Abstract
Vitellin (Vt) was purified from eggs of parthenogenetic bush tick Haemaphysalis longicornis by gel filtration and ion exchange chromatography. Our results revealed that only one single Vt existed in parthenogenetic bush tick, and the purified Vt was proved to be a hemoglycolipoprotein consisting of nine polypeptides with molecular weights of 203, 147, 126, 82, 74, 70, 61, 47 and 31 kDa, respectively. Polyclonal antibody and monoclonal antibody against Vt were produced using the purified Vt. The change in vitellogenin (Vg) and Vt levels over time of the parthenogenetic H. longicornis was established, and the Vg content in haemolymph and Vt in ovary at different feeding or engorgement statuses was also determined using a double antibody sandwich enzyme-linked immunosorbent assay. The Vg level in haemolymph was distinctly increased on the day of engorgement (1.785 mg/mL) and continued to increase until 2nd day post-engorgement (5.611 mg/mL). There was a slight decrease in Vg level after 4 days of engorgement, and a second peak was observed on day 2 post-oviposition (10.774 mg/mL). Subsequently, Vg content continuously decreased and reached a low level on the 10th day post-oviposition. The Vt content in ovary continuously increased once the female reached its critical weight (0.024 mg per female), and reached the maximum level on day 2 post-oviposition (1.942 mg per female). Afterwards, Vt content rapidly decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ticks are well known vectors for a great variety of infectious pathogens, including viruses, rickettsia, bacteria, spirochetes protozoa and nematoda, all of which can cause severe damage to both animals and humans as well as significant economic losses (Deng and Jiang 1991; Sanches et al. 2012). Therefore, ticks have attracted much scientific and public attention due to their medical and veterinary importance (Jongejan and Uilenberg 2004).
Although most described tick species reproduce bisexually, a few species are able to reproduce by parthenogenesis (Oliver 1974, 1977). Parthenogenesis in ticks has been recognized in several genera, including Amblyomma, Hyalomma and Haemaphysalis, with certain species of ticks lacking males altogether (Aragão 1912; Pervomaisky 1949; Zhmaeva 1950; Kiszewski et al. 2001).
The bush tick Haemaphysalis longicornis Neumann is widely distributed in Australia, New Zealand, Korea, Japan (Tenquisf and Charleston 2001) and 17 provinces of China (Deng and Jiang 1991). Moreover, it can transmit a large variety of pathogens, including Theileria (Li et al. 2009), Babesia (Guan et al. 2010) and Rickettsia (Zou et al. 2011). Most interestingly, H. longicornis consists of a diploid bisexual population, a triploid obligatory parthenogenetic population and an aneuploid population, which are capable of parthenogenetic and bisexual reproduction (limited in Cheju Do) (Oliver 1977; Chen et al. 2012).
Reproductive success in ticks highly relies on the vitellogenesis and ovarian development (Boldbaatar et al. 2010). However, most studies have been conducted on bisexual ticks rather than parthenogenetic species (Sonenshine 1991; James and Oliver 1996; Denardi et al. 2004; Friesen and Kaufman 2004; Saito et al. 2005; Sanches et al. 2010). We have previously described several characteristics of the parthenogenetic population of H. longicornis, including some important micro-structures and synchronized life-cycle features (Chen et al. 2012). However, only limit research on vitellin (Vt) or vitellogenesis in the parthenogenetic population has been performed. In this study, Vt was purified from eggs of parthenogenetic H. longicornis, and the change in Vt levels over time was also investigated, providing basic background for further studies.
Materials and methods
Sample collection and tick rearing
Nymphs of parthenogenetic H. longicornis were collected from vegetation by flag dragging in Cangxi County (31°37′–32°10′N, 105°43′–106°28′E) of Sichuan Province, Southwest China, and reared in our laboratory for two generations. The emerged adults were fed on rabbits and removed once a suitable feeding status or engorgement stage was achieved. Unfed and engorged ticks were kept in cotton-plugged glass tubes in an incubator (27 ± 1 °C, 90 % RH and 12-h photoperiod).
