Abstract
Haematococcus pluvialis is known as the richest natural source of carotenoid astaxanthin, whose synthesis is related to changes in cells according its complex life cycle and the availability of nutrients present in the culture media. This study’s objective was to evaluate the influence of different culture media under photoautotrophic and mixotrophic conditions on the growth, life cycle, biochemical composition, and astaxanthin production in H. pluvialis. Cultures were performed using the following media: KM1, MM2, Provasoli, modified Provasoli, and how to control the BBM, all kept at 24 ± 1 °C, with constant aeration, light/dark photoperiod 24:0 and irradiance of 60 μmol photons/m2/s. Growth and life cycle were evaluated by daily cell counts under an optical microscope; values of maximum cell density (MCD) and dry biomass were also assessed. Biochemical analyses were chlorophyll (a and b), astaxanthin, total proteins, and fatty acids. Cultivation under photoautotrophic conditions in BBM medium showed higher concentration of chlorophylls a (33 ± 4.0 μg/mL) and b (18 ± 1.6 μg/mL) and protein (62.7 ± 3.38%). Cultivation in modified Provasoli medium, under mixotrophic conditions, showed higher MCD (84.5 ± 11.7), biomass production (4.9 ± 0.0004 mg/mL), fatty acid methyl esters, and astaxanthin productivity (9.28 ± 0.4 mg/L/day). These results demonstrate that the culture media and the culture conditions to which H. pluvialis is submitted stimulate specific metabolic and biosynthetic pathways of the cells, which directly affect the biochemical composition of the microalgae. Modified Provasoli medium proved to be the most efficient in increasing astaxanthin productivity for this species, as it has shown better growth parameters and biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are microscopic and photosynthetic organisms that produce biomolecules such as proteins, amino acids, lipids, polyunsaturated fatty acids, and carotenoids. These substances have applications in several areas such as biotechnology and can be applied in aquaculture, biochemistry, pharmaceutics, and nutraceuticals, as well as in bioenergy production (Katiyar et al. 2017). The production of biocompounds from the microalgae is strictly linked to the culture conditions to which they are submitted (Mustafa et al. 2013). Several studies have evaluated factors such as light, light intensity, photoperiod, salinity, temperature, pH, composition, and quantity and quality of the nutrients of the culture medium, which can significantly alter the production of a particular biocompound of interest (Wahidin et al. 2013; Mandotra et al. 2016; Shen et al. 2016).
Astaxanthin is a carotenoid which belongs to the xanthophylls group and is produced by aquatic organisms such as microalgae. It is well-known for its high antioxidant, anti-inflammatory, anticancer, and antiproliferative activities which increase the efficiency of conventional chemotherapy drugs in subjacent tumor cells (Faraone et al. 2020). It is a reddish orange pigment which can be extracted from natural sources or synthetically produced from petrochemicals. Synthetic astaxanthin contains a mixture of three isomers: 3S,3′S; 3R,3′S; and 3R,3′R. In contrast, the predominant isomer in natural astaxanthin is 3S,3′S, which is preferable as an additive in aquaculture due to its significantly greater antioxidant activity when compared to synthetic astaxanthin, giving, for example, a greater extent of pigmentation in rainbow trout (Barbosa et al. 1999), in goldfish Carassius auratus (Gouveia et al. 2003; Cunha et al. 2020), in the ornamental marine fish Pseudochromis fridmani (Jiang et al. 2019), in the crab Eriocheir sinensis (Su et al. 2020), and in the shrimp Penaeus monodon (Angell et al. 2018), and for improving immunological activity and zootechnical performance of cultivated animals (An et al. 2020; Wang et al. 2020).
A point of concern related to the safety of synthetic astaxanthin consumers is the inherent toxicity of its raw material; moreover, it has not yet been approved for direct human consumption as food supplements (Li et al. 2010). The increased demand for the consumption of natural products and the growing concerns regarding the safety of synthetic astaxanthin for aquaculture or human consumption make these types of unnatural pigments less desirable, providing a great opportunity for the production of natural astaxanthin from Haematococcus pluvialis (Jiang et al. 2018). The aforementioned microalgae are the biological source that accumulates the highest astaxanthin content in nature, its cells being able to contain up to 3.8% of carotenoid in dry biomass weight.
Astaxanthin synthesis in H. pluvialis cells is related to morphological, physiological, and biochemical changes in cells due to the complex life cycle of the species and environmental factors, in addition to the nutrients and their concentrations present in the culture media (Shah et al. 2016). Culture media can be chemically differentiated by the presence or absence of certain nutrients (nitrogen, phosphorus, potassium, and carbon as an organic substrate) and/or their different concentrations. Varying carbon/nitrogen ratios, for example, stimulates the specific metabolic pathways and biosynthesis of cells, which directly affect the biochemical composition, growth, and productivity of microalgae biomass.
Also, the microalgae metabolism can be influenced by the conditions of the culture to which they are submitted; based on the energy sources (light or carbon) present in the growth medium, the crops are classified into photoautotrophic, heterotrophic, and mixotrophic. In photoautotrophic cultivation, algae use light as a source of energy and CO2 as the main source of carbon (Pang et al. 2019a), whereas in the heterotrophic cultivation, the main source of carbon is organic, and under these conditions, microalgae do not use light energy for their growth (Perez-Garcia et al. 2011). In mixotrophic cultivation (a combination of both autotrophic and heterotrophic), microalgae use light energy and both organic and inorganic compounds simultaneously present in the medium as carbon sources for their growth (Perez-Garcia et al. 2011). Cultivation under mixotrophic conditions can be an effective way to increase growth, improving algae productivity, and the accumulation of biomolecules (Pang and Chen 2017a; Doria et al. 2018). Compared to other species of commercially grown microalgae (Chlorella vulgaris, Dunaliella salina), H. pluvialis has some unfavorable characteristics for cultivation, such as low growth rate (0.2 per day), low biomass productivity (0.01 to 4.8 g/L/day), complex life cycle, and little knowledge about the optimal growth and induction conditions of astaxanthin in cells (Zhang et al. 2017). However, under mixotrophic conditions, the growth rate and biomass productivity of H. pluvialis can be increased (Pang and Chen 2017b).
