use, 2010), endosulfan (2012), and aldicarb (2012), fuelling the need for alternative remedies, including EPN–based products.
5 Conclusions and Future Directions
South Africa relies heavily on chemical pesticides with several hundred registered pesticides available on the local market (CropLife South Africa, [s.d.]). Not surprisingly, South Africa is also one of the four largest importers of pesticides in sub–Saharan Africa (Osibanjo et al., 2002). The economic implications and potential environmental impact thereof was reviewed by Quinn et al. (2011). Over the past 10 years much research input has been directed towards the base–line characterization of indigenous EPN species as well as the verification of target pest suitability for biocontrol with EPN. Ideally, such endeavours should be supported by the commercialization of indigenous species/populations, thereby negating the need for importation and release of exotic organisms. Interest in mass production of indigenous species/populations of EPN is evident from recent work by Fasemore (2012), Van Zyl (2012), Ferreira and Addison & Malan (2014), Ferreira and Malan 2014b), Ramakuwela, Hatting, Laing, and Hazir (2014), and Van Zyl and Malan (2014a, 2014b). According to the USA–based company ‘MarketsandMarkets’ the global market for biopesticides was valued at $1,796.56 Million in 2013 and is expected to reach $4,369.88 Million by 2019, growing at a CAGR of 16.0 % from 2014 to 2019. The need, in South Africa, for safer, environmentally sound, alternatives to chemical pesticides is expected to contribute to the abovementioned market expansion.
References
Addison, M. F. (2005). Suppression of codling moth Cydia pomonella L. (Lepidoptera: Tortricidae) populations in South African apple and pear orchards using sterile insect release. Acta Horticulturae, 671, 313–327.
Ali, S. S., Pervez, R., Abid Hussain, M., & Ahmad, A. (2007). Effect of temperature on survival of Steinernema seemae, S. masoodi and S. carpocapsae (Rhabditida: Steinernematidae) and their subsequent infectivity to prepupa of Helicoverpa armigera (Hubner). Archives of Phytopathology and Plant Protection, 40(3), 183–187.
Allsopp, E., Barnes, B. N., Blomefield, T. L., & Pringle, K. L. (2015). Grapevine. In G. L. Prinsloo & V. M. Uys (Eds.), Insects of cultivated plants and natural pastures in Southern Africa (pp. 420–437). Petroria, South Africa: Entomological Society of Southern Africa.
Annecke, D. P., & Moran, V. C. (1982). Insects and mites of cultivated plants in South Africa. Durban/Pretoria, South Africa: Butterworths.
Barnes, B. M., Eyles, D. K., & Franz, G. (2002). South Africa’s fruit fly SIT programme – the Hex River Valley pilot project and beyond. In B. M. Barnes (Ed.), Proceedings of the 6th international symposium on fruit flies of economic importance (pp. 131–141). Isteg Scientific Publications, Irene, South Africa.
Barnes, B. N. (1987). Bionomics, behaviour and monitoring of the vine snoutbeetle, Phlyctinus callosus Boh., indeciduous fruit orchards, with proposals for an improved control strategy. PhD thesis, Stellenbosch University, Stellenbosch.
Barnes, B. N. (1989). Embryonic and immature stages of Phlyctinus callosus Boh. (Coleoptera, Curculionidae) – Aspects of biology and behavior with respect to control in deciduous fruit orchards. Journal of the Entomological Society of Southern Africa, 52, 165–178.
Barnes, B. N., & Giliomee, J. H. (1992). Fruit–feeding behavior of banded fruit weevil, Phlyctinus callosus (Schönherr) (Col, Curculionidae), in apple orchards. Journal of Applied Entomology Zeitschrift für Angewandte Entomologie, 113, 407–415.
Barnes, B. N., Knipe, M. C., & Calitz, F. J. (1994). Trunk barriers provide effective control of banded fruit weevil on apples and nectarines. Deciduous Fruit Grower, 44, 327–340.
Barnes, B. N., Knipe, M. C., & Calitz, F. J. (1996). Latest results with trunk exclusion barriers for weevil control on apples (Jongste resultate met stamsperbande vir kalanderbeheer op appels). Deciduous Fruit Grower, 46, 284–287.
Barnes, B. N., & Pringle, K. L. (1989). Oviposition by the banded fruit weevil, Phlyctinus callosus (Schoenherr) (Coleoptera, Curculionidae), in deciduous fruit orchards in South Africa. Bulletin of Entomological Research, 79, 31–40.
Barnes, M. M. (1991). Codling moth occurrence, host race formation, and damage. In L. P. S. Van der Geest & H. H. Evenhuis (Eds.), Tortricid pests: Their biology, natural enemies and control (pp. 313–327). Amsterdam: Elsevier.
Bastidas, B., Edgar, P., & San-Blas, E. (2014). Size does matter: The life cycle of Steinernema spp. in micro–insect hosts. Journal of Invertebrate Pathology, 121, 46–55.
Basson, S. (1993) Biological control of Phlyctinus callosus by entomogenous nematodes. Proceedings of the 11th Symposium of the Nematological Society of Southern Africa, Rustenburg, South Africa. Phytophylactica, 25, 303.
