Abstract
The aim of this study was to evaluate the effects of the antioxidant astaxanthin on the immune response, growth, and reproduction of the crayfish Procambarus clarkii (Girard, 1852) stressed with the toxin microcystin-leucine-arginine (MC-LR). P. clarkii exposed to MC-LR were treated with one of four concentrations of dietary astaxanthin supplement (0, 2.5, 5, or 10 mg/g) for 36 consecutive days in tanks. In crayfish exposed to 0.05 mg/l MC-LR, activity levels of superoxide dismutase and alkaline phosphatase in the hepatopancreas significantly increased (P < 0.05) following dietary supplementation with 10 mg/g astaxanthin. The survival and fecundity of P. clarkii decreased significantly with exposure to 0.01 mg/l MC-LR, but astaxanthin supplementation significantly improved survival (P < 0.05). The average daily oxygen consumption rate of P. clarkii stressed with 0.01 mg/l MC-LR was significantly higher than that of unstressed P. clarkii (P < 0.05); astaxanthin supplementation did not significantly affect the average daily oxygen consumption rate of MC-LR-stressed P. clarkii. In conclusion, the antioxidant astaxanthin was found to be a suitable dietary supplement for P. clarkii as it improved immune activity and alleviated MC-LR-induced oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Procambarus clarkii (Girard, 1852), the most widespread and aggressive crayfish worldwide (Gherardi and Holdich 1999), is highly tolerant of extreme environments and commonly survives cyanobacterial blooms (Gherardi and Lazzara 2006). Indeed, Vasconcelos et al. (2001) showed that P. clarkii could feed on microalgae and accumulate algal toxins within its own organs. Field investigations indicate that water contaminated with cyanobacteria often contains relatively high levels of microcystins (MCs) (Carmichael 1996). Although P. clarkii feeds normally when exposed to MCs, its growth is slowed and its fecundity declines, thus the mechanisms causing these effects are of scientific interest.
MCs are generally considered to disrupt the redox balance (Cazenave et al. 2006), and exposure to them can cause toxicity by the inhibition of phosphatase, causing oxidative stress, and by a change in mitochondrial membrane permeability (Prieto et al. 2007). MCs stimulate the production of intracellular reactive oxygen species (ROS), which leads to cell damage and lipid peroxidation and possibly induces apoptosis via various pathways (Guzman and Solter 1999; Zhang et al. 2008). Smith and Haney (2006) showed that MC levels gradually decrease in the organs and tissues of fish and other aquatic organisms over time. In another study, the most common MC, leucine-arginine (MC-LR) was found to accumulate primarily in the hepatopancreas (Huang et al. 2003) and secondarily in the ovaries (An et al. 2015) of aquatic organisms, and caused lipid peroxidation in both. Thus, the accumulation of MC-LR may explain the slow growth and low fecundity of P. clarkii in waters contaminated by cyanobacterial blooms.
Astaxanthin, one of the most potent antioxidants known, effectively eliminates oxygen free radicals from cells (Pei and Dong 2007). Astaxanthin is known to affect coloration, increase growth, and enhance immunity in juvenile kuruma shrimp, Marsupenaeus japonicus (Wang et al. 2018). In this study, we evaluated the effects of dietary supplementation with astaxanthin on the growth and development of P. clarkii under MC-LR stress, and then investigated the mechanisms by which astaxanthin alleviated the effects of this stress. This paper provides a theoretical basis and technical information for the safe and efficient culture of P. clarkii.
Materials and methods
Experimental materials
The P. clarkii used in this study originated from Baoying Lake (Baoying County, YangZhou City) and were purchased in Yangzhou City, Jiangsu Province, China. Healthy, active crayfish (with all limbs intact) of roughly the same size were selected and temporarily cultured at 15 ± 3 °C under natural light for 1 month. The biochemical composition and nutritional values of the commercial feed purchased from Jiangsu Fuyuda Food Products (Yangzhou City, Jiangsu Province, China) are shown in Table 1. MC-LR, purchased from Beijing Express Technology (Beijing), was dissolved in purified water, refrigerated, and directly added to the crayfish cultures in amounts proportional to tank size (800 mm × 600 mm × 400 mm) to give the appropriate experimental concentrations. Astaxanthin, purchased from Fujian Corona Technology (Fuzhou City, Fujian Province, China) was stored in 500-mg capsules (capacity 34.5 kJ/g), each of which contained 60 mg astaxanthin with 440 mg soybean oil as the adjuvant. During application, the astaxanthin capsules were punctured and their contents mixed well with the P. clarkii commercial feed. The difference in calorific value between the astaxanthin-supplemented and non-supplemented feeds was eliminated by the addition of a calorifically equivalent amount of soybean oil.