Faced with this, research aimed at improving and seeking more knowledge about these factors is necessary. The objective of this work was to evaluate the influence of different culture media and photoautotrophic and mixotrophic conditions of cultivation, on the growth, life cycle, biochemical composition, and astaxanthin production of the microalgae H. pluvialis.
Materials and methods
Microalgae
Haematococcus pluvialis, from the Laboratorio de Produção de Alimento Vivo of Departmento de Pesca e Aquicultura in Universidade Federal Rural de Pernambuco, was maintained in Bold’s Basal medium (Bold 1949), at 24 ± 1 °C, pH 7, photoperiod 24:0 h (light/dark), constant aeration, and irradiance of 60 μmol photons/m/s (supplied from artificial lighting with 40 W type fluorescent lamps “daylight”).
Medium
Four different culture media were tested, Bold’s Basal medium (BBM) (Bold 1949) used as a control and Provasoli medium (Provasoli 1968), in photoautotrophic conditions, and KM1, MM2 (Tripathi et al. 1999), and modified Provasoli in mixotrophic conditions (medium compositions are summarized in Table 1). The experiment was carried, in Erlenmeyer flasks containing 2 L of the culture media out in a completely randomized design with three independent replicates for each treatment. Culture mediums were prepared using treated freshwater and sterilized. The experimental units were then inoculated from an exponentially growing culture with an initial cell density of 10 × 104 cells/mL. All the flasks were kept at a temperature of 24 ± 1 °C, constant aeration (flow rate of 2 L/min), and under an irradiance of 60 μmol photons/m2/s (fluorescent lamps) with continuous illumination.
Determination of the rate of cell growth and productivity
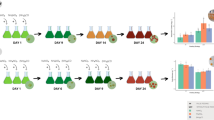
To evaluate the growth of H. pluvialis, samples collected every 24 h after the start of the experiment were submitted to cells counting under an optical microscope (objective × 40) in a Neubauer chamber. Cells were counted according to the life cycle in which they were (Fig. 1), according to the characteristics mentioned by Shah et al. (Shah et al. 2016) and Butler et al. (Butler et al. 2017) and Cui et al. (Cui et al. 2020).
Life cycle of the microalga Haematococcus pluvialis. a Zoospores, spherical, ellipsoidal, or pear-shaped vegetative cells, with two flagella; b Palmela, vegetative cells at rest and without flagella; c Palmela in transition to aplanospores; d Aplanospores, cells in red cysts; e Cells in the intermediate stage, with flagella and initiating the production of astaxanthin characterized by the red cytoplasm; f Red cells with flagella, without forming aplanospores
For each treatment of the experiment, a growth curve was drawn based on the mean daily cell density of three replicates using CurveExpert software version 1.4, and each curve was adjusted by the approximation to the logistic curve according to the formula developed by Pindyck and Rubinfeld (Pindyck and Rubinfeld 1990).
Besides growth curves, five other parameters were measured: Maximum cell density (MCD) was evaluated considering the day of cultivation in which the algal population reached maximum cell density; yield or production of each culture based on the different media through dry biomass per mL in the culture (mg/mL); time of cultivation was determined by the number of days passed from the beginning of the experiment to the day the population reached the stationary phase of growth; doubling time (Dt) and growth rate (k), the number of cell divisions of the population per day, were determined by the equation quoted in Stein (Stein 1973):
where k = growth rate 3.322 = conversion factor of logarithm base 2 to base 10; (T2 - T1) = time interval in days; N1 = initial cell density; N2 = final cell density; and log = base 10 logarithm.
To obtain final dry biomass, 1 mL samples were centrifuged at 5000×g for 2 min and washed twice with 0.5 M ammonium formate (3%) for the removal of the salts from the culture medium (Zhu and Lee 1997) and one wash with 1 mL of distilled water (Chioccioli et al. 2014). The obtained biomass was lyophilized, and after drying, the samples were weighed to determine the final dry biomass.
Biochemical analyses
Biochemical analysis of the microalgae was performed concomitantly with the life cycle. Samples were collected after 24 h of the beginning of the experiment and every 3 days for the evaluation of dry biomass, chlorophyll a and b, and astaxanthin. For analyses of gross protein product and fatty acids, samples were collected after the first day of culture and at the end of the experiment.
Chlorophylls
For determination of chlorophyll a and b, samples of 15 mL of the cultures were centrifuged (2200×g, 10 min), and the obtained biomass was subjected to extraction according to Humphrey’s method (Humphrey 1979). Cells were mixed with 3 mL of 90% acetone and submitted to ultrasonic bath in ice for 5 min, using an Elmasonic P 60 H (Elma Schmidbauer GmbH). Samples were then kept at 4 °C for 12 h for the complete extraction of chlorophyll. Subsequently, samples were centrifuged (2200×g, 10 min) again, and the extracts obtained were read at the absorbance of 664 and 647 nm for chlorophyll a and b, respectively. The spectrophotometer (UV/Vis Biospectro sp-220) was previously calibrated with 90% acetone. Then, the chlorophyll concentrations were calculated following the equations defined by Jeffrey and Humphrey (Jeffrey and Humphrey 1975):
Total proteins
The determination of total proteins used the extraction method proposed by Barbarino and Lourenço (Barbarino and Lourenço 2005) from 5 mg of lyophilized cells and the quantification by the method describe in Lowry et al. (Lowry et al. 1951), and bovine serum albumin (31.25–500 μg/mL, Sigma-Aldrich, USA) was used as standard.