Collection of eggs and preparation of crude egg extract
Eggs were collected and weighed daily from ovipositing females. Briefly, 1 g of eggs was homogenized in 2 mL distilled water containing protease inhibitor (PMSF) using a glass-Teflon homogenizer, and the homogenate was centrifuged at 9,000 rpm for 15 min at 4 °C. Subsequently, crude egg extract (supernatant) was obtained by discarding the fat layer and then frozen at −20 °C prior to further purification.
Purification of Vt from crude egg extract
Crude egg extract (1 mL) was applied onto a Sepharose CL-4B gel filtration column (1.0 × 90 cm) and eluted with water at 4 °C, followed by the collection of 5-mL fractions. Proteins and heme proteins in the eluate were photometrically monitored at wavelengths of 280 and 400 nm, respectively (Chinzei et al. 1983). Fractions containing Vt (the largest peak, Fig. 1a) were pooled and dialyzed against dialysis buffer (0.02 M Tris–HCl, pH 8.0) at 4 °C for 12 h. The dialyzed Vt was applied onto a diethyl-amino-ethyl (DEAE)-cellulose column (1.6 × 30 cm), which was previously equilibrated with same buffer. Vt purification was conducted using a stepwise gradient elution (0.0–0.8 M NaCl). Similar to gel filtration, the eluate of Vt was collected and monitored at 280 and 400 nm. The Vt fractions were pooled and dialyzed against water at 4 °C for 24 h and then stored at −20 °C. The Vt concentration was determined using the Bradford assay (Bradford 1976).
Polyacrylamide gel electrophoresis (PAGE)
Purity of Vt was examined by native-PAGE and sodium dodecyl sulfate (SDS)-PAGE. Native PAGE was carried out on 7.5 % separating gels (1.5 M Tris–HCl buffer, pH 8.8) with 4 % stacking gels (0.5 M Tris–HCl buffer, pH 6.8). Samples (5–20 μg protein) were dissolved in 0.1 M Tris–HCl buffer (pH 6.8) containing 10 % glycerol and bromophenol blue. Electrophoresis was performed in 0.05 M Tris–glycine buffer (pH 8.3) at a constant voltage (120 V) for 4–5 h. SDS-PAGE was carried out on 7.5 % separating gels with 4 % stacking gels containing 0.1 % SDS. Samples were dissolved in 0.1 M Tris–HCl (pH 6.8) containing 2 % SDS, 5 % 2-mercaptoethanol, 10 % glycerol and 0.05 % bromophenol blue, and then boiled for 2 min. Electrophoresis was carried out in 0.05 M Tris–glycine buffer (pH 8.3) containing with 0.1 % SDS at a constant voltage (120 V) until the tracking dye reached to the end of gel.
After electrophoresis, both native-PAGE and SDS-PAGE gels were stained with Coomassie blue, whereas the native-PAGE gel was further stained with periodic acid-Schiff (PAS) for carbohydrates or Sudan black B for lipids.
Antibody preparation and immunological tests
Polyclonal antibodies (PcAb) were prepared according to James and Oliver (1997). The purified Vt (500 μg) was emulsified in Freund’s complete adjuvant and then subcutaneously injected into rabbits. Two booster injections of Vt were given with the same procedure but using Freund’s incomplete adjuvant. Serum was collected after 4–6 weeks, when high antibody titer was observed against Vt. IgG fractions were isolated from antisera using caprylic acid ammonium sulfate precipitation (Li et al. 2008).
Monoclonal antibody (McAb) was produced in BALB/c mice, which were subcutaneously injected with 50 μg Vt antigens emulsified in Freund’s complete adjuvant. After that, booster injection was performed twice using the same antigen emulsified in Freund’s incomplete adjuvant at intervals of 14 days. Splenocytes from immunized mice were fused to myeloma cells, SP2/0, using polyethylene glycol. Hybridomas were selected in HAT medium, and the hybridoma culture supernatants were screened by enzyme-linked immunosorbent assay (ELISA) in 96-microtiter plates and Western blot.
ELISA was performed according to Li et al. (2008). The positive hybridoma cells were cloned three times. The immunoglobulin of ascites was purified with the method of caprylic acid ammonium sulfate precipitation, while titers and specificity of McAbs were analyzed as previously described (Li et al. 2008). The immunoglobulin isotype was determined using Mouse Monoclonal Antibody Isotyping Reagents.