Experimental methods
Measurement of indices of immunity in P. clarkii
The feed of P. clarkii exposed to MC-LR was supplemented with one of four concentrations of astaxanthin (0, 2.5, 5, or 10 mg/g). The total number of P. clarkii (average body weight 27.6 ± 5.9 g) in this experiment was 144. Each treatment was in triplicate, and each of the replicates comprised 12 P. clarkii of the same size (1:1 male:female ratio, with no significant difference in body weight between the sexes). P. clarkii were cultured in aquaria (800 mm × 600 mm × 400 mm) at room temperature for 36 consecutive days. Stress was induced using 0.05 mg/l MC-LR. An additional identical aquarium was established simultaneously as a blank control in which the P. clarkii were not exposed to MC-LR stress. The immune indices of the crayfish were measured three times throughout the experiment, on days 12, 24, and 36. On each of these days, three P. clarkii were randomly selected from each aquarium [on day 36, two aquaria had less than three crayfish left (in one aquarium under 0 mg/g astaxanthin only one crayfish remained, and in an aquarium under 2.5 mg/g astaxanthin only two crayfish remained), so the survivors were combined with those of another replicate of the same test group]. For each aquarium, the hepatopancreas of each crayfish was dissected out and the hepatopancreases homogenized in bulk in 6 ml of distilled water in a grinder. Grinders were numbered according to aquaria. The homogenized samples were kept on ice, and an 1.8-ml aliquot of each homogenized sample was centrifuged at 12,000 g for 15 min in an Eppendorf 5415R Refrigerated Centrifuge to collect the supernatants. For each sample, alkaline phosphatase (AKP) and superoxide dismutase (SOD) levels were measured using a microenzyme labeling method and the WST-1 assay, respectively, using assay kits from Nanjing Jiancheng Bio-Engineering Institute (Jiangsu Province, China). The protein concentrations were measured using a bicinchoninic acid assay, and absorbance was determined using an IMark 168–1130 Microplate Reader. The activities of AKP and SOD were calculated as follows:
Oxygen consumption rate, growth and accumulation experiments
To investigate rates of oxygen consumption and growth, 160 healthy P. clarkii were selected and divided into five groups. Each group consisted of four replicates, and each replicate comprised eight adult crayfish. Before experimentation, each P. clarkii was weighed on an electronic scale. The average initial weight of the 160 P. clarkii was 20.61 ± 4.92 g, with no significant differences in body weights among the five groups. The concentrations of the dietary astaxanthin supplement were 0, 2.5, 5, and 10 mg/g, and the MC-LR concentration during the stress experiment was 0.01 mg/l. An additional identical aquarium, in which the P. clarkii were not treated with MC-LR and were not fed astaxanthin, was established simultaneously as a blank control. After 36 consecutive days of the experiment, the survival rate, body weight, and fecundity of P. clarkii were determined.
The oxygen consumption rates of crayfish (untreated, treated with MC-LR alone and treated with MC-LR plus astaxanthin) were measured three times a day, at 0600, 1400, and 2200 hours. The oxygen consumption rate was determined using an improved static method in which the individuals of each group were submerged in a storage box sealed with plastic wrap. The dissolved oxygen in the water of the box was measured using an Oxi330i Dissolved Oxygen Meter (WTW Laboratories, Germany), and the average value was used to estimate the average daily oxygen consumption rate. After each measurement, aeration was used to ensure that the dissolved oxygen content was maintained at a concentration above 3.0 mg/l. Dissolved oxygen was also measured in a water box without crayfish at the same time points as the background readings to eliminate confounding effects, such as those due to the respiration of microorganisms in the water.