Fatty acids
Lipids were extracted according to Bligh and Dyer (Bligh and Dyer 1959) modified where 1 g of dry biomass was mixed with 20 mL of chloroform/methanol (1:1 v/v) and vortexed for 2 min and incubated twice in an ultrasonic bath for 15 min and cooled on ice for 5 min. The sample was then centrifuged (10.000×g; 15 min). The supernatant was transferred to a new tube, and 7.5 mL of Milli-Q water was added and again vortexed for 2 min, followed by centrifugation (10.000×g; 5 min). The sample was carefully transferred to a separatory funnel, and after 1 h, the organic phase was collected. The solvent was evaporated, and the lipid sample was transesterified with 0.5 mL of KOH in methanol and vortexed for 2 min. Subsequently, 2 mL of vortexed n-hexane was added for 2 min and centrifuged (4.500×g; 6 min). The nonpolar phase was collected, filtered (Millipore 0.22 μm), and stored in vials.
The analysis of fatty acid methyl esters (FAMEs) was performed in a gas chromatograph (Agilent Technologies model 7890A) equipped with a flame ionization detector and coupled to the capillary column DB5-MS (30 m × 0.32 mm × 0.25 μm; Agilent Technologies). The injection volume was 1 μL with a split rate of 100:1. The heating ramp was started with an isotherm of 150 °C for 5 min, followed by a heating rate of 4 °C/min until the temperature reached 280 °C maintained for 5 min. The temperature of the detector and the injector was 300 °C.
The identification of the FAMEs was performed by comparison with the retention time of an authentic standard (Fatty Acid Methyl Ester mix SupelcoTM C4-C24) and the quantification by a percentage based on the area normalization of the obtained peaks.
Astaxanthin
The determination of astaxanthin followed the extraction method with hydrochloric acid pretreatment followed by acetone extraction (HCl-ACE) described by Dong et al. (Dong et al. 2014), and the extract obtained was filtered in filter 0.22 μm and analyzed by LC-MS. The analyses were carried out in ultra-performance liquid chromatography (UPLC) Acquity H-Class (Waters), column C18 HSS T3 2.1 × 100 mm, and particle size of 1.8 μm. The mobile phases used consisted of acetonitrile solution containing 0.1% formic acid (eluent A) and a methanolic solution containing 0.1% formic acid (eluent B) and ethyl acetate solution containing 0.1% of formic acid (eluent C) which were pumped at a flow rate of 0.37 mL/min. Elution was performed in isocratic (10% A, 50% B, 40% C) for 5 min. Ten microliters of sample was injected. The column temperature was maintained at 40 °C and the auto-injector at 10 °C. The UPLC system was coupled to a single quadrupole mass spectrometer SQ Detector 2 (Waters). The capillary voltage was 3.5 kV, the voltage of the 50 V cone; the desolvation temperature was 350 °C, with a gas flow of the source of 650 L/h. The data acquisition was done in Selected Ion Recording (SIR) mode, searching the mass of astaxanthin, in positive ionization. The acquisition of the chromatograms and mass spectra was done through the software MassLynx™ (Waters).
A calibration curve was used, using astaxanthin commercial standard with 97% purity (SML0982 Sigma, St. Louis, MS, USA), for quantification of the same in the extracts. The entire procedure was performed in triplicate and protected from light.
The astaxanthin productivity (mg/L/day) was computed according to the equation (Ct – C0)/t, where Ct and C0 are astaxanthin concentration by final dry biomass (mg/L) in the after final induction of carotenoid (Ct) and the initial astaxanthin concentration (C0) at the time (t) of the induction stage during the cultivation of H. pluvialis (Wan et al. 2015).
Water quality
Every 3 days during the microalga cultivation, water quality parameters such as temperature, pH (Q400AS), and nitrate (APHA/AWWA/WEF 2012) are evaluated.
Statistic
The response variables of the treatments were submitted to Bartlett’s variance homogeneity test and to the Shapiro-Wilk normality test. Data which did not present normality were transformed by sin (x). For each treatment, cell density, growth rate, and doubling time were determined and subjected to one-way ANOVA. The factors culture media and days of cultivation were submitted to factorial ANOVA with a posteriori Tukey test to establish differences between combinations and/or interactions and treatments. Data that did not obtain normal distribution even after the transformations were submitted to the Kruskal-Wallis test and/or the Friedman test followed by the Conover test for one- and two-way ANOVA, respectively. To elucidate the relationship between all the response variables concerning the different culture media and the cultivation days, a multivariate analysis of the main components is performed. All analyses were performed in the program R Statistical software version 3.4.2 (RCoreTeam 2017) and with significance index p < 0.05.
Results
It was observed that in each of the media tested, the life cycle of the microalga H. pluvialis was altered (Fig. 2). It is noted that in BBM, Provasoli, and modified Provasoli media, the zoospore phase was longer than that observed in the other media. Only in KM1 medium was it possible to visualize all cell types of the life cycle. In the KM1 and MM2 media, a faster transition to the astaxanthin accumulation phases is observed; however, when cultivated in modified Provasoli medium, about three times more biomass was obtained, despite reaching the accumulation phase of astaxanthin later, when compared to the KM1 and MM2 media. Regarding the life cycle, mean, and day factors, as well as the interaction between them, presented significant differences.
The growth curves of BBM, Provasoli, and modified Provasoli treatments were similar, with the highest growth on the first day and reaching the stationary phase around the eighth day. However, some differences were noticeable in the treatments with the KM1 and MM2 media, which showed a reduction in the microalga cultivation time, entering the stationary phase on the fourth day (Fig. 3). For these two media cultivations were terminated at 6 days due to microscopic observation of cell mortality.
Logistic curves of the growth of the microalga Haematococcus pluvialis for the different culture media. The points indicate the values obtained, and the solid line represents the adjusted curve applying the logistic model. Each curve represents the average of three replicates in cell numbers per milliliter
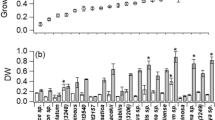
Significant differences were found in all growth parameters (Table 2). Concerning doubling time, KM1 medium presented a longer time interval leading to a decrease in the growth rate, which is statistically different from the other means studied. In contrast, MM2 presented lower duplication time and higher growth velocity. The highest MCD was observed in the modified Provasoli medium, and the MM2 and KM1 media had the lowest recorded cell densities. The modified Provasoli medium presented the highest dry biomass per mL of culture (4.9 ± 0.0004 mg/mL), which is significantly higher than the others.