Bell, M. R. (1995). Effects of an entomopathogenic nematode and nuclear polyhedrosis virus on emergence of Heliothis virescens (Lep: Noctuidae). Journal of Entomological Science, 30, 243–250.
Bell, M. R., & Hardee, D. D. (1994, January 5–8). Tobacco budworm: Possible use of various entomopathogens in large area pest management. Proceedings of the Beltwide Cotton Production conference (pp. 1168–1171), San Diego, CA: Beltwide Cotton Production.
Bellini, L. L., & Dolinski, C. (2012). Foliar application of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) for the control of Diatraea saccharalis in greenhouse. Ciências Agrárias Londrina, 33, 997–1004.
Bong, C. F. J., & Sikorowski, P. P. (1983). Use of DD136 strain of Neoaplectana carpocapsae Weiser (Rhabditidae: Steinernematidae) for control of corn earworm (Lepidoptera: Noctuidae). Journal of Economic Entomology, 76, 590–593.
Bredenhand, E., Van Hoorn, A., May, F., Ferreira, T., & Johnson, S. (2010). Evaluation of techniques for monitoring banded fruit weevil, Phlyctinus callosus (Schoenherr) (Coleoptera: Curculionidae), infestation in blueberry orchards. African Entomology, 18, 205–209.
Cabanillas, H. E., Poinar, G. O., & Raulston, J. R. (1994). Steinernema riobravis n.sp. (Rhabditida: Steinernematidae) from Texas. Fundamental and Applied Nematology, 17, 123–131.
Cabanillas, H. E., & Raulston, J. R. (1995). Impact of Steinernema riobravis (Rhabditida: Steinernematidae) on the control of Helicoverpa zea (Lepidoptera: Noctuidae) in corn. Journal of Economic Entomology, 88, 58–64.
Cabanillas, H. E., & Raulston, J. R. (1996). Effects of furrow irrigation on the distribution and infectivity of Steinernema riobravis against corn earworm in corn. Fundamental and Applied Nematology, 19, 273–281.
CABI. (2011). Crop protection compendium. Thaumatotibia leucotreta. Wallingford, CT: CABI. http://www.cabi.org/cpc
Carnegie, A. J. M. (1981). Combatting Eldana saccharina Walker: A progress report. Proceedings of the International Society of Sugar Cane Technologists, 55, 116–119.
Carpenter, J., Bloem, S., & Hofmeyer, H. (2007). Area–wide control tactics for the false codling moth Thaumatotibia leucotreta in South Africa: A potential invasive species. In: M. J. B. Vreysen, A. S. Robertson, & J. Hendrichs (Eds.), Area–wide control of pest insects (pp. 351–359). Springer Science & Business Media, The Netherlands.
CIBC. (1984). Possibilities for the control of false codling moth Cryptophlebia leuctreta (Lepidoptera: Tortricidae). Biocontrol News and Information, 5, 217–220.
Çimen, H. C., Lee, M. M., Hatting, J., Hazir, S., & Stock, S. P. (2014a). Steinernema innovationi n. sp. (Panagrolaimomorpha: Steinernematidae), a new entomopathogenic nematode species from South Africa. Journal of Helminthology.
Çimen, H. C., Lee, M.-M., Hatting, J., Hazir, S., & Stock, P. S. (2014b). Steinernema tophus sp. n. (Nematoda: Steinernematidae), a new entomopathogenic nematode from South Africa. Zootaxa, 3821, 337–353.
Conlong, D. E., & Rutherford, R. S. (2009). Conventional and new biological and habitat interventions for integrated pest management systems: Review and case studies using Eldana saccharina Walker (Lepidoptera: Pyralidae). In R. Peshin & A. K. Dhawan (Eds.), Integrated pest management: Innovation–development process (pp. 241–260). Dordrecht, The Netherlands: Springer Science and Business Media B.V.
Conlong, D. E., & Way, M. J. (2015). Sugarcane. In G. L. Prinsloo & V. M. Uys (Eds.), Insects of cultivated plants and natural pastures in Southern Africa (pp. 156–176). Petroria, South Africa: Entomological Society of Southern Africa.
Cossentine, J. E., Sholberg, P. L., Jensen, L. B. J., Bedford, K. E., & Sheperd, T. C. (2004). Fumigation of empty fruit bins with carbon dioxide to control diapausing codling moth larvae and Penicillium expansum Link. ex Tom spores. HortScience, 39, 429–432.
CropLife South Africa. [s.d.]. Agricultural remedies database. http://www.croplife.co.za/
Daiber, C. C. (1979a). A study of the biology of the false codling moth [(Cryptophlebia leucotreta (Meyr.)]: The cocoon. Phytophylactica, 11, 151–157.
Daiber, C. C. (1979b). A study of the biology of the false codling moth [(Cryptophlebia leucotreta (Meyr.)]: The larva. Phytophylactica, 11, 141–144.
Daiber, C. C. (1980). A study of the biology of the false codling moth Cryptophlebia leucotreta (Meyr.): The adult and generations during the year. Phytophylactica, 12, 187–193.