The oxygen consumption rate (R) of each P. clarkii was calculated as R = (Rt − R0) × V/W × T, where R0 and Rt are the background oxygen consumption of the blank control and the oxygen consumption of the P. clarkii in the experimental aquarium (mg O2/l), respectively. V is the water volume (liters), W is the body weight of the P. clarkii individual (grams), and T is the duration of the assay (0.5 h).
Fecundity was determined by counting the number of eggs in the ovaries of a female P. clarkii (revealed by cutting the breastplate). The specific growth rate (SGR) was calculated as (lnY2 − lnY1)/t × 100%, where Y1 and Y2 represent the body weights of the crayfish at the beginning and end of the experiment, respectively, and t (days) is the growth period. The survival rate was calculated as the number of crayfish alive at the end of the experiment.
At the end of the experiment, 1.5 g of ovarian tissue was collected from each female P. clarkii and the tissue from three females within one group was pooled as one sample. Thus, each treatment was represented by three pooled samples. The MC-LR concentrations in crayfish ovaries were measured at the end of the experiment using a commercial ELISA kit (J&Q Environment, Beijing), with a detection sensitivity of 0.1 ng/ml. To ensure that the toxin had been fully released and not oxidized by exposure to air, the freshly dissected ovarian tissue samples were quickly weighed and completely homogenized. The homogenates were centrifuged at 15,000 g for 25 min in a 5415R Refrigerated Centrifuge (Eppendorf China). An IMark 168–1130 micro plate reader (Bio-Rad, USA) was used to detect the absorbance.
Management of daily feeding
The biochemical composition and calorific value of the formulated feed were determined before the experiment. During the two experiments, P. clarkii were fed the prepared feed twice daily, at 0800 and 1800 hours. The water temperature of the aquaria holding the P. clarkii was maintained at 15 ± 3 °C. One-third of the water volume in each aquarium was changed daily, and the dissolved oxygen content was maintained at above 3.0 mg/l. To maintain a constant concentration of MC-LR, the concentrations in different aquaria were adjusted while changing the water. Daily food intake and molting were recorded for all P. clarkii. Dead crayfish were recorded and removed quickly.
Statistical analysis
The data were analyzed by SPSS for Windows (version 19.0; SPSS, Chicago, IL). Inter-treatment differences of the data were analyzed with one-way ANOVA followed by post hoc Tukey multiple range tests. Differences were considered significant when P < 0.05.
Results
Effects of astaxanthin on the immunity of P. clarkii under MC-LR stress
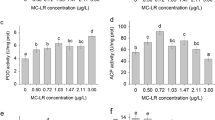
MC-LR stress significantly increased AKP activity in the P. clarkii hepatopancreas (Fig. 2), but had no significant effect on SOD activity (Fig. 1). After 12 days of MC-LR stress, the SOD activity increased with increasing concentrations of the astaxanthin dietary supplement; however, the increase was only significant at 10 mg/g astaxanthin (P < 0.05; Fig. 1). By day 24, the SOD activity of the 5 mg/g astaxanthin supplemented group was significantly higher than that of the 2.5 mg/g astaxanthin supplemented group and the control group. Although stress due to MC-LR had rapidly and significantly affected AKP activity in P. clarkii by day 12 (P < 0.05; Fig. 2), the effects of the astaxanthin dietary supplement on AKP activity were relatively slow, with no significant effect of astaxanthin at any concentration by day 12 (Fig. 2). However, by day 24, AKP activity was significantly elevated at 10 mg/g astaxanthin (P < 0.05). On day 36, the AKP activity level was significantly (P < 0.05) elevated at 2.5 and 10 mg/g compared with 0 mg/g astaxanthin.