Regarding water quality parameters, temperature remained constant throughout the crop (24 ± 1 °C). However, a declining trend in nitrate (NO3-N) concentrations was observed, influenced by days of cultivation and type of media, from the first day, and alkalinization was observed in all treatments. However, their values were influenced only by the day factor and not between the culture media (Fig. 4).
Chlorophyll a and b and carotenoid astaxanthin were influenced by the culture media and days (Table 3). The BBM medium presented the highest concentration of chlorophyll on the first day of cultivation, while KM1 and MM2 had the lowest amount. Throughout the cultures, concentration of these pigments progressively decreases in all treatments. At the end of the cultivation time, chlorophyll content was reduced, the BBM medium maintained higher values, and the media KM1 (7 ± 0.3 μg/mL) and MM2 (9 ± 0.8 μg/mL) had the lowest values. Astaxanthin values increased significantly from day one (p < 0.05), mainly under mixotrophic condition in media supplemented with sodium acetate when compared to media without acetate in photoautotrophic condition. On the first day of cultivation, after 24 h of growth, the modified Provasoli medium obtained the highest concentration (46 ± 3.1 μg/mL), and after finishing the cultures, it was observed that concentration of this pigment in KM1 medium on day 6 and modified Provasoli media on day 9 showed equivalent values of astaxanthin (~ 85 μg/mL). However, modified Provasoli medium obtained the maximum final biomass, 4.08 times higher than that obtained for the microalgae grown in the KM1 medium. The cultures with the modified Provasoli medium had the highest productivity of astaxanthin (9.28 ± 0.4 mg/L/day) than those cultivated with the KM1 medium (3.37 ± 0.2 mg/L/day), presenting the lowest values recorded for astaxanthin productivity, differing statistically from the others (Table 3).
The protein content of the microalgae was influenced both by the culture medium used and by the day of culture, and there were also significant differences in the interaction between the two factors (Table 4). A reduction in the amount of protein in the biomass varied from 25 to 61% between the media. The KM1 medium presented the lowest values of proteins from the first to the last day of culture with a higher percentage of reduction in the protein content.
Table 5 shows the mean values obtained for each culture medium studied for the concentration of FAMEs of H. pluvialis biomass, which was influenced both by the culture medium and the duration of cultivation, with significant differences between the factors. The most significant fatty acids identified were palmitic acid (C16:0) with 16.5–25.3%, oleic acid (C18:1) with 20–29.8%, and linoleic acid (C18:2) with 8.28–24.2%, showing the highest concentrations in relation to the last day of culture for all media studied.
The principal component analysis (PCA) (Fig. 5) reports the distribution of these variables according to the culture media (Fig.5a) and the first and last day (Fig. 5b), in which ellipses delimit the 95% confidence perimeter.
When evaluating the different culture media (Fig. 5a), it can be detected that the principal component analysis has a cumulative effect of 74.30% of the variables. In the first component, it had an effect of 52.5% provided by the correlations of the variables number of cells, C16:0, C18:1, C18:2, astaxanthin, chlorophyll a and b, and proteins, while in the second component, it had an effect of only 21.8% provided by the correlations of the variables C12:0, C14:0, C18:0, and biomass.
By evaluating first and last day of cultivation (Fig. 5b), it can be detected that the principal component analysis has a cumulative effect of 75.68% of the variables, where for the first component had a 56.94% effect provided by the correlations of the nitrate variables, C16:0, C18:1, C18:2, unidentified fatty acids, astaxanthin, chlorophyll a and b, number of cells, and proteins, while in the second component, it had an effect of only 18.74% provided by the correlations of the variables C12:0, C14:0, C18:0, and biomass.
Thus, the highest values of nitrate, unidentified fatty acids, proteins, chlorophylls a and b, and C14:0 were found on the first day and mainly for BBM, Provasoli, and modified Provasoli media. In contrast, the highest values of C18:1, C16:0, astaxanthin, dry biomass, and the number of cells were detected on the last day of cultivation, mainly for KM1, MM2, and Provasoli modified to C18:1, C16:0, and astaxanthin. In the graph, it is possible to observe that the values of astaxanthin are inversely correlated with the values of proteins (r = − 0.75), chlorophyll a (− 0.70), nitrate (− 0.72), and chlorophyll b (− 0.67), as well as fatty acids not identified with the values of C16:0 (− 0.92), C18:2 (− 0.91), and the number of cells (− 0.62). The variables nitrate, protein, and chlorophylls (a and b) are highly directly correlated, with values of r > 0.86, similarly to variables C18:2 and C16:0 (r = 0.88).
This indicates that increased astaxanthin production in H. pluvialis cells, with higher indices on the last day of cultivation, leads to a decrease in nitrate concentrations of water, proteins, and chlorophyll in cells.
Discussion
The life cycle of the microalgae is determined by the culture conditions to which they are submitted, mainly the nutritional factors, such as varied organic and inorganic nutrients, the addition of sodium acetate, and the nitrogen and phosphorus restriction (Borowitzka et al. 1991; Aflalo et al. 2007; Tocquin et al. 2012; Butler et al. 2017; Pan-utai et al. 2017; Wang et al. 2017; Cheng et al. 2018). In the present study, cells in the zoospores (green vegetative) phase were observed mainly in photoautotrophic conditions, BBM, and Provasoli media and at the beginning of the culture in modified Provasoli. On the other hand, astaxanthin-inducing cells (palmela, intermediate cells, and aplanospores) were more observed in the KM1 medium. Inorganic nitrogen and phosphorus are key ingredients for H. pluvialis flagellate propagation cells (Borowitzka et al. 1991). H. pluvialis zoospores were found in nutrient-rich crops, mainly nitrate and phosphate by Aflalo et al. (Aflalo et al. 2007), Tocquin et al. (Tocquin et al. 2012), and Wang et al. (Wang et al. 2017) as well as in this study. The change in cell morphology from the vegetative stage to the cysts after the addition of acetate to the culture medium was obtained by Kobayashi et al. (Kobayashi et al. 1991).