Daiber, C. C. (1989). The false codling moth, Cryptophlebia leucotreta (Meyr) (Lepidoptera, Tortricidae), in Southern Africa. Journal of Plant Diseases and Protection, 96, 71–80.
De Waal, J. Y., Addison, M. F., & Malan, A. P. (2013). A superabsorbent polymer formulation for improved efficacy of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) control of codling moth larvae, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biocontrol Science & Technology, 23, 62–78.
De Waal, J. Y., Malan, A. P., & Addison, M. F. (2011a). Efficacy of entomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) against codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in temperate regions. Biocontrol Science & Technology, 20, 489–502.
De Waal, J. Y., Malan, A. P., & Addison, M. F. (2011b). Evaluating mulches together with Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) for the control of diapausing codling moth larvae, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biocontrol Science & Technology, 21, 225–270.
De Waal, J. Y., Malan, A. P., Levings, J., & Addison, M. F. (2010). Key elements in the successful control of diapausing codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) in fruit bins with a South African isolate of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae). Biocontrol Science and Technology, 20, 489–502.
Dick, J. (1945). Some data on the biology of the sugarcane borer Eldana saccharina Walker. Proceedings of the South African Sugar Technologists Association, 19, 75–79.
Dolinski, C., Del Valle, E. E., Burla, R. S., & Machado, I. R. (2007). Biological traits of two native Brazilian entomopathogenic nematodes (Rhabditida: Heterorhabditidae). Nematologia Brasileira, 31(32), 180–185.
Elawad, S., Ahmad, W., & Reid, A. P. (1997). Steinernema abbasi sp. n. (Nematoda: Steinernematidae) from the Sultanate of Oman. Fundamental and Applied Nematology, 20, 435–442.
El-Rahman, R. M. A., El-Razzik, M. I. A., Osman, E. A., & Mangoud, A. A. H. (2012). Efficacy of the entomopathogenic nematodes and their based–product on some species of mealybugs (Hemiptera: Pseudococcidae) in Egypt. Egyptian Academic Journal of Biological Sciences Entomology, 5, 193–196.
Fasemore, O. A. (2012). An investigation of the possiblities of scaling–up and mass–producing entomopathogenic nematodes (EPNs) (154 p.). PhD thesis, University of the Witwatersrand, Johannesburg, South Africa.
Feaster, M. A., & Steinkraus, D. C. (1996). Inundative biological control of Helicoverpa zea (Lepidoptera: Noctuidae) with the entomopathogenic nematode Steinernema riobravis (Rhabditida: Steinernematidae). Biological Control, 7, 38–43.
Ferreira, T., Van Reenen, C. A., Endo, A., Spröer, C., Malan, A. P., & Dicks, L. M. T. (2013). Description of Xenorhabdus khoisanae sp. nov., a symbiont of the entomopathogenic nematode Steinernema khoisanae. International Journal of Systematic and Evolutionary Microbiology, 63, 3220–3224.
Ferreira, T., Van Reenen, C. A., Pagès, S., Patrick Tailliez, P., Malan, A. P., & Dicks, L. M. T. (2013). Description of Photorhabdus luminescens subsp. noenieputensis subsp. nov., a symbiotic bacterium associated with a new Heterorhabditis species related to Heterorhabditis indica. International Journal of Systematic and Evolutionary Microbiology, 63, 1853–1858.
Ferreira, T., & Malan, A. P. (2014a). Potential of entomopathogenic nematodes for the control of the banded fruit weevil, Phlyctinus callosus (Schönherr) (Coleoptera: Curculionidae). Journal of Helminthology, 88, 293–301.
Ferreira, T., & Malan, A. P. (2014b). Xenorhabdus and Photorhabdus, bacterial symbionts of the entomopathogenic nematodes Steinernema and Heterorhabditis and their in vitro liquid mass culture: a review. African Entomology, 22(1), 1–14.
Ferreira, T., Addison, M. F., & Malan, A. P. (2014). In vitro liquid culture of a South African isolate of Heterorhabditis zealandica for the control of insect pests. African Entomology, 22, 80–92.
Ferreira, T., Van Reenen, C. A., Endo, A., Spröer, C., Malan, A. P., & Dicks, L. M. T. (2014). Description of Photorhabdus zealandica sp. nov., a symbiont of the entomopathogenic nematode Heterorhabditis zealandica. International Journal of Systematic and Evolutionary Microbiology, 64(Pt 5), 1540–1545.
Ferreira, T., Van Reenen, C. A., Tailliez, P., Pagès, S., Malan, A. P., & Dicks, L. M. T. (2014). First report of the symbiotic bacterium, Xenorhabdus indica, associated with the entomopathogenic nematode Steinernema yirgalemense. Journal of Helminthology.
Fitt, G. P. (1989). The ecology of Heliothis species in relation to agroecosystems. Annual Review of Entomology, 34, 17–52.
Flores-Lara, Y., Renneckar, D., Forst, S., Goodrich-Blair, H., & Stock, P. (2007). Influence of nematode age and culture conditions on morphological and physiological parameters in the bacterial vesicle of Steinernema carpocapsae (Nematoda: Steinernematidae). Journal of Invertebrate Pathology, 95, 110–118.