Effects of astaxanthin supplementation on superoxide dismutase (SOD) activity (U/mg protein) in the hepatopancreas of Procambarus clarkii under microcystin-leucine-arginine (MC-LR) stress (0.05 mg/l) after 12, 24, and 36 days. Four concentrations of astaxanthin were examined: 0, 2.5, 5, and 10 mg/g. For each time point, bars with different letters indicate significant difference (P < 0.05)
Effects of astaxanthin supplementation on alkaline phosphatase (AKP) activity (U/mg protein) in the hepatopancreas of P. clarkii under MC-LR stress (0.05 mg/l) after 12, 24, and 36 days. Four concentrations of astaxanthin were examined: 0, 2.5, 5, and 10 mg/g. For each time point, bars with different letters indicate significant difference (P < 0.05)
Changes in the oxygen consumption rate of MC-LR-stressed P. clarkii over time
For the first 18 days of the experiment, the average 6-day oxygen consumption rates of P. clarkii treated with 0.01 mg/l MC-LR were significantly higher than those of unstressed P. clarkii (P < 0.05; Fig. 3). The oxygen consumption rates of the experimental groups (no MC-LR stress, 0.01 mg/l MC-LR stress, and 0.01 mg/l MC-LR + 2.5/5/10 mg/g astaxanthin) decreased at a similar rate over time. Dietary supplementation with astaxanthin did not have a significant effect on the average 6-day oxygen consumption rate of P. clarkii under MC-LR stress (P > 0.05).
Effects of astaxanthin on the fecundity of P. clarkii under MC-LR stress
The fecundity of P. clarkii treated with MC-LR was lower than that of the untreated group (Table 2). Although dietary supplementation with astaxanthin increased average and relative fecundity compared with those of the 0 mg/g treatment, no significant differences in fecundity were detected among the four astaxanthin treatments after 36 days (P > 0.05). In addition, after 36 days, the fecundity of crayfish treated with astaxanthin remained significantly lower than that of crayfish not subjected to MC-LR stress (P < 0.05; Table 2).
The accumulation of MC-LR in the ovaries of P. clarkii decreased significantly with an increase in dietary supplementation with astaxanthin (Fig. 4). Although the decrease was not significant at 2.5 mg/g astaxanthin, the MC-LR concentrations in the ovaries of the P. clarkii fed 5 and 10 mg/g astaxanthin were significantly lower than those in the ovaries of the P. clarkii supplied with non-supplemented feed (P < 0.05).
Effects of astaxanthin on the SGR and survival rate of P. clarkii under MC-LR stress
Exposure to MC-LR significantly reduced the SGR of P. clarkii (P < 0.05), from 0.327 ± 0.032 (% day−1) to 0.159 ± 0.034 (% day−1), and also reduced crayfish survival rate, from 95.8 ± 7.2 to 55.8 ± 7.2% (Table 3). SGR increased significantly (P < 0.05) with astaxanthin supplementation, and at the two highest concentrations of astaxanthin, SGRs were not significantly different from those of crayfish without MC-LR stress (P < 0.05; Table 3). The highest survival rate was at 10 mg/g astaxanthin (89.7 ± 14.4%), although there were no significant differences in survival rates among the astaxanthin treatments (2.5, 5, and 10 mg/g).
Discussion
Effects of astaxanthin on the growth and development of P. clarkii
Previous research showed that astaxanthin significantly increased growth rate in the giant freshwater prawn Macrobrachium rosenbergii (14.48% increase) (Lv et al. 1999). Other research suggested that dietary supplementation with astaxanthin increased the growth of Penaeus japonicus, as well as shortening its molting cycle and increasing its resistance to external environmental stressors (Petit et al. 1997). Dietary supplementation with 80 mg/kg astaxanthin reduced the respiratory metabolism of post-larval Litopenaeus vannamei inhabiting low-salinity seawater, which indicated that astaxanthin reduced the energy consumption, and thereby increased the growth of, juvenile L. vannamei under low-salinity stress (Medina 2006). Chien et al. (2003) supplemented the diets of Penaeus merguiensis larvae with 80 mg/kg astaxanthin and then stressed the larvae by exposing them to rapidly changing environments (i.e., a decrease in salinity from 32 to 0%; a decrease in temperature from 27 to 5 °C) for 5 consecutive minutes, and found that the mean survival of the experimental group was 1.17-fold greater than that of the control group. In the current study, astaxanthin supplementation strongly affected the survival rate of P. clarkii under MC-LR stress (Table 3), and the SGR of P. clarkii under MC-LR stress was highest in the groups fed 5 and 10 mg/g astaxanthin. These results suggest that astaxanthin increased the resistance of P. clarkii to MC-LR stress. In addition, dietary supplementation with astaxanthin also reduced the accumulation of MC-LR in the ovaries of P. clarkii. Thus, astaxanthin may not only alleviate MC-LR-induced oxidative stress in P. clarkii, but also reduce MC-LR accumulation in certain target organs (e.g., the ovary). Thus, some degree of dietary supplementation with astaxanthin may improve the growth and development of farmed P. clarkii as MC-LR contamination is common in ponds used for their aquaculture.