The results showed that the microalgae H. pluvialis had better growth in the media BBM, Provasoli, and modified Provasoli than in media KM1 and MM2. Nitrogen and phosphorus are important nutrients for microalgae cultivation, and in some culture media, the availability of these and/or their sources may become a limiting factor, as observed for KM1 and MM2 media which, even with organic nitrogen sources did not promote better growth to microalgae compared to the modified Provasoli. The right proportion of N:P is essential for active cell division because both nitrogen and phosphorus are essential for the synthesis of cellular structures, cell growth, and metabolism (Tocquin et al. 2012). Nitrogen is one of the essential elements for the growth of microalgae and a fundamental component of cell structural substances, such as proteins, amino acids, genetic material (DNA, RNA), and photosynthetic pigments such as chlorophylls (Berman-Frank et al. 2001). Thus, nitrogen is extremely important because it is a component of several substances in the primary metabolism of microalgae. The nitrate salts (NaNO3; KNO3) and the ammonium salts (NH4Cl; (NH4)2SO4) are the main sources of inorganic nitrogen used in the culture medium. Organic sources, such as urea, amino acids, yeast extract, and L-asparagine, can also be applied but with less relevance to microalgae than inorganic forms of nitrogen (Chiranjeevi and Mohan 2016). Like nitrogen, phosphorus is considered a major limiting factor for microalgae because it regulates growth and metabolism in all processes involving energy exchanges and forms the structural molecules of cells, such as ATP synthesis, phosphate sugars, nucleic acids, and the Calvin cycle. In response to phosphorus limitation, microalgae are not capable of synthesizing the ATP necessary for several cellular processes, including chlorophyll biosynthesis. In cultures, phosphorus can be added to culture media such as NaH2PO4H2O, K2HPO4, and sodium glycerophosphate (Roopnarain et al. 2014).
Some studies highlight the importance of nitrogen and phosphorus sources on the growth of microalgae. Concentrations of 0 to 0.4 g/L of potassium nitrate (KNO3) were tested in Chlorella pyrenoidosa, and the worst growth results were observed when the concentration was less than 0.05 g/L of KNO3 (Nigam et al. 2011). Scenedesmus sp. cultivated with NaNO3 (0 to 247 mg/L) reached the highest growth rates and biomass productivity in the concentrations from 30.8 mg/L and still observed that the low concentrations of nitrate significantly decreased the photosynthetic activity and affected the cellular morphology of the microalga (Pancha et al. 2014). Different sources of nitrogen (sodium nitrate, ammonium chloride, and urea) on the growth in Haematococcus demonstrated the best results with sodium nitrate (Mazumdar et al. 2019). The present data corroborate with those verified by Sarada et al. (Sarada et al. 2002), where the ideal nitrogen source for the growth of H. pluvialis is sodium nitrate. Even the MM2 and KM1 media, which have organic nitrogen sources, had the lowest growth parameters compared to BBM, Provasoli, and modified Provasoli, which had sodium nitrate as their main source of nitrogen. Orosa et al. (Orosa et al. 2005) demonstrated that different concentrations of sodium nitrate do not have significant interference in the growth of microalgae H. pluvialis; however, it is essential since in the absence of it there was none. Nahidian et al. (Nahidian et al. 2018) investigated H. pluvialis growth in BBM medium with three times higher phosphorus concentrations resulted in a 32% increase in specific growth rate. Tocquin et al. (Tocquin et al. 2012) observed a drastic decrease in cell densities due to cell death in H. pluvialis cultivation in the absence of phosphorus, similarly to our results, in which the KM1 medium had the lowest growth speed, the highest doubling time and the lowest MCD, and dry biomass.
Higher cell densities (84.50 × 104 cells/mL) and biomass production were observed under mixotrophic conditions in modified Provasoli medium, which besides sodium glycerophosphate, sodium nitrate, and L-asparagine has an addition of yeast extract and organic carbon (sodium acetate). Cultured microalgae exhibit higher MCD in culture media having the same conditions. Cheng et al. (Cheng et al. 2018) achieved the highest biomass and higher MCD in mixotrophic media with acetate and yeast extract (5.8 × 106 cells/mL) than in the photoautotrophic medium BG11 (4.3 × 104 cells/mL). Butler et al. (Butler et al. 2017) cultivated H. pluvialis in EG/JM medium containing sodium acetate and yeast extract which resulted in the highest MCD (3.64 × 105 cells/mL). Pang et al. (Pang et al. 2019b) achieved better growth results of H. pluvialis in media under mixotrophic conditions compared to media in photoautotrophic and heterotrophic conditions. However, regarding the carbon sources tested (ribose and acetate), they observed that ribose exerted the best algal growth (1.325 × 106 cells/mL). Under mixotrophic conditions, microalgae may grow so photoautotrophic and heterotrophic concomitantly and may use inorganic (CO2) and organic carbon sources (acetate, ribose, glucose). CO2 fixation is driven by photosynthesis through light capture, while simultaneously organic compounds are assimilated through aerobic respiration, which is influenced by the availability of organic carbon exogenous and oxygen (Pang et al. 2019a). When microalgae are subjected to mixotrophic conditions, growth rates are higher because of the chemical energy of organic carbon prolonged exponential growth phase, resulting in higher biomass. In addition, mixotrophic growth ignores the limitations of photosynthesis, as self-shading in high-density crops; decreases photoinhibition; and converts the CO2 produced by carbon respiration and O2 produced by photosynthesis for the biosynthesis of cellular components (Pang et al. 2019b; Zhang et al. 2019).