For Western blot analysis, 10 μg Vt antigens were separated on a 7.5 % SDS-PAGE gel under the reduced condition, and then the gel was electro-transferred onto polyvinilidene difluoride (PVDF) membranes at 4 °C for 1 h. The PVDF membranes were incaubated with McAb and then reacted with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG at room temperature for 1.5 h. Reactive bands were revealed using DAB reagent (3, 3′-diaminobenzidine).
Collection of haemolymph and ovary samples
Haemolymph and ovary of female ticks at different feeding durations or different periods after engorgement were prepared as follows.
Briefly, haemolymph was collected using a calibrated glass micropipette, which could measure the sample volume based on the length of the fluid column, and then samples were diluted in 0.01 M Tris–HCl (pH 8.0) containing 0.02 M Ethylene glycol bis (2-aminoethyl) tetraacetic acid (EGTA) and a small amount of phenylmethanesulfonyl fluoride (PMSF).
Ovaries were dissected and homogenized in 100 μL of 0.01 M Tris–HCl (pH 8.0). The ovarian homogenates were centrifuged at 9,000 g for 10 min. Supernatant was collected into a clean Eppendorf tube and stored at −20 °C until analysis.
Determination of vitellogenin (Vg) content
Vg content was determined using double antibody sandwich ELISA (DAS-ELISA). Briefly, rabbit anti-Vt polyclonal IgG (21.0 μg/mL) was used as capture antibody to coat microtiter plates at 4 °C overnight. After washing three times with phosphate-buffered saline containing 0.05 % (v/v) Tween-20 (PBST), wells were blocked with 10 % fetal bovine serum (FBS) in PBST at 37 °C for 2 h. Subsequently, the plates were washed three times with PBST, and then incubated with 100 μL test samples at 37 °C for 45 min. After six washes with PBST, the anti-Vt McAb was added into each well at 37 °C for 45 min. Wells were washed again, and then the plates were incubated with HRP-conjugated goat anti-mouse IgG at 37 °C for 30 min. After three washes with PBS, chromogen and substrate were added (10 μg 3,3′,5,5′-Tetramethylbenzidine, 5 μL H2O2 in 0.1 M citrate–phosphate buffer, pH 5.0) and incubated at 37 °C for 15 min. The reaction was terminated with 2 M H2SO4. Finally, optical density (OD) was determined at 450 nm. Meanwhile, a serial dilution of the purified Vt (from 20.48 μg/mL to 1.25 ng/mL) was used to generate a standard curve in order to quantitatively evaluate test samples.
Chemicals
All chemicals were purchased from Sigma Chemical (USA), except for DEAE-cellulose (Whatman Chemical, USA).
Results
Purification of Vt
Vt was purified from crude egg extract by gel filtration (Sepharose CL-4B) and ion exchange chromatography on DEAE-cellulose. The soluble fractions of crude egg extract were eluted from Sepharose gel in several peaks, and the first peak was the only peak containing a heme protein, which was proved by its absorbance at 280 and 400 nm (Fig. 1a). Heme proteins (fractions from the first peak) were pooled and subsequently loaded onto a DEAE-cellulose ion exchange column. The pooled sample was eluted as one peak from column with 0.2 M NaCl, and it was designated as Vt (Fig. 1b).
Purity of Vt was assessed under non-reducing and non-denaturing conditions (native-PAGE) (Fig. 2a). Positively stained Vt revealed the presence of carbohydrates and lipids (Fig. 2b, c), indicating that it was a hemoglycolipoprotein.
Polypeptides of Vt
Purified Vt was tested under reducing conditions (SDS-PAGE) (Fig. 3), and nine polypeptide bands of Vt were detected. The molecular weights of these polypeptides were 203, 147, 126, 82, 74, 70, 61, 47 and 31 kDa, respectively.
Production of McAb against Vt
In the present study, we generated seven hybridoma cell lines secreting McAb against Vt by fusing myeloma cells (SP2/0) with splenocytes from BALB/c mouse immunized with Vt antigen. The hybridoma cell line that produced IgG isotype with the highest specificity and titer was named as 4G11.