Glazer, I., & Navon, A. (1990). Activity and persistence of entomoparasitic nematodes tested against Heliothis armigera (Lepidoptera: Noctuidae). Journal of Economic Entomology, 83(5), 1795–1800.
Glazer, I., & Salame, L. (2000). Osmotic survival of the entomopathogenic nematode Steinernema carpocapsae. Biological Control, 18, 251–257.
Goebel, F. R., & Sallam, N. (2011). New pest threats for sugarcane in the new bioeconomy and how to manage them. Current Opinion in Environmental Sustainability, 3, 81–89.
Goebel, F. R., & Way, M. J. (2003). Investigation of the impact of Eldana saccharina (Lepidoptera: Pyralidae) on sugarcane yield in field trials in Zululand. Proceedings of the South African Sugar Technologists Association, 77, 256–265.
Grenier, E., Bonifassi, E., Abad, P., & Laumond, D. (1996). Use of species specific satellite DNAs as diagnostic probes in the identification of Steinernematidae and Heterorhabditidae entomopathogenic nematodes. Parasitology, 113, 483–489.
Grenier, E., Laumond, C., & Abad, P. (1996). Molecular characterization of two species–specific tandemly repeated DNAs from entomopathogenic nematodes Steinernema and Heterorhabditis (Nematoda: Rhabditida). Molecular and Biochemical Parasitology, 83, 47–56.
Grewal, P. S., Selvan, S., & Gaugler, R. (1994). Thermal adaptation of entomopathogenic nematodes: Niche breadth for infection, establishment, and reproduction. Journal of Thermal Biology, 19, 245–253.
Harington, J. S. (1953). Observation on the biology, the parasites and the taxonomic position of the maize beetle – Heteronychus san–helenae Blanch. South African Journal of Science, 50, 11–14.
Hatting, J., Stock, P. S., & Hazir, S. (2009). Diversity and distribution of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) in South Africa. Journal of Invertebrate Pathology, 102, 120–128.
Hatting, J. L., & Kaya, H. K. (2001, July 2–5). Entomopathogenic nematodes: prospects for biological control in South Africa. In: Proceedings of the 13th Entomological Congress, Entomological Society of Southern Africa (pp 28–29). Pietermaritzburg, South Africa: Entomological Society of Southern Africa. ISBN 0–620–27806–4.
Hattingh, V., & Moore, S. D. (2003). Mealybugs. In T. G. Grout (Ed.), Integrated production guidelines for export citrus. Integrated pest and disease management (pp. 65–69). Nelspruit, : Citrus Research International.
Hominick, W. (2002). Biogeography. In R. Gaugler (Ed.), Entomopathogenic nematology (pp. 115–143). Wallingford, UK: CABI.
Hussain, M. A., Ahmad, R., & Ahmad, W. (2014). Evaluation of Steinernema masoodi (Rhabditida: Steinernematidae) against soil–dwelling life stage of Helicoverpa armigera (Lepidoptera: Noctuidae) in laboratory and microplot study. Canadian Journal of Plant Protection, 2(1), 4–8.
Jankielsohn, A., & Hatting, J. L. (2005, August 7–11). Efficacy of entomopathogenic nematodes, applied in an insect cadaver, as biological control agent against soil-dwelling stages of bollworm (Helicoverpa armigera Hübner). Proceedings of the 38th Annual Meeting of the Society for Invertebrate Pathology. Anchorage, Alaska.
Jansson, R. K., Lecrone, S. H., & Gaugler, R. (1993). Field efficacy and persistence of entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) for control of sweet potato weevil (Coleoptera: Apionidae) in southern Florida. Journal of Economic Entomology, 86, 1055–1063.
Judd, G. J. R., & Gardiner, M. G. T. (2005). Towards eradication of codling moth in British Columbia by complimentary actions of mating disruption, tree banding and sterile insect technique: Five–year study in organic orchards. Crop Protection, 24, 718–733.
Kanga, F. N., Trinh, P. Q., Waeyenberge, L., Spiridonov, S. E., Hauser, S., & Moens, M. (2012). Two new species of Steinernema Travassos, 1972 from the humid forest of southerm Cameroon. Russian Journal of Nematology, 20, 15–36.
Kanga, F. N., Waeyenberge, L., Hauser, S., & Moens, M. (2012). Distribution of entomopathogenic nematodes in Southern Cameroon. Journal of Invertebrate Pathology, 109, 41–51.
Karunakar, G., Easwaramoorhy, S., & David, H. (1999). Susceptibility of nine lepidopteran insects to Steinernema glaseri S. feltiae and Heterorhabditis indicus infection. International Journal of Nematology, 9(1), 68–71.
Kaya, H. K. (1985). Susceptibility of early larval stages of Pseudaletia unipuncta and Spodoptera exigua (Lepidoptera: Noctuidae) to the entomogenous nematode Steinernema feltiae (Rhabditida: Steinernematidae). Journal of Invertebrate Pathology, 46, 58–62.
Keeping, M. G. (2006). Screening of South African sugarcane cultivars for resistance to stalk borer, Eldana saccharina Walker (Lepidoptera: Pyralidae). African Entomology, 14, 277–288.