The temperature remained within the recommended range (20 to 27 °C) for the growth of H. pluvialis (Giannelli et al. 2015). Depending on the type of nitrogen sources used in the culture media, these systematically affect the pH in different ways (ammonia-based media decreases the pH and nitrate-based media increases the pH), especially in algae cultivation where no source additional carbon is provided during the growing days (Nguyen and Rittmann 2015; Wang and Curtis 2016). The assimilation of nitrate involves a reduction in nitrite and, ultimately, in ammonia, thus capturing approximately one proton per NO3− consumed, resulting in the alkalinization of the medium (Wang and Curtis 2016). An increase in pH is also observed when sodium acetate or potassium acetate is provided as a carbon source (Perez-Garcia et al. 2011), as was observed for the KM1 and MM2 media, which had low nitrate concentrations but had sodium acetate as a carbon source. This happens with the remaining Na+ or K+ couples with hydroxyl ions (OH−) or other anions to form alkalis (Perez-Garcia et al. 2011). This may have led to increased pH in the cultures, reaching 9 at the end of cultivation. The ideal pH for the growth of H. pluvialis is in the range of 7–8; above these values, cell growth and biomass production can be significantly altered (Cheng et al. 2016). In this study, nitrate concentration decreased, considering that this is one of the nitrogen sources preferable to the species (Göksan et al. 2011). When the nitrate concentrations reached very low values, the microalgae reached their stationary phase and began to accumulate more astaxanthin, reducing the number of chlorophylls, as observed by Pan-utai et al. (Pan-utai et al. 2017).
Regarding the cellular protein content, the BBM medium presented the best result, with more than 60% at the end of the culture. This result is commonly observed in chlorophytes grown with high concentrations of nitrogen (Lourenço et al. 2004). According to these authors, the nitrogen source does not affect the protein content, but on the amount of this nutrient, which is why the higher protein values (> 80%) found on the first day of cultivation can be explained and the higher mean values for BBM medium which remained until the last day of cultivation with the highest concentrations of nitrate.
The astaxanthin/chlorophyll ratio increased with culture time and was higher in sodium acetate containing mixotrophic media. The highest carotenoid concentrations were obtained in KM1 medium on the sixth day of cultivation (85 ± 6.1 μg/mL) and a ninth day for modified Provasoli medium (85 ± 6.1 μg/mL). However, in relation to astaxanthin productivity, since the highest cell densities and biomass production were observed for modified Provasoli (for reasons already explained above), the microalgae grown with this medium presented the highest astaxanthin productivity (9.28 ± 0.4 mg/L/day). It has been observed that under conditions of astaxanthin accumulation in cells, chlorophyll biosynthesis is decreased (Kim et al. 2013; Zhang et al. 2019). Previous studies on the cultivation of H. pluvialis have shown that acetate is an important source of carbon by enhancing astaxanthin synthesis (Borowitzka et al. 1991; Tripathi et al. 1999; Butler et al. 2017; Pan-utai et al. 2017; Zhang et al. 2019). Acetylation assimilation begins by acetylating coenzyme A through acetyl-CoA synthetase, forming coenzyme A (acetyl-CoA) in a one-step catalyst reaction using an ATP molecule (Boyle and Morgan 2009). With the addition of acetate in the cultures, the glyoxylate metabolic flow rate in microalgae cells increases, along with the TCA cycle for mitochondrial citrate, providing carbon and NADPH skeletons, indispensable for astaxanthin biosynthesis (Boyle and Morgan 2009; Perez-Garcia et al. 2011). With the formation of coenzyme (acetyl-CoA), it is also possible to observe an improvement in fatty acid synthesis. The most expressive fatty acids were oleic (C18:1), palmitic (C16:0), and linoleic (C18:2) acids. Similarly and corroborating with the present study, Zhekisheva et al. (Zhekisheva et al. 2002) also observed a linear correlation between the accumulation of oleic acid and astaxanthin.
Conclusion
This work brings as perspective the possibility of optimizing the production of biomolecules of interest by promoting changes in the culture medium from H. pluvialis. The evaluated parameters for growth, life cycle, dry biomass, chlorophylls, astaxanthin, proteins, and methyl esters of fatty acids were directly influenced by the culture media. The microalga H. pluvialis is highly capable of adapting to different cultivation conditions and nutrients present in the culture media, by stimulating its metabolic pathways, in which it is possible to modify the biochemical composition of its biomass according to the applicability of a specific biocompounds of interest. The BBM medium produced more chlorophylls and proteins. The modified Provasoli medium, under mixotrophic conditions, was the most suitable for growth, dry biomass production, methyl ester of fatty acids, and productivity of the carotenoid astaxanthin in H. pluvialis.