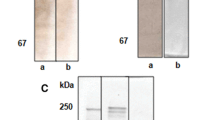
The IgG isotype of 4G11 was identified, and data showed that 4G11 was of the isotype IgG2b. The IgG of 4G11 was purified, and SDS-PAGE revealed that molecular weights of its heavy chain and light chain were 55.8 and 20.9 kDa, respectively (Fig. 4).
Western blot analysis showed that purified 4G11 had specific immunological reaction with only two polypeptides (82, 61 kDa) of Vt (Fig. 5).
Vg dynamics of the haemolymph at different feeding or engorgement statuses
Vg level in haemolymph of the parthenogenetic H. longicornis obviously varied at different feeding statuses or after different periods post-engorgement. Vg content in haemolymph was extremely low at unfed stage and at early stage of attachment (0.112, 0.137 mg/mL). However, it distinctly increased at the engorgement stage (1.785 mg/mL) and then reached a relatively high level on 2nd day post-engorgement (5.611 mg/mL). Shortly afterwards, the Vg content exhibited a gradually decrease from 4th day post-engorgement to the beginning of oviposition (3.559 mg/mL), but increased again and rapidly reached its maximum level on 2nd day post-oviposition (10.774 mg/mL). Subsequently, the Vg content continued to decrease and reached a low level on 10th day post-oviposition (0.107 mg/mL) (Table 1; Fig. 6).
Dynamics of Vg or Vt content in haemolymph or ovary at different feeding or engorgement statuses. The different feeding or engorgement statuses are represented by the following capital letters or the combination of the letters and numbers. U: Unfed stage; F: The 3rd day after feeding; E0: Day of engorgement; E2, E4: The 2nd and 4th day after engorgement; O0: Day of oviposition; O2, O4, O6, O8, O10: The 2nd, 4th, 6th, 8th, 10th day after oviposition
Vt dynamics of the ovary at different feeding or engorgement statuses
The content of Vt in ovary was very low at the early stage of attachment, leading to difficulty in detection until the day of engorgement (0.024 mg/ovary). After engorgement, ovarian Vt level was obviously increased, exhibiting a 46-fold increase on the day of oviposition (1.115 mg/ovary), and it reached the maximum level on 2nd day post-oviposition (1.942 mg/ovary). Afterwards, the Vt content began to decline rapidly (0.364 mg/ovary) and reached a lower level on 10th day post-oviposition (0.024 mg/ovary) (Table 1; Fig. 6).
Discussion
Vt from eggs of the parthenogenetic tick, H. longicornis was purified and partially characterized in the current study. The property of Vt is similar to that reported in bisexual population of H. longicornis (Yang et al. 2004). The Vt here also contains heme, lipid and carbohydrate components. Derived from the digestion of host hemoglobin, the heme component is the characteristic of Vt found in ticks (Diehl et al. 1982). In general, Vts from ticks are similar to insect Vts in terms of carbohydrate and lipid composition (James and Oliver 1997).
Previous studies have shown that many bisexual ixodid ticks in general have two Vts, such as Rhipicephalus appendiculatus and Dermacentor variabilis (Dhadialla 1986; Rosell and Coons 1991). However, the argasid tick, Ornithodoros moubata, possesses only one single Vt (Chinzei et al. 1983), and the prostriate tick, Ixodes scapularis, which is thought to be phylogenetically intermediate between argasids and metastriates, has only one single Vt (James and Oliver 1997). Similarly, we showed that only one single Vt existed in bisexual population of H. longicornis. Whether there is a relationship between the number of Vts and the species of ticks remains unknown.
Vt of the parthenogenetic H. longicornis consisted of nine polypeptides with molecular weights ranging from 31 to 203 kDa, which was inconsistent with other bisexual tick species as previously reported. Vt of the bisexual H. longicornis is composed of eight subunits, and their molecular weights are 112, 103, 80, 78, 71, 68, 62 and 52 kDa, respectively (Yang et al. 2004). Whereas both D. variabilis and R. appendiculatus have two Vts with polypeptides, of which the molecular weights range from 35 to 135 kDa and from 43 to 160 kDa, respectively (Rosell and Coons 1991; Dhadialla 1986). O. moubata has only one Vt with six polypeptides, and the molecular weight of its subunits ranges from 52 to 112 kDa (Chinzei et al. 1983). The Vt in I. scapularis contains seven subunits with molecular weights ranging from 35 to 154 kDa (James and Oliver 1997).
In insects, Vgs are large (200–700 kDa) homologous phosphoglycolipoproteins with monmers consisting of one to four subunits. The Vg monomers of most insects are composed of one large (>150 KDa) and one small (<65 KDa) subunit (Kunkel and Nordin 1985; Raikhel and Dhadialla 1992; Valle 1993; Sappington and Raikhel 1998). However, in ticks, no such similar rule was observed in terms of the molecular weight and subunit number of Vt or Vg.
Since the first full-length Vg cDNA sequence for tick was reported from D. variabilis, multiple Vg genes have been isolated from ticks, with full-length sequence available from D. variabilis, H. longicornis, and O. moubata, and partial sequence available from R. microplus and I. scapularis (Thompson et al. 2007; Boldbaatar et al. 2010; Khalil et al. 2011). Notably, three potential Vg sequences have been characterized from parthenogenetic H. longicornis (HlVg1–3) (Boldbaatar et al. 2010), but further analyses cast doubt on HlVg2 and HlVg3 actually being Vg sequences due to their similarity to the common tick storage protein CP (Khalil et al. 2011). In the current study, Vt subunit sizes were compared with the possible products from the predicted RXXR cleavage sites of the three previously described HlVgs. The 61 KDa Vt subunit resolved on the SDS-PAGE was presumably consistent with the expected cleavage product of 60.8 KDa found in HlVg1, from the first cleavage site (RGTR, aa 253–256) to the second cleavage site (RAIR, aa 779–782), whereas no other Vt subunits were found consistent with the expected products obtained from the suggested cleavage sites of HlVg2 and HlVg3. Undoubtedly, those results strengthened the idea that HlVg1 is the precursor to Vt in H. longicornis. However, further analysis remains necessary to explore their relationship.
In arthropods, Vg is transported from its original site by haemolymph to ovary and specifically accumulates in oocytes, and Vg becomes Vt with further processing (Raikhel and Dhadialla 1992; Friesen and Kaufman 2004). In ticks, it has been widely recognized that Vg is produced from fat body and gut and regulated by ecdysteroids (Horigane et al. 2010; Khalil et al. 2011).
The Vt makes up 80 % of the total protein present in eggs (Chinzei et al. 1983), which can be used as a source of nutrient during embryonic development. In ticks, reproductive success is closely related to the process of Vg transportation and uptaking (Boldbaatar et al. 2010). Vgs and Vts are structurally, biochemically and immunologically similar in majority of insects and ticks (Kunkel and Nordin 1985; Kaufman 2004). For the first time, PcAb and McAb against Vt of parthenogenetic population of H. longicornis was produced. In addition, a series of identifications were performed on the purified McAb, providing an important tool for further elucidating vitellogenesis and its regulatory mechanism in the parthenogenetic H. longicornis.
The Vg or Vt content of the haemolymph and ovary in the parthenogenetic H. longicornis was determined using DAS-ELISA. The Vg content of haemolymph was extremely low at the unfed stage and the early stage of attachment, while it was distinctly increased after engorgement. This indicated that feeding initiated Vg production, and Vg was synthesized at an accelerating rate and rapidly released into haemolymph after engorgement.
There was a correlation between Vg content in haemolymph and Vt content in ovaries of the parthenogenetic H. longicornis. The Vg content was distinctly increased in haemolymph on the day of engorgement, when Vt began to appear in ovary with a very low content. Then Vg content in haemolymph reached a relatively high level on 2nd day post-engorgement. Shortly afterwards, Vg content was gradually decreased from 4th day post-engorgement to the beginning of oviposition. In contrast, the ovarian Vt level was continuously increased, and it reached a maximum level on 2nd day post-oviposition. Therefore, the decrease of Vg content in haemolymph at 4 days after engorgement was attributed to an increasing rate of Vg uptake by oocytes.