Kung, S.-P., Gaugler, R., & Kaya, H. K. (1990). Soil type and entomopathogenic nematode persistence. Journal of Invertebrate Pathology, 55, 401–406.
Kuschel, G. (1972). The foreign Curculionidae established in New Zealand (Insecta: Coleoptera). New Zealand Journal of Science, 15, 273–289.
Lacey, L. A., & Unruh, T. R. (1998). Entomopathogenic nematodes for control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Effect of nematode species, concentration, temperature, and humidity. Biological Control, 13, 190–197.
Le Vieux, P. D., & Malan, A. P. (2013a). An overview of the vine mealybug (Planococcus ficus) in South African vineyards and the use of entomopathogenic nematodes as potential biocontrol agents. South African Journal of Enology and Viticulture, 34, 34–45.
Le Vieux, P. D., & Malan, A. P. (2013b). The potential use of entomopathogenic nematodes to control Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). South African Journal of Entomology and Viticulture, 34, 296–306.
Le Vieux, P. D., & Malan, A. P. (2014). Prospects for using entomopathogenic nematodes to control the vine mealybug, Planococcus ficus, in South African vineyards. South African Journal of Enology and Viticulture, 36, 59–70.
Le Vieux, P. D., & Malan, A. P. (2015). Prospects for using entomopathogenic nematodes to control the vine mealybug, Planococcus ficus, in South African vineyards. South African Journal of Enology and Viticulture, 36(1), 59–70.
Leslie, G. (2004). Pests of sugarcane. In G. James (Ed.), Sugarcane (pp. 78–100). Oxford, U.K.: Blackwell Science.
Leslie, G. W. (2009). Estimating the economic injury level and the economic threshold for the use of alpha–cypermethrin against the sugarcane borer, Eldana saccharina Walker (Lepidoptera: Pyralidae). International Journal of Pest Management, 55, 37–44.
Lewis, E. E., Campbell, J. F., & Gaugler, R. (1997). The effects of aging on the foraging behaviour of Steinernema carpocapsae (Rhabdita: Steinernematidae). Nematologica, 43, 355–362.
Malan, A. P., Knoetze, R., & Moore, S. D. (2011). Isolation and identification of entomopathogenic nematodes from citrus orchards and their biocontrol potential against false codling moth. Journal of Invertebrate Patholology, 108, 115–125.
Malan, A. P., Knoetze, R., & Tiedt, L. R. (2014). Heterorhabditis noenieputensis n. sp. (Rhabditida: Heterorhabditidae), a new entomopathogenic nematode from South Africa. Journal of Helminthology, 88, 138–151.
Malan, A. P., & Manrakhan, A. (2009). Susceptibility of the Mediterranean fruit fly (Ceratitis capitata) and the Natal fruit fly (Ceratitis rosa) to entomopathogenic nematodes. Journal of Invertebrate Pathology, 100, 47–49.
Malan, A. P., Nguyen, K. B., & Addison, M. F. (2006). Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from the southwestern parts of South Africa. African Plant Protection, 12, 65–69.
Malan, A. P., Nguyen, K. B., De Waal, J. Y., & Tiedt, L. (2008). Heterorhabditis safricana n. sp. (Rhabditida: Heterorhabditidae), a new entomopathogenic nematode from South Africa. Nematology, 10, 381–396.
Mamiya, Y. (2008). Steinernema kushidai n. sp. (Nematoda: Steinernematodae) associated with scarabaeid beetle larvae from Shizuoca, Japan. Applied Entomology and Zoology, 23, 313–320.
Manrakhan, A., Daneel, J.-H., & Moore, S. D. (2013). The impact of naturally occurring entomopathogenic nematodes on false codling moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae), in citrus orchards. Biocontrol Science and Technology, 24, 241–245.
Mekete, T., Gaugler, R., Nguyen, K. B., Mandefro, W., & Tessera, T. (2005). Biogeography of entomopathogenic nematodes in Ethiopia. Nematropica, 35, 31–35.
Mironidis, G. K., & Savopoulou-Soultani, M. (2008). Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environmental Entomology, 37(1), 16–28.
Molyneux, A. S. (1985). Survival of infective juveniles of Heterorhabditis spp., and Steinernema spp. (Nematoda: Rhabditida) at various temperatures and their subsequent infectivity for insects. Revue de Nematologie, 8(2), 165–170.
Moore, S. B. (2002). Entomopathogens and microbial control of citrus pests in South Africa: A review. South African Fruit Journal, 1, 30–32.
Moore, S. D., & Hattingh, V. (2012). A review of current pre–harvest control options for false codling moth in citrus in southern Africa. South African Fruit Journal, 11, 82–85.
Moore, S. D., Kirkman, W., & Stephen, P. (2004). Cryptogran: A virus for the biological control of false codling moth. South African Fruit Journal, 3, 35–39.
Mwaniki, S. W., Nderitu, J. H., Olubayo, F., Kimenju, J. W., & Nguyen, K. (2008). Factore influencing the occurrence of entomopathogenic nematodes in the Central Rift Valley Region of Kenya. African Journal of Ecology, 46(Suppl. 1), 79–84.
Myburgh, A. C. (1980). Infestation potential of the codling moth. The Deciduous Fruit Grower, 30, 368–377.
Newton, P. J. (1998). False codling moth Cryptophlebia leuctreta (Meyrick). In E. C. G. Bedford, M. A. Van den Berg, & E. A. De Villiers (Eds.), Citrus pests in the Republic of South Africa (pp. 194–200). Nelspruit, South Africa: Dynamic Ad.
Nguyen, K. B., Hunt, D. J., & Mráček, Z. (2007). Steinernematidae: Species descriptions. In K. B. Nguyen & D. J. Hunt (Eds.), Entomopathogenic nematodes: Systematics, phylogeny and bacterial simbionts (Nematology Monographs and Perspectives, pp. 121–609). Leiden, The Netherlands/Boston: Brill.
Nguyen, K. B., Malan, A. P., & Gozel, U. (2006). Steinernema khoisanae n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology, 8, 157–175.
Nguyen, K. B., Tesfamariam, M., Gozel, U., Gaugler, R., & Adams, B. J. (2004). Steinernema yirgalemense n. sp. (Rhabditida: Steinernematidae) from Ethiopia. Nematology, 6, 839–856.
Nthenga, I., Knoetze, R., Berry, S., Tiedt, L. R., & Malan, A. P. (2014). Steinernema sacchari n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology, 16, 475–494.
O’Leary, S. A., Stack, C. M., Chubb, M. A., & Burnell, A. M. (1998). The effect of day of emergence from the insect cadaver on the behavior and environmental tolerances of infective juveniles of the entomopathogenic nematode Heterorhabditis megidis (strain UK211). Journal of Parasitology, 84(4), 665–672.
Osibanjo, O., Bouwman, H., Bashir, N. H. H., Okond’Ahoka, J., Choong Kwet Yve, R., & Onyoyo, H. A. (2002). Regionally based assessment of persistent toxic substances (Sub–Saharan Africa Regional Report). Geneva, Switzerland: UNEP Chemicals.
Perez, E. E., Lewis, E. E., & Shapiro-Ilan, D. I. (2003). Impact of host cadaver on survival and infectivity of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under desiccating conditions. Journal of Invertebrate Pathology, 82, 111–118.
Pillay, U., Martin, L. A., Rutherford, R. S., & Berry, S. D. (2009). Entomopathogenic nematodes in sugarcane in South Africa. Proceedings of the South African Sugar Technologists Association, 82, 538–541.
Polaszek, A., & Khan, Z. R. (1998). Host plants. In: A. Polaszek (Ed.), African cereal stem borers: Economic importance, taxonomy, natural enemies and control (pp. 3–10). Wallington, UK: CAB International, in association with ACP–EU Technical Centre for Agricultural and Rural Co–operation (CTA).
Pringle, K. L., Eyles, D. K., & Brown, L. (2003). Trends in codling moth activity in apple orchards under mating disruption using pheromones in the Elgin area, Western Cape province of South Africa. African Entomology, 11, 65–75.
Prinsloo, G. L., & Uys, V. M. (2015). Insects of cultivated plants and natural pastures in Southern Africa. Pretoria, Southern Africa: Entomological Society of Southern Africa.
Quinn, L. P., De Vos, B. J., Fernandes-Whaley, M., Roos, C., Bouwman, H., Kylin, H., et al. (2011). Pesticide use in South Africa: One of the largest importers of pesticides in Africa, In M. Stoytcheva (Ed.), Pesticides in the modern world – Pesticides use and management. InTech ISBN: 978–953–307–459–7. Available from: http://www.intechopen.com/books/pesticides–in–the–modern–world–pesticidesuse–and–management/pesticide–use–in–south–africa–one–of–the–largest–importers–of–pesticides–in–africa
Ramakuwela, T., Erasmus, A., & Hatting, J. (2011). Maize stalk borer, Busseola fusca, as potential target for control with entomopathogenic nematodes. South African Journal of Plant and Soil, 28(4), 255–256.
Ramakuwela, T., Hatting, J., Laing, M.D., Hazir, S. (2014, May 4–9). Cost effective solid-state production of an entomopathogenic nematode (Steinernematidae). Proceedings, 6th International Congress of Nematology (pp. 32). Cape Town.
Raulston, J. R., Pair, S. D., Loera, J., & Cabanillas, H. E. (1992). Prepupal and pupal parasitism of Helicoverpa zea and Spodoptera frugiperda (Lepidoptera: Noctuidae) by Steinernema sp. in cornfields in the lower Rio Grande Valley. Journal of Economic Entomology, 85(5), 1666–1670.
Regupathy, A., Kranthi, K., Singh, J., Iqbal, A. & Russell, D. (2003, March 9–13). Patterns and magnitude of insecticide resistance levels in India, Pakistan and China. Proceedings of World Cotton Research Conference. Cape Town. Abst. PS30.9.
Richter, A. R., & Fuxa, J. R. (1990). Effect of Steinernema feltiae on Spodoptera frugiperda and Heliothis zea (Lepidoptera: Noctuidae) in corn. Journal of Economic Entomology, 83, 1286–1291.
Riedl, H., Blomefield, T. L., & Giliomee, J. H. (1998). A century of codling moth control in South Africa: II. Current and future status of codling moth management. Journal of South African Society of Horticultural Science, 8, 32–54.
Rutherford, R. S., & Conlong, D. E. (2010). Combating sugarcane pests in South Africa: From researching biotic interactions to bio–intensive integrated pest management in the field. Proceedings of the International Society of Sugar Cane Technologists, 27, 1–17.
Shamseldean, M. M., Abou-El-Sooud, A. B., Abd-Elgawad, M. M., & Saleh, M. M. (1996). Identification of a new heterorhabditid species from Egypt, Heterorhabditis tayserae n. sp. (Rhabditidae: Heterorhabditidae). Egypt Journal of Biological Control, 6, 129–138.
Shamseldean, M. M., Adb-Elgawad, M. M., & Atwa, A. A. (1998). Effects of soil temperature, exposure time and host introduction on pathogenicity and recycling of an Egyptian strain of Heterhabditis bacteriophora strain EASD98 and its comparison with H. bacteriophora strain HP88 and Steinernema riobravae from USA. Journal of Nematology, 8, 71–76.
Shamseldean, M. M., El-Sadawy, H., & Allam, S. F. M. (2004). Comparative safety of entomopathogenic nematodes on honeybee workers. Egyptian Journal of Biological Pest Control, 14(1), 147–153.
Shapiro, D. I., & Glazer, I. (1996). Comparison of entomopathogenic nematode dispersal from infected hosts versus aqueous suspension. Environmental Entomology, 25, 1455–1461.
Shapiro, D. I., & Lewis, E. E. (1999). Comparison of entomopathogenic nematode infectivity from infected hosts versus aqueous suspension. Environmental Entomology, 28, 907–911.
Shapiro-Ilan, D. I. (2001). Virulence of entomopathogenic nematodes to pecan weevil larvae, Curculio caryae (Coleoptera: Curculionidae), in the laboratory. Journal of Economic Entomology, 94(1), 7–13.
Shapiro-Ilan, D. I., Han, R., & Dolinksi, C. (2012). Entomopathogenic nematode production and application technology. Journal of Nematology, 44(2), 206–217.
Shapiro-Ilan, D. I., Lewis, E. E., Tedders, W., & Son, Y. (2003). Superior efficacy observed in entomopathogenic nematodes applied in infected–host cadavers compared with application in aqueous suspension. Journal of Invertebrate Pathology, 83, 270–272.
Shapiro-Ilan, D. I., Stuart, R. J., & McCoy, C. W. (2006). A comparison of entomopathogenic nematode longevity in soil under laboratory conditions. Journal of Nematology, 38(1), 119–129.
Spaull, V. W. (1988). A preliminary evaluation of the entomogenous nematodes to control the African sugarcane stalk borer Eldana Saccharina (Lepidoptera: Pyralidae). Proceedings of the South African Sugar Technologists Association, 62, 120–123.
Spaull, V. W. (1990). Field tests to control the phylarid, Eldana saccharina, with an entomogenous nematode, Heterorhabditis sp. Proceedings of the South African Sugar Technologists Association, 64, 103–106.
Spaull, V. W. (1991). Heterorhabditis and Steinernema species (Nematoda: Rhabditida) for the control of a sugarcane stalk borer in South Africa. Phytophylactica, 23, 213–215.
Spiridonov, S. E., Reid, A. P., Podrucka, K., Subbotin, S. A., & Moens, M. (2004). Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analyses of sequences of the ITSI–5.8S–ITS2 region of rDNA and morphological features. Nematology, 6, 547–566.
Stack, C. M., Easwaramoorthy, S. G., Metha, U. K., Downes, M. J., Griffin, C. T., & Burnell, A. M. (2000). Molecular characterisation of Heterorhabditis indica isolates from India, Kenya, Indonesia and Cuba. Nematology, 2, 477–487.
Steenkamp, S., Erasmus, A., & Malan, A. (2011). The effect of South African entomopathogenic nematodes on Bt-resistant and non-resistant lifestages of Busseola fusca. South African Journal of Plant & Soil, 28(4), 268–269.
Stock, S. P., Rivera-Orduño, B., & Flores-Lara, Y. (2009). Heterorhabditis sonorensis n. sp. (Nematoda: Heterorhabditidae), a natural pathogen of the seasonal cicada Diceroprocta ornea (Walker) (Homoptera: Cicadidae) in the Sonoran Desert. Journal of Invertebrate Pathology, 100, 175–184.
Stokwe, N. F. (2009). Entomopathogenic nematodes: Characterization of a new species, long–term storage and control of obscure mealybug, Pseudococcus viburni (Hemiptera: Pseudococcidae) under laboratory conditions. MScAgric thesis, Department of Conservation Ecology and Entomology, Stellenbosch University, Stellenbosch.
Stokwe, N. F., Malan, A. P., Nguyen, K. B., Knoetze, R., & Tiedt, L. (2011). Steinernema citrae n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology, 13, 567–587.
Stuart, R. J., Lewis, E. E., & Gaugler, R. (1996). Selection alters the pattern of emergence from the host cadaver in the entomopathogenic nematode, Steinernema glaseri. Parasitology, 113(02), 183–189.