References
Aflalo C, Meshulam Y, Zarka A, Boussiba S (2007) On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol Bioeng 98:300–305. https://doi.org/10.1002/bit.21391
An Z, Yang H, Liu X, Zhangv Y (2020) Effects of astaxanthin on the immune response and reproduction of Procambarus clarkii stressed with microcystin-leucine-arginine. Fish Sci 1:1–8. https://doi.org/10.1007/s12562-020-01434-0
Angell A, de Nys R, Mangott A, Vucko MJ (2018) The effects of concentration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon. Algal Res 35:577–585. https://doi.org/10.1016/j.algal.2018.09.031
APHA/AWWA/WEF (2012) Standard Methods for the Examination of Water and Wastewater. Stand Methods 541. doi: ISBN 9780875532356
Barbarino E, Lourenço SO (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:447–460. https://doi.org/10.1007/s10811-005-1641-4
Barbosa MJ, Morais R, Choubert G (1999) Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 176:331–341. https://doi.org/10.1016/S0044-8486(99)00115-5
Berman-Frank I, Lundgren P, Chen YB et al (2001) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science (80- ) 294:1534–1537. https://doi.org/10.1126/science.1064082
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Bold HC (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull Torrey Bot Club 76:101–108
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304. https://doi.org/10.1007/BF02392882
Boyle NR, Morgan JA (2009) Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Syst Biol 3:1–14. https://doi.org/10.1186/1752-0509-3-4
Butler T, McDougall G, Campbell R, Stanley M, Day J (2017) Media screening for obtaining Haematococcus pluvialis red motile macrozooids rich in astaxanthin and fatty acids. Biology (Basel) 7:2. https://doi.org/10.3390/biology7010002
Cheng J, Li K, Yang Z, Zhou J, Cen K (2016) Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour Technol 204:49–54. https://doi.org/10.1016/j.biortech.2015.12.076
Cheng T, Xu X, Zhang W, Chen L, Liu T (2018) Protoplast preparation from enriched flagellates and resting cells of Haematococcus pluvialis. J Appl Microbiol 124:469–479. https://doi.org/10.1111/jam.13643
Chioccioli M, Hankamer B, Ross IL (2014) Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weight in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS One 9:1–12. https://doi.org/10.1371/journal.pone.0097269
Chiranjeevi P, Mohan SV (2016) Critical parametric influence on microalgae cultivation towards maximizing biomass growth with simultaneous lipid productivity. Renew Energy 98:64–71. https://doi.org/10.1016/j.renene.2016.03.063
Cui D, Hu C, Zou Z, Sun X, Shi J, Xu N (2020) Comparative transcriptome analysis unveils mechanisms underlying the promoting effect of potassium iodide on astaxanthin accumulation in Haematococcus pluvialis under high light stress. Aquaculture 525:735279. https://doi.org/10.1016/j.aquaculture.2020.735279
Cunha L, Besen K, Ha N et al (2020) Biofloc technology (BFT) improves skin pigmentation of goldfish (Carassius auratus). Aquaculture 522:735132. https://doi.org/10.1016/j.aquaculture.2020.735132
Dong S, Huang Y, Zhang R, Wang S, Liu Y (2014) Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. ScientificWorldJournal 2014:694305–694307. https://doi.org/10.1155/2014/694305
Doria E, Temporiti MEE, Damiani MC et al (2018) Influence of light stress on the accumulation of xanthophylls and lipids in Haematococcus pluvialis CCALA 1081 grown under autotrophic or mixotrophic conditions. J Mar Biol Aquac 4:36–40. https://doi.org/10.15436/2381-0750.18.1799
Faraone I, Sinisgalli C, Ostuni A, Armentano MF, Carmosino M, Milella L, Russo D, Labanca F, Khan H (2020) Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: a systematic review. Pharmacol Res 155:104689. https://doi.org/10.1016/j.phrs.2020.104689
Giannelli L, Yamada H, Katsuda T, Yamaji H (2015) Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J Biosci Bioeng 119:345–350. https://doi.org/10.1016/j.jbiosc.2014.09.002
Göksan T, Ak I, Kılıç C (2011) Growth characteristics of the alga Haematococcus pluvialis flotow as affected by nitrogen source, vitamin, light and aeration. Turkish J Fish Aquat Sci 11:377–383. https://doi.org/10.4194/1303-2712-v11
Gouveia L, Rema P, Pereira O, Empis J (2003) Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquac Nutr 9:123–129. https://doi.org/10.1046/j.1365-2095.2003.00233.x
Humphrey GF (1979) Photosynthetic characteristics of algae grown under constant illumination and light-dark regimes. J Exp Mar Bio Ecol 40:63–70. https://doi.org/10.1016/0022-0981(79)90034-0
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem und Physiol der Pflanz 167:191–194. https://doi.org/10.1016/S0015-3796(17)30778-3
Jiang X, Yan Q, Liu F, Jing C, Ding L, Zhang L, Pang C (2018) Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci Lett 662:36–43. https://doi.org/10.1016/j.neulet.2017.09.064
Jiang J, Nuez-Ortin W, Angell A, Zeng C, de Nys R, Vucko MJ (2019) Enhancing the colouration of the marine ornamental fish Pseudochromis fridmani using natural and synthetic sources of astaxanthin. Algal Res 42:101596. https://doi.org/10.1016/j.algal.2019.101596
Katiyar R, Gurjar BR, Biswas S, Pruthi V, Kumar N, Kumar P (2017) Microalgae: an emerging source of energy based bio-products and a solution for environmental issues. Renew Sust Energ Rev 72:1083–1093. https://doi.org/10.1016/j.rser.2016.10.028
Kim S, Park JE, Cho YB, Hwang SJ (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13. https://doi.org/10.1016/j.biortech.2013.06.068
Kobayashi M, Kakizono T, Nagai S (1991) Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J Ferment Bioeng 71:335–339. https://doi.org/10.1016/0922-338X(91)90346-I
Li Y, Sommerfeld M, Chen F, Hu Q (2010) Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J Appl Phycol 22(3):253–263
Lourenço SO, Barbarino E, Lavín PL, Lanfer Marquez UM, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur J Phycol 39:17–32. https://doi.org/10.1080/0967026032000157156
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0304-3894(92)87011-4
Mandotra SK, Kumar P, Suseela MR, Nayaka S, Ramteke PW (2016) Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour Technol 201:222–229. https://doi.org/10.1016/j.biortech.2015.11.042
Mazumdar N, Novis PM, Visnovsky G, Gostomski PA (2019) Effect of nutrients on the growth of a new alpine strain of Haematococcus (Chlorophyceae) from New Zealand. Phycol Res 67:21–27. https://doi.org/10.1111/pre.12344
Mustafa Y, Fagiri A, Salleh A, El-nagerabi SAF (2013) Influence of chemical and environmental factors on the growth performance of Spirulina platensis strain SZ100. J Algal Biomass Util 4:7–15
Nahidian B, Ghanati F, Shahbazi M, Soltani N (2018) Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU. Bioresour Technol 255:229–237. https://doi.org/10.1016/j.biortech.2018.01.130
Nguyen BT, Rittmann BE (2015) Predicting dissolved inorganic carbon in photoautotrophic microalgae culture via the nitrogen source. Environ Sci Technol 49:9826–9831. https://doi.org/10.1021/acs.est.5b01727
Nigam S, Rai MP, Sharma R (2011) Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am J Biochem Biotechnol 7:126–131. https://doi.org/10.3844/ajbbsp.2011.126.131
Orosa M, Franqueira D, Cid A, Abalde J (2005) Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour Technol 96:373–378. https://doi.org/10.1016/j.biortech.2004.04.006
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154. https://doi.org/10.1016/j.biortech.2014.01.025
Pang N, Chen S (2017a) Effects of C5 organic carbon and light on growth and cell activity of Haematococcus pluvialis under mixotrophic conditions. Algal Res 21:227–235. https://doi.org/10.1016/j.algal.2016.12.003
Pang N, Chen S (2017b) Effects of C5 organic carbon and light on growth and cell activity of Haematococcus pluvialis under mixotrophic conditions. Algal Res 21:227–235. https://doi.org/10.1016/j.algal.2016.12.003
Pang N, Gu X, Chen S, Kirchhoff H, Lei H, Roje S (2019a) Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew Sust Energ Rev 112:450–460. https://doi.org/10.1016/j.rser.2019.06.001
Pang N, Xie Y, Oung HMO, Sonawane BV, Fu X, Kirchhoff H, Cousins AB, Chen S (2019b) Regulation and stimulation of photosynthesis of mixotrophically cultured Haematococcus pluvialis by ribose. Algal Res 39:101443. https://doi.org/10.1016/j.algal.2019.101443
Pan-utai W, Parakulsuksatid P, Phomkaivon N (2017) Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: organic and inorganic. Biocatal Agric Biotechnol 12:152–158. https://doi.org/10.1016/j.bcab.2017.10.004
Perez-Garcia O, Escalante FME, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Pindyck RS, Rubinfeld DL (1990) Econometric models and economic forecasts, 3rd edn. Mcgraw-Hill College, New York
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe H, Hattori A (eds) Cultures and collections of algae, proceeding. Hakone, Japanese Society of Plant Physiology, pp 63–75
RCoreTeam (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Roopnarain A, Gray VM, Sym SD (2014) Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour Technol 156:408–411. https://doi.org/10.1016/j.biortech.2014.01.092
Sarada R, Bhattacharya S, Ravishankar GA (2002) Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J Microbiol Biotechnol 18:517–521. https://doi.org/10.1023/A:1016349828310
Shah MMR, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci 7:1–28. https://doi.org/10.3389/fpls.2016.00531
Shen XF, Liu JJ, Chauhan AS, Hu H, Ma LL, Lam PKS, Zeng RJ (2016) Combining nitrogen starvation with sufficient phosphorus supply for enhanced biodiesel productivity of Chlorella vulgaris fed on acetate. Algal Res 17:261–267. https://doi.org/10.1016/j.algal.2016.05.018
Stein JR (1973) Handbook of phycological methods: culture methods and growth measurements, 1rd edn. Cambridge University, Cambridge
Su F, Yu W, Liu J (2020) Comparison of effect of dietary supplementation with Haematococcus pluvialis powder and synthetic astaxanthin on carotenoid composition, concentration, esterification degree and astaxanthin isomers in ovaries, hepatopancreas, carapace, epithelium of adult. Aquaculture 523:735146. https://doi.org/10.1016/j.aquaculture.2020.735146
Tocquin P, Fratamico A, Franck F (2012) Screening for a low-cost Haematococcus pluvialis medium reveals an unexpected impact of a low N/P ratio on vegetative growth. J Appl Phycol 24:365–373. https://doi.org/10.1007/s10811-011-9771-3
Tripathi U, Sarada R, Ramachandra Rao S, Ravishankar GA (1999) Production of astaxanthin in Haematococcus pluvialis cultured in various media. Bioresour Technol 68:197–199. https://doi.org/10.1016/S0960-8524(98)00143-6
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11. https://doi.org/10.1016/j.biortech.2012.11.032
Wan M, Zhang Z, Wang J, Huang J, Fan J, Yu A, Wang W, Li Y (2015) Sequential heterotrophy-dilution-photoinduction cultivation of Haematococcus pluvialis for efficient production of astaxanthin. Bioresour Technol 198:557–563. https://doi.org/10.1016/j.biortech.2015.09.031
Wang J, Curtis WR (2016) Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: toward metabolic pH control. J Appl Phycol 28:43–52. https://doi.org/10.1007/s10811-015-0551-3
Wang N, Guan B, Kong Q, Duan L (2017) A semi-continuous cultivation method for Haematococcus pluvialis from non-motile cells to motile cells. J Appl Phycol 30:773–781. https://doi.org/10.1007/s10811-017-1337-6
Wang Y, Wang B, Liu M, Jiang K, Wang M, Wang L (2020) Comparative transcriptome analysis reveals the potential influencing mechanism of dietary astaxanthin on growth and metabolism in Litopenaeus vannamei. Aquac Reports 16:100259. https://doi.org/10.1016/j.aqrep.2019.100259
Zhang C, Liu J, Zhang L (2017) Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin J Oceanol Limnol 35:1205–1211. https://doi.org/10.1007/s00343-017-6103-8
Zhang C, Zhang L, Liu J (2019) Exogenous sodium acetate enhances astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. J Appl Phycol 31:1001–1008. https://doi.org/10.1007/s10811-018-1622-z
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331. https://doi.org/10.1046/j.1529-8817.2002.01107.x
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194. https://doi.org/10.1023/A:1007914806640
Acknowledgements
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support provided to this the research and partners involved in its execution and to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship granted to the first author. We would also like to thank the Labpratório de Bioprocessos and Central Análitica do Centro de Tecnologias Estratégicas do Nordeste (CETENE).
Code availability
Not applicable.
Funding
The financial support granted to this research and the scholarships for the researchers involved were made by the following institutions: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yllana Ferreira Marinho, Carolina Barbosa Malafaia, Katarynna Santos de Araújo, Túlio Diego da Silva, Ana Paula Felipe dos Santos, Laenne Barbara de Moraes, and Alfredo Oliveira Gálvez. The first draft of the manuscript was written by Yllana Ferreira Marinho, and all authors commented on the previous versions of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript and are in agreement with the submission and publication of this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marinho, Y.F., Malafaia, C.B., de Araújo, K.S. et al. Evaluation of the influence of different culture media on growth, life cycle, biochemical composition, and astaxanthin production in Haematococcus pluvialis. Aquacult Int 29, 757–778 (2021). https://doi.org/10.1007/s10499-021-00655-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00655-z