When ovipositing began, Vg content in haemolymph was increased again and reached its highest level on 2nd day post-oviposition, and this increase might be due to a decrease of Vg uptake into ovaries after oviposition. The reduced Vg uptake by ovaries could induce an accumulation of a high content of Vg in haemolymph, resulting in termination of Vg production. Subsequently, Vg content in haemolymph and Vt content in ovaries were continuously decreased and reached a low level on 10th day post-oviposition.
Even though the vitellogenesis and ovarian development of the parthenogenetic and bisexual populations of H. longicornis were similar to each other, some important differences also existed. Early studies on bisexual population showed that Vt is detected in ovary at 2 days after engorgement (Yang et al. 2004). The current work revealed that Vt appeared in ovary of the parthenogenetic population 2 days earlier than that of bisexual population. This could be regarded as an adaptative strategy due to the absence of copulation and lack of male factor transfer.
References
Aragão H (1912) Contribuição para a sistematica e biolojia dos ixódidas: partenojeneze em carrapatos: Amblyomma agamum n. sp. Mem Inst Oswaldo Cruz 4:96–119
Boldbaatar D, Umemiva-Shirafuji R, Xuan X, Tanakan T, Fujisaki K (2010) Multiple vitellogenins from the Haemaphysalis longicornis ticks are crucial for ovarian development. J Insect Physiol 56:1587–1598
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Z, Yang XJ, Bu F, Yang XH, Liu JZ (2012) Morphological, biological and molecular characteristics of bisexual and parthenogenetic Haemaphysalis longicornis. Vet Parasitol 189:344–352
Chinzei Y, Chino H, Takahashi K (1983) Purification and properties of vitellogenin and vitellin from a tick Ornithodoros moubata. J Comp Physiol 152:13–21
Denardi SE, Bechara GH, Oliveira PR, Nunes ET, Saito KC, Camargo-Mathias MI (2004) Morphological characterization of the ovary and vitellogenesis dynamics in the tick Amblyomma cajennense (Acari: Ixodidae). Vet Parasitol 125:379–395
Deng GF, Jiang ZJ (1991) Economic insect fauna of China. Fasc 39, Acari: Ixodidae. Science Press, Beijing
Dhadialla TS (1986) Purification and some biochemical properties of vitellins from Rhipicephalus appendiculatus eggs and their use as antigens to induce type II immune resistance in rabbits. J Cell Biochem (Suppl) 10:77
Diehl PA, Aeschlimann A, Obenchain FD (1982) Tick reproduction: oogenesis and oviposition. In: Obenchain FD, Galun R (eds) Physiology of ticks. Pergamon Press, Oxford
Friesen KJ, Kaufman WR (2004) Effects of 20-hydroxyecdysone and other hormones on egg development, and identification of a vitellin-binding protein in the ovary of the tick, Amblyomma hebraeum. J Insect Physiol 50:519–529
Guan G, Moreau E, Liu J, Hao X, Ma M, Luo J, Chauvin A, Yin H (2010) Babesia sp. BQ1 (Lintan): molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol Int 59:265–267
Horigane M, Shinoda T, Honda H, Taylor D (2010) Characterization of a vitellogenin gene reveals two phase regulation of vitellogenesis by engorgement and mating in the soft tick Ornithodoros moubata (Acari: Argasidae). Insect Mol Biol 19:501–515
James AM, Oliver JH Jr (1996) Vitellogenin concentrations in the heamolymph and ovaries of Ixodes scapularis ticks during vitellogenesis. Exp Appl Acarol 20:639–647
James AM, Oliver JH Jr (1997) Purification and partial characterization of vitellin from the black-legged tick, Ixodes scapularis. Insect Biochem Mol Biol 27:639–649
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:3–14
Kaufman WR (2004) Assuring paternity in a promiscuous world: are there lessons for ticks among the insects. Parasitology 129:145–160
Khalil SM, Donohue KV, Thompson DM, Jeffers LA, Ananthapadmanaban U, Sonenshine DE, Mitchell RD, Roe RM (2011) Full-length sequence, regulation and developmental studies of a second vitellogenin gene from the American dog tick, Dermacentor variabilis. J Insect Physiol 57:400–408
Kiszewski AE, Matuschka FR, Spielman A (2001) Mating strategies and spermiogenesis in ixodid ticks. Annu Rev Entomol 46:167–182
Kunkel JG, Nordin JH (1985) Yolk proteins. In: Kerkut GA, Gilbert LJ (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon Press, Oxford
Li XM, Zhang ZP, Yang XL, Wang D, Liu JZ (2008) Preparation and identification of monoclonal antibodies to the vitellin from the hard tick Haemaphysalis longicornis Neumann (Arachnida: Ixodidae). Acta Entomol Sin 51:1028–1032
Li Y, Luo J, Guan G, Ma M, Liu A, Liu J, Ren Q, Niu Q, Lu B, Gao J (2009) Experimental transmission of Theileria uilenbergi infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol Res 104:1227–1231
Oliver JH Jr (1974) Symposium on reproduction of arthropods of medical and veterinary importance. Reproduction of ticks (Ixodoidea). J Med Entomol 11:26–34
Oliver JH Jr (1977) Cytogenetics of mites and ticks. Annu Rev Entomol 22:407–429
Pervomaisky GS (1949) Parthenogenetic development in ticks of the family Ixodidae. Zool Zh 28:523–526
Raikhel AS, Dhadialla TS (1992) Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 37:217–251
Rosell R, Coons LB (1991) Purification and partial characterization of vitellin from the eggs of the hard tick, Dermacentor variabilis. Insect Biochem 21:871–875
Saito KC, Bechara GH, Oliveira PR, Nunes ET, Denardi SE, Camargo-Mathias MI (2005) Morphological, histological, and ultrastructural studies of the ovary of the tick Boophilus microplus (Canestrini 1887) (Acari: Ixodidae). Vet Parasitol 129:299–311
Sanches GS, Bechara GH, Camargo-Mathias MI (2010) Ovary and oocyte maturation of the tick Amblyomma brasiliense Aragão, 1908 (Acari: Ixodidae). Micron 41:84–89
Sanches GS, Araujo AM, Martins TF, Bechara GH, Labruna MB, Camargo-Mathias MI (2012) Morphological records of oocyte maturation in the parthenogenetic tick Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae). Ticks Tick Borne Dis 3:59–64
Sappington TW, Raikhel AS (1998) Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol 28:277–300
Sonenshine DE (1991) Biology of ticks, vol 1. Oxford University Press, New York
Tenquisf JD, Charleston WAG (2001) A revision of the annotated checklist of ectoparasites of terrestrial mammals in New Zealand. J Roy Soc N Z 31:481–542
Thompson DM, Khalil SMS, Jeffers LA, Sonenshine DE, Mitchell RD, Osgood CJ, Roe RM (2007) Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem Molec Biol 37:363–374
Valle D (1993) Vitellogenesis in insects and other groups-a review. Mem Inst Oswaldo Cruz 88:1–26
Yang XL, Gao ZH, Hu YH, Liu JZ (2004) Purification and properties of vitellin from the bush tick, Haemaphysalis longicornis Neumann (Arachnida: Ixodidae). Acta Entomol Sin 47:316–319
Zhmaeva ZM (1950) Parthenogenetic development of Haemaphysalis bispinosa Neum (Acarina: Ixodidae). Entomol Obozr 31:121–122
Zou Y, Wang Q, Fu Z, Liu P, Jin H, Yang H, Guo H, Xi Z, Liu Q, Chen L (2011) Detection of spotted fever group Rickettsia in Haemaphysalis longicornis in Hebei Province, China. J Parasitol 97:960–962
Acknowledgments
This project was supported by National Natural Science Foundation of China (31472050; 31071979; 30970406), Research Fund for the Doctoral Program of Higher Education of China (20101303120001), Natural Science Foundation of Hebei Province of China (C2014205021), Natural Science Research Programs of Educational Department of Hebei Province (Q2012072), and Science Foundation of Hebei Normal University (L2009Z07; L2012Z05; L2011B13).
Conflict of interest
All the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaolong Yang and Zhijun Yu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, X., Yu, Z., He, Y. et al. Purification of vitellin and dynamics of vitellogenesis in the parthenogenetic tick Haemaphysalis longicornis (Acari: Ixodidae). Exp Appl Acarol 65, 377–388 (2015). https://doi.org/10.1007/s10493-014-9866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9866-z