Effects of astaxanthin on the immunity of P. clarkii
Previous studies have confirmed that the increased activity of antioxidant enzymes such as glutathione S-transferase, SOD, and catalase, weakens the antioxidant defense systems of organisms (Prieto et al. 2007), thereby affecting their normal growth and respiratory functions (Racotta et al. 2002). Antioxidants such as N-acetylcysteine, selenium, and astaxanthin effectively maintain activity levels of antioxidant enzymes by reducing the production of ROS and malondialdehyde levels, thereby alleviating cytoskeletal damage (Weng et al. 2003). Thus, it is thought that antioxidants can be effective in preventing oxidative stress induced by toxins such as MC (Ana et al. 2008). In this study, dietary supplementation with astaxanthin increased AKP and SOD activity levels in P. clarkii. SOD activity increased rapidly and significantly in the P. clarkii fed 10 mg/g astaxanthin during the early stage of the experiment (within 12 days), and the effect of this was revealed at a later stage of the experiment (within 36 days), with a reduction in oxidative stress. By contrast, AKP activity levels increased significantly in P. clarkii fed astaxanthin in the mid (10 mg/g on day 24) and late (2.5 and 10 mg/g on day 36) stages of the experiment. These results are consistent with those of Xie et al. (2008), who showed that astaxanthin treatment increased SOD activity in Macrobrachium nipponense and improved its immunity. In addition, Wen et al. (2011) showed that dietary supplementation with astaxanthin significantly increased phenoloxidase activity in Penaeus monodon, which increased slowly with increasing levels of astaxanthin dietary supplementation up to 160 mg/kg. In agreement with these previous studies, our results suggest that astaxanthin dietary supplementation increased overall cellular antioxidant capacity and improved immunity in P. clarkii.
Changes in the oxygen consumption rate of P. clarkii under MC-LR stress
Oxygen consumption rate is an important indicator of metabolic activity in animals, as an increase in the former indicates an increase in the latter (Cebrian et al. 2008). In aquatic environments, temperature, body weight, and toxins significantly affect oxygen consumption rate (Yan et al. 2002). Zou et al. (2013) showed that the respiratory rate and the circadian rhythm of M. nipponense were not consistently affected by high concentrations of ammonia-nitrogen. With an increase in ammonia nitrogen level an inverted U-shaped curve was observed in the average oxygen consumption rate of M. nipponense, i.e., it first increased, then decreased. These results indicated that exposure to certain concentrations of ammonia–nitrogen stimulated the oxygen consumption rate of M. nipponense. In our study, exposure to the toxin MC-LR increased the oxygen consumption rate of P. clarkii, and in the early stages of the experiment, its average oxygen consumption rate under MC-LR stress was higher than that of the control group. Although oxygen consumption rates also decreased slightly in the control group with time, the decrease in oxygen consumption to control levels with exposure to MC-LR over time suggested that the crayfish eventually adapted to the test level of MC-LR toxicity.
Conclusion
In the present study, dietary supplementation with a specific concentration of astaxanthin alleviated MC-LR-induced oxidative stress in P. clarkii through an increase in the activity of antioxidant enzymes. Thus, this study provides a theoretical basis for the reduction of MC-LR-induced oxidative stress in aquatic organisms via astaxanthin dietary supplementation.
References
An ZH, Sun LS, Wang P (2015) Acute toxicity and accumulation of microcystin-leucine-arginine in the crayfish Procambarus clarkii (Girard, 1852). Crustaceana 88:397–404
Ana IP, Angeles J, Silvia P, Isabel M, Ana MC (2008) Protective role of vitamin E on the microcystin-induced oxidative stress in tilapia fish (Oreochromis niloticusz). Environ Toxicol Chem 27:1152–1159
Carmichael WW (1996) Toxic microcystis and the environment. In: Watanabe MF, Harada K, Carmichael WW, Fujiki H (eds) Toxic microcystis. CRC, Boca Raton, p 111
Cazenave J, Bistoni MA, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquatic Toxicol 76:1–12
Cebrian C, Andreu-Moline E, Fernandez CA, Ferrando MD (2008) Changes of oxygen consumption on Procambarus clarkii exposed to methidathion: effect on isolated gills. J Environ Sci Health Part B 25:767–775
Chien YH, Pan CH, Hunter B (2003) The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 216:177–191
Gherardi F, Holdich DM (1999) Crayfish in Europe as alien species: how to make the best of a bad situation. Balkema, Rotterdam
Gherardi F, Lazzara L (2006) Effects of the density of an invasive crayfish (Procambarus clarkii) on a pelagic and surface microalgae in a Mediterranean wetland. Arch Hydrobiol 165:401–414
Guzman RE, Solter PF (1999) Hepatic oxidative stress following prolonged sub lethal microcystin LR exposure. Toxicol Pathol 27:582–588
Huang CH, Chang RJ, Huang SL (2003) Dietary vitamin E supplementation affects tissue lipid peroxidation of hybrid tilapia Oreochromis niloticus × O. aureus. Comp Biochem Phys Part B 134:265–270
Lv YH, Jin ZY, Xu XM (1999) Effects of the yeast Phaffia rhodozyma and astaxanthin in it on pigmentation and growing of the prawn M. rosenbergii. J Aquacult 4:15–18
Medina ZR (2006) Metabolismo respiratorio en juveniles de Litopenaeus vannamei. CIC ESE, Mexico
Pei LH, Dong FH (2007) Effect of astaxanthin on osteoblasts injury caused by H2O2. Chin J Basic Med Tradit Chin Med 13:585–587
Petit H, Ngre-Sadargues G, Castillo R (1997) The effects of dietary astaxanthin on growth and molting cycle of post larval stages of the prawn, Penaeus japonicus (Crustacea, Decapoda). Comp Biochem Phys 117:539–544
Prieto AI, Pichardo S, Jos A, Moreno I, Camean AM (2007) Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquatic Toxicol 84:337–345
Racotta IS, Palacios E, Mendez L (2002) Metabolic responses to short and long-term exposure to hypoxia in white shrimp (Penaeus vannamei). Mar Freshwater Behav Physiol 35:269–275
Smith JL, Haney JF (2006) Food web transfer, accumulation, and depuration of microcystins, a cyanobacterial toxin, in pumpkinseed sunfish (Lepomis gibbosus). Toxicon 48:580–589
Vasconcelos V, Oliveira S, Teles FO (2001) Impact of a toxic and non-toxic strain of Microcystis aeruginosa on the crayfish Procambarus clarkii. Toxicon 39:1461–1470
Wang W, Ishikawa M, Koshio S, Yokoyama S, Hossain MS, Moss AS (2018) Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture 491:197–204
Wen WG, Lin HZ, Wu KC (2011) Effects of dietary with astaxanthin on growth and immunological parameters of black tiger shrimp, Penaeus monodon. Acta Sci Natur Univ Sunyatseni 50:144–146
Weng D, Lu Y, Wei YN, Liu Y, Shen PP (2003) The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 232:15–23
Xie JH, Guan YQ, Wang JB (2008) Effects of astaxanthin on density and phagotrophic ability of blood lymphocyte of prawn Macrobrachium rosenbergii. Hebei Fish 169:8–11
Yan MC, Shan LZ, Shao XB (2002) Influences of temperature and weight on respiration and excretion of Miichthys miiuy juvenile. J Trop Oceanogr 13:739–742
Zhang H, Zhang J, Chen Y, Zhu Y (2008) Microcystin—RR induces apoptosis in fish lymphocytes by generating reactive oxygen species and causing mitochondrial damage. Fish Physiol Biochem 34:307–312
Zou LC, Ren SY, Wang ZZ (2013) Acute effects of ammonia exposure on mortality, oxygen consumption, and suffocation point in freshwater shrimp Macrobrachium nipponensis. Oceanol Limnol Sin 46:206–211
Acknowledgement
This study was funded by the Jiangsu-Agricultural-Industry-Technology System (Red Swamp Crayfish).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
An, Z., Yang, H., Liu, X. et al. Effects of astaxanthin on the immune response and reproduction of Procambarus clarkii stressed with microcystin-leucine-arginine. Fish Sci 86, 759–766 (2020). https://doi.org/10.1007/s12562-020-01434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01434-0