Tahir, H. I., Otto, A. A., & Hague, N. G. M. (1995). The susceptibility of Helicoverpa (Heliothis) armigera and Erias vitella larvae to entomopathogenic nematodes. Afro Asian Journal of Nematology, 5(2), 161–165.
Tamiru, T., Waeyenberge, L., Tesfaye, H., Ehlers, R. U., Puza, V., & Mráček, Z. (2012). Steinernema ethiopiense sp. n. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Ethiopia. Nematology, 14, 741–757.
Tarasco, E., Triggiani, O., Sai, K., & Zamoum, M. (2009). Survey on entomopathogenic nematodes in Algerian soils and their activity at different temperatures. Frustula Entomologica, 32(45), 31–42.
Van Niekerk, S., & Malan, A. P. (2012). Potential of South African entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) for control of the citrus mealybug, Planococcus citri (Pseudococcidae). Journal of Invertebrate Pathology, 111, 166–176.
Van Niekerk, S., & Malan, A. P. (2013). Adjuvants to improve control of Planococcus citri (Hemiptera: Pseudococcidae) using entomopathogenic nematodes (Heterorhabditidae and Steinernematidae). Journal of Helminthology, 89, 189–195.
Van Niekerk, S., & Malan, A. P. (2014a). Compatibility of Heterorhabditis zealandica and Steinernema yirgalemense with agrochemicals and biological control agents. African Entomology, 22, 49–56.
Van Niekerk, S., & Malan, A. P. (2014b). Evaluating the efficacy of a polymer–surfactant formulation to improve control of Planococcus citri (Risso) (Hemiptera: Pseudococcidae) under simulated natural conditions. African Plant Protection, 17, 1–8.
Van Zyl, C. (2012). The in vivo production of Heterorhabditis zealandica and Heterorhabditis bacteriophora (99 p). MSc thesis, University of Stellenbosch.
Van Zyl, C., & Malan, A. P. (2014a). The role of entomopathogenic nematodes as biological control agents of insect pests, with emphasis on the history of their mass culturing and in vivo production. African Entomology, 22(2), 235–249.
Van Zyl, C., & Malan, A. P. (2014b). Optimisation of inoculation techniques for in vivo mass culture of entomopathogenic nematodes through nematode and insect host manipulation. African Entomology, 24, 405–416.
Vyas, R. V., Patel, N. B., Yadav, P., Ghelani, Y. H., & Patel, D. J. (2003). Performance of entomopathogenic nematodes for management of gram pod borer, Helicoverpa armigera. Annals of Plant Protection Sciences, 11, 107–109.
Wakgari, W. M., & Giliomee, J. H. (2004). Insect pests of astringent persimmon in the Western and Eastern Cape provinces of South Africa. African Plant Protection, 10, 1–5.
Walton, V. M. (2003). Development of an integrated pest management system for vine mealybug, Planococcus ficus (Signoret), in vineyards in the Western Cape province, South Africa. PhD thesis, Department of Conservation Ecology and Entomology, Stellenbosch University, Stellenbosch, South Africa.
Walton, V. M., & Pringle, K. L. (2004). Vine mealybug, Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae), a key pest in South African vineyards: A review. South African Journal of Enology and Viticulture, 25, 54–62.
Waturu, C. N. (1998). Entomopathogenic nematodes (Steinernmatidae and Heterorhabditidae) from Kenya. PhD thesis, University of Reading, Reading, U.K.
Waturu, C. N., Hunt, D. J., & Reid, A. P. (1997). Steinernema karii sp. n. (Nematoda: Steinernematidae), a new entomopathogenic nematode from Kenya. International Journal of Nematology, 7, 65–75.
Welter, S. C. (2008). Codling moth. In H. C. R. T. Resh (Ed.), Encyclopedia of insects (pp. 189–199). Amsterdam: Elsevier.
Witt, A. B. R., Little, R. M., & Crowe, T. M. (1995). The effectiveness of helmeted guineafowl Numida meleagris (Linnaeus 1766) in controlling the banded fruit weevil Phlyctinus callosus (Schönherr, 1826), and their impact on other invertebrates in apple orchards in the Western Cape province, South Africa. Agriculture, Ecosystems & Environment, 55, 169–179.
Yan, X., Liu, X., Han, R., Chen, S., De Clerc, P., & Moens, M. (2010). Osmotic induction of anhydrobiosis in entomopathogenic nematodes of the genera Heterorhabditis and Steinernema. Biological Control, 53, 325–330.
Zadji, L., Baimey, H., Afouda, L., Houssou, F. G., Waeyenberge, L., De Sutter, N., et al. (2013). First record on the distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Southern Benin. Russian Journal of Nematology, 21, 117–130.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Malan, A.P., Hatting, J.L. (2015). Entomopathogenic Nematode Exploitation: Case Studies in Laboratory and Field Applications from South Africa. In: Campos-Herrera, R. (eds) Nematode Pathogenesis of Insects and Other Pests. Sustainability in Plant and Crop Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-18266-7_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-18266-7_20
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18265-0
Online ISBN: 978-3-319-18266-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)