Abstract
Two forms of vitellogenin (Vg: Vg1 and Vg2) were purified from the plasma of estradiol-17β (E2)-treated Indian walking catfish, Clarias batrachus, by gel filtration and adsorption chromatography. Native Vg1 and Vg2 had apparent molecular masses of 375 and 450 kDa, respectively, and both Vgs resolved into two similar major bands (95 and 67 kDa) in SDS-PAGE under reducing condition. Polyclonal antisera raised against each form of Vg were absorbed with a combination of hypophysectomized male catfish serum proteins and alternate Vg to ensure specificity. Immunological analyses verified the presence of Vg1 and Vg2 in the plasma of female catfish. Homologous ELISAs were developed for Vg1 and Vg2 using their respective harvested antisera, which exhibited the detection limit of 100 ng ml−1 for Vg1 and 40 ng ml−1 for Vg2, and low level of cross-reactivity (not parallel to the standard) was found with alternate Vg in each assay. Treatment of male catfish with E2 induced both Vgs showing a proportionate ratio of Vg1 to Vg2 at 5.6:1. Plasma concentrations of both Vgs measured by ELISAs at different reproductive phases of field collected female catfish increased in accordance with the ovarian development, keeping the proportionate ratio of Vg1 to Vg2 at about 2:1 in fish undergoing vitellogenesis during prespawning period and 1:20 during spawning period, suggesting that Vg1 may be the major Vg to contribute in yolk formation, whereas Vg2, besides its role in yolk formation, may facilitate other physiological functions. The present study, thus, demonstrates the occurrence of two unequally synthesized Vgs in the catfish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well established that vitellogenin (Vg), the egg yolk precursor glycolipophosphoprotein, is a prerequisite for the oocyte growth during oogenesis (see Wallace and Selman 1981). Vg is synthesized in the liver under the influence of ovarian estradiol-17β (E2). Then, it is secreted into circulation to reach the ovary and gets incorporated into the growing oocytes by receptor-mediated endocytosis (Sawaguchi et al. 2006 and references therein). It is then cleaved into yolk proteins (YPs: lipovitellin (Lv), phosvitin (Pv), and β′ component (β′-c) and deposited in the cytoplasm (for reviews, see Mommsen and Walsh 1988; Selman and Wallace 1989; Nath 1999; Nath et al. 2007). While in teleosts, occurrence of multiple Vg genes has been confirmed earlier (Finn and Kristoffersen 2007), two distinct Vg proteins have been isolated in different species of tilapia (Ding et al. 1989; Kishida and Specker 1993; Buerano et al. 1995; Takemura and Kim 2001; Ndiaye et al. 2006), zebrafish, Danio rerio (Wang et al. 2000); haddock, Melanogrammus aeglefinus (Reith et al. 2001); medaka, Oryzias latipes (Shimizu et al. 2002); Japanese common goby, Acanthogobius flavimanus (Ohkubo et al. 2003); Indian major carp, mrigal, Cirrhinus mrigala (Maitra et al. 2007); and three forms in white perch, Morone americana (Hiramatsu et al. 2002a); mosquito fish, Gambusia affinis (Sawaguchi et al. 2005); and red seabream, Pagrus major (Sawaguchi et al. 2006).
The presence of different forms of Vg may be the norm in teleost fishes, and YPs (Lv, Pv, and β′-c) derived from these proteins may play different roles in relation to oocyte maturation and embryonic nutrition (Hiramatsu et al. 2002b; Sawaguchi et al. 2005, 2006). In barfin flounder, haddock, and brackish water fishes, it appears that different processing of YPs derived from each type of Vg generates free amino acids, which drive oocyte hydration during maturation and may provide a pool of diffusible nutrients to support early embryonic nutrition (see reviews of Matsubara et al. 2003; Patino and Sullivan 2002; Sawaguchi et al. 2006). Previously, we have shown that two forms of Vg, HAI (lipid rich and less phosphorus content), and HAII (phosphorus rich and less lipid content) purified from the plasma of E2-treated mrigal (Maitra et al. 2007) may have different roles during vitellogenesis; while administration of HAI could enhance both synthesis and incorporation of Vg, HAII could accelerate Vg synthesis but not its uptake into the growing oocytes of the catfish, Clarias batrachus, during the prespawning period (Nath and Maitra 2001).
Therefore, to have some knowledge on the differential role of different types of Vgs individual measurement of Vg in plasma in relation to ovarian growth during different reproductive phases is necessary. Various methods like RIA, ELISA, single radial immunodiffusion, and chemiluminescent immunoassay (CLIA) have been developed and used to measure either one or two Vgs in the plasma of different fish species (Takemura and Kim 2001; Ohkubo et al. 2003; Fujiwara et al. 2005; Maitra et al. 2007; Braathen et al. 2009; see Specker and Sullivan 1994 for earlier references). Keeping in mind the findings in our earlier research (see Nath and Maitra 2001), we are interested to measure different forms of Vg (if present) in the plasma in relation to ovarian development in Indian walking catfish, C. batrachus, which is a seasonal breeder and breeds during summer monsoon. The annual ovarian cycle of this catfish has been divided into four phases: preparatory (Feb–Apr), prespawning (May–Jun), spawning (Jul–Aug), and post-spawning (Sep–Jan) (Ghosh and Nath 2005). The walking catfish is much in demand in Southeast Asian countries including India because of its meat quality and medicinal value (Debnath 2011). So it is having a high market value. This fish is an air breathing fish and well adapted to adverse ecological conditions. Because of its importance to the society, culture of this catfish has been received much attention in recent years. For successful culture of fish, production of quality fertilizable eggs is one of the prerequisites which depends on the amount of Vg-derived yolk proteins deposited in the developing oocytes through the physiological process, vitellogenesis. Therefore, vitellogenesis is vital to the reproductive success of female fish. To understand the role of Vg in the formation of yolky oocytes its quantification in relation to ovarian development is essential, and this can be done through the development of sensitive Vg ELISA. In the catfish, prior investigation has revealed the identification and characterization of a single immunoreactive form of Vg (Garnayak et al. 2013). Although this catfish is an established model for reproductive physiology and is considered to be a valuable commercial fish in India, no step has been taken to explore the potential multiplicity of Vg. Keeping this in view, the objectives of the present study were to (i) purify and characterize two forms of Vg possibly present in the catfish and (ii) raise antibodies and develop homologous ELISA for each form of Vg so as to quantify them in the plasma of female catfish at different reproductive phases and also after E2 treatment in male catfish.
Materials and methods
Collection and care of fish
Sexually mature males (body weight range 100–115 g) and females (body weight range 110–125 g) of Indian walking catfish were collected from the local market in and around Santiniketan (Lat. 23° 41′ 30″ N and long. 87° 30′ 47″ E) and maintained in the laboratory keeping 10 fishes per aquarium (size 45 × 30 × 30 cm) under natural photoperiod and temperature. Fish were fed ad libitum with laboratory-made fish food, containing rice-bran, oil cake, and commercial animal food pellets (Epic, India). Water of the aquarium was replenished daily with tap water. Fortified procaine penicillin (Allembic chemical works, Vadodara, India) was added occasionally to the aquarium water (5000 units L−1) as a prophylactic against skin infection. Fishes were acclimatized for 7 days prior to their use in experiments.
Preparation of catfish Vgs
Catfish Vgs were purified from the plasma of E2-treated male catfish following the procedure of Maitra et al. (2007) with a slight modification. In brief, to induce Vg synthesis, stock solution of E2 (Sigma, USA) was made in ethanol (10 mg in 0.2 ml) and just before injection, a working suspension was made with 0.64 % saline and injected (i.m.) at the dose level of 0.5 mg/kg to a group of 20 male catfish on every alternate day for 30 days. Control males were maintained in separate aquarium and received equal volume (0.1 ml) of 0.64 % saline only. The day following the last injection, both E2- and saline-treated fish were bled separately by caudal puncture using heparinized syringes. Blood was collected in tubes (kept in ice) containing heparin (130 IU ml−1 blood), (0.05 %) phenylmethylsulfonyl fluoride (PMSF, purchased from SRL, India), and aprotinin (0.5 TIU ml−1 of blood, Sigma, USA) dissolved in 10 mM phosphate buffer (pH 7.4) containing 0.9 % NaCl (PBS). Plasma was collected by centrifugation at 1500×g for 10 min at 4 °C in a refrigerated centrifuge (Indian Equipment Corporation, Mumbai) and either used immediately or kept at −30 °C until its use for the purification of Vgs.
Protein was precipitated from the plasma with 55 % ammonium sulfate saturation and then dissolved in eluting buffer, 50 mM Tris–HCl buffer (pH 8) containing 2 % KCl, 0.05 % PMSF, and aprotinin (0.5 TIU L−1). The undissolved materials (if any) were removed by centrifugation and the clear protein solution was dialyzed against the same buffer and designated as “crude Vg.” The crude Vg solution was then fractionated by gel filtration using Ultrogel AcA 34 (Sigma, U8878) column (size 73 × 2.6 cm), which was previously equilibrated with the eluting buffer. Five-milliliter fractions were collected at the flow rate of 25 ml h−1. The absorbance of each fraction was read at 280 nm on a spectrophotometer (JASCO). Alkali-labile phosphorus (ALP), an index of Vg, was estimated in every alternate fraction as described by Nath and Sundararaj (1981) (data not shown here). Fractions of peak I (fraction nos. 12–22) had contained ALP (data not shown here) and hence pooled and dialyzed extensively against chilled 10-mM potassium phosphate buffer (pH 8). Protein was estimated by Lowry et al. (1951), and 30 mg of protein was loaded on the HA-Ultrogel (Sigma, H9890) column (12 × 1.5 cm) previously equilibrated with 10 mM phosphate buffer (pH 8) for adsorption chromatography. Bound proteins were eluted by increasing the concentration of phosphate buffer (50 and 100 mM, pH 8) in a stepwise manner. The protein eluted at 50 mM was referred as Vg1 and that eluted at 100 mM as Vg2.
The molecular masses of Vg1 and Vg2 were determined by native 4–15 % PAGE along with high molecular weight marker proteins (Pharmacia). The total phosphorus, ALP, lipid, and carbohydrate contents of Vg1 and Vg2 were estimated following the methods of Bartlett (1959), Nath and Sundararaj (1981), Folch et al. (1957), and Umbreit et al. (1958), respectively. In each determination, 5 mg each of Vg1 and Vg2 was used.
Preparation of specific antiserum against Vg1 and Vg2
Antibodies against Vg1 (a-Vg1) and Vg2 (a-Vg2) were raised in rabbits by intra-dermal injection at multiple sites with 1 mg of either Vg1 or Vg2 protein emulsified with Freund’s complete adjuvant (Sigma, USA). Rabbits were rechallenged after 15 days of the first immunization with 500 μg of respective Vg emulsified with Freund’s incomplete adjuvant. Booster injection of 100 μg either of Vg1 or Vg2 was given to respective rabbits after 3 weeks through an ear vein. Blood was collected from the ear vein 1 week after the booster and serum was separated by clotting the blood at 37 °C for 30 min and then kept at 4 °C overnight. Antiserum was collected by centrifugation at 2000×g for 10 min at 4 °C. Both a-Vg1 and a-Vg2 antisera were cross-reacted with 21-day post-hypophysectomized male catfish serum, Vg1, Vg2, and crude egg yolk extract in Ouchterlony immunodiffusion test (Ouchterlony 1953), as precipitin lines formed against each sample tested (data not shown). Therefore, preincubation of both antisera was performed separately with lyophilized 21-day post-hypophysectomized male catfish serum proteins (10 mg ml−1), incubated at 37 °C for 30 min and kept overnight at 4 °C followed by centrifugation at 2000×g. This resulted in removal of IgG directed to other proteins as revealed by Ouchterlony immunodiffusion test (data not shown here). Afterward, to avoid cross-reactions of antibodies that recognize common epitopes on different Vgs, each Vg antiserum was preincubated three times (1.5 mg ml−1 each time) with alternate Vgs (a-Vg1 with Vg2 and a-Vg2 with Vg1) in a similar manner as described above and the resulting harvested antisera (h-a-Vg1 and h-a-Vg2) were tested in the same condition as before. Thereafter, each harvested antiserum was kept separately in aliquots at −30 °C.
Electrophoresis
Native PAGE of plasma samples from control male, vitellogenic female, E2-treated male catfish, and the Vg protein of peak I (pooled fractions) after gel filtration was performed in 4–15 % gradient gel using electrophoresis buffer (0.025 M Tris, 0.192 M Glycine, pH 8.3). Sodium dodecyl sulfate (SDS)-PAGE (stacking gel 4 %, separating gel 12.5 %) was performed for purified Vg (Vg1 and Vg2) following the procedure of Laemmli (1970) under denatured and reduced (with β-mercaptoethanol) conditions. Gels were run at constant 70 V in cold (4 °C) and stained with Coomassie Brilliant Blue R250 (SRL, India) for protein. A separate native PAGE (4–15 %) of Vg protein (peak I) was performed as described above, and the gel was stained with Sudan Black B for lipid and with PAS for carbohydrate (data not shown).
Western blot analysis
Normal male catfish plasma and purified Vg1 and Vg2 were separated by Native PAGE (4–15 % gradient gels) and electroblotted separately onto a nitrocellulose membrane according to the method of Towbin et al. (1979). After electroblotting, the membrane was blocked with 3 % BSA and 5 % casein in Tris-buffered saline (TBS; 0.02 M Tris and 0.5 M NaCl, pH 7.5) for 3 h with constant shaking. Membranes were incubated with respective harvested primary antisera, h-a-Vg1 (1:5000) and h-a-Vg2 (1:5000) in TBS for 3 h followed by incubation with the secondary antibody [goat anti-rabbit IgG conjugated with horseradish peroxidase, PAP (Sigma, USA, lot 58H4337) diluted 1:4000] for 2 h at room temperature. After washing thrice with TBS containing 0.05 % Tween 20, the antigen–antibody reactions were visualized by DAB. Purified Vg1 and Vg2 resolved through SDS-PAGE, were subjected to Western blot analysis using h-a-Vg1 and h-a-Vg2, respectively, following the same protocol as described above (see Fig. 5).
ELISA for catfish Vgs (Vg1 and Vg2)
Competitive ELISAs for catfish Vg1 and Vg2 using their respective harvested antisera (h-a-Vg1 and h-a-Vg2) were developed following the procedure described by Maitra et al. (2007) for mrigal Vg. In brief, before developing the ELISA for each Vg, the optimal concentrations of Vg coating and dilution of antibodies for each Vg were determined by testing various dilutions (1:10,000; 1:25,000; 1:50,000; and 1:100,000) of antisera with different concentrations (50–200 ng well−1) of respective Vg coating (data not shown). Finally, for Vg1 ELISA, 200 ng well−1 of Vg1 coating and 1:10,000 dilution of h-a-Vg1 (O.D. range 1.0–1.3) and for Vg2 ELISA, 200 ng well−1 of Vg2 coating and 1:25,000 dilution of h-a-Vg2 (O.D. range 1.0–1.5) were chosen for making standard curve for each Vg. Thereafter, 96-well microtiter plates (Costar, Cambridge, MA, USA) were coated with 200 μl well−1 containing 1 μg ml−1 of either Vg1, Vg2, or BSA (non-specific binding) in 0.05 M sodium carbonate buffer, pH 9.6. Plates were incubated overnight at 4 °C. The non-specific binding sites were saturated by incubating the plates with 200 μl of 1 % BSA in PBS containing 0.05 % Tween 20 (PBS-T) for 1 h at room temperature (25 °C). Blocking solution was removed by washing the plates three times with PBS-T. Serially diluted Vg1 or Vg2 standard solution (100 μl) (1.56 to 1600 ng ml−1) or individual plasma samples (diluted 1:2 to 1:1000 in PBS-T–BSA) were mixed with equal volume (100 μl) of harvested antibodies, either h-a-Vg1 or h-a-Vg2, in separate tubes and incubated overnight at 4 °C. Wells were loaded with 200 μl of sample-primary antibody solution and incubated for 3 h at room temperature (25 °C). Standard and plasma samples were assayed in triplicate. After the plates were washed as above, each well received 200 μl of second antibody [goat anti-rabbit IgG conjugated with horseradish peroxidase, PAP (Sigma, USA, lot 58H4337] (1:4000 in PBS-T–BSA) was incubated for 2 h at room temperature and washed thrice with PBS-T. For color development, each well received 200 μl of freshly prepared coloring reagent containing 10 mg o-phenylenediamine (OPD) (Sigma, USA) in 25 ml of 0.1 M citrate–phosphate buffer, pH 5.0 and 3 μl of H2O2. After 30 min of incubation in the dark at room temperature, the reaction was stopped by adding 50 μl well−1 of 2 N H2SO4, and the absorbance was measured at 492 nm using an Anthos 2020 microplate reader (Anthos Labtec, Austria). Absorbance measurements were expressed by a non-linear model [(B i − NSB/B 0 − NSB) × 100 = f (log (dose or dilution))], where B i represented the binding of sample or standard Vg; B 0, the maximum binding and NSB, the non-specific binding. The analysis of competition curves was performed after linearization of the binding percentage values (B) through logit–log transformation [logit B = loge (B/100 − B)]. The parallelism between regression lines was tested by analysis of covariance (ANCOVA) following the method described by Snedecor and Cochran (1957). Parallelism of standard Vg preparations (Vg1 and Vg2) to their counterparts in circulation was demonstrated by serially diluting the plasma samples from vitellogenic female and E2-injected male. Cross-reactivity of h-a-Vg1 with Vg2 and h-a-Vg2 with Vg1 was estimated by running ELISAs three times. Within (intra) and between (inter) assays, coefficients of variation (CVs) were calculated from the estimated concentration of Vgs among all sample replicates and quality control means, respectively, using the following formula: %CV = (standard deviation of plate means (σ) / mean of plate means (μ)) × 100. Absorption values from reciprocal assay (Vg1 in Vg2 ELISA and vice versa) were converted to %B/B 0 and finally transformed to logit values for linearization as described above (Oubina et al. 1996).
E2 induction of Vg synthesis in male catfish
In the late preparatory period (April), male catfish were collected and acclimatized in the laboratory as described above. E2 dissolved in ethanol and brought to suspension in 0.64 % saline was injected at the dose level of 0.5 mg/kg body wt. into a group of four fishes on every alternate day for 10 days. Simultaneously, the control group received 0.64 % saline. The day following the last injection each fish was bled, plasma separated and kept in aliquots at −30 °C until use for estimation of Vg1 and Vg2 with respective ELISA.
Sampling of female catfish during different reproductive phases
Sexually mature female catfish were collected during different reproductive phases, preparatory (March), prespawning (June), spawning (August), and post-spawning (December) and sacrificed on the day of collection. At the time of sampling, each fish was weighed to the nearest gram, bled, and plasma separated as described above. Plasma samples were diluted 1:10 with PBS, divided into small aliquots and stored at −30 °C for estimation of Vg1 and Vg2 by respective ELISA. After blood collection fish were killed by decapitation, ovaries dissected out and weighed to the nearest mg. Gonadosomatic index (GSI) was calculated by using the following formula:
Ovaries were then fixed in Bouin’s solution for histological studies. Transverse sections of ovaries were cut at 6 μm and stained with Ehrlich’s hematoxylin and eosin. The different stages of oogenesis were characterized and identified in the ovaries of catfish following the method described by Ghosh and Nath (2005). There are three stages of oogenesis, stage I (SI), the non-yolky oocytes present throughout the year; stage II (SII), the vitellogenic oocytes characterized either by the appearance of cortical alveoli or yolk vesicles in the cytoplasm; and stage III (SIII), the fully formed yolky oocytes. The diameter of the yolky oocytes (SIII) during prespawning and spawning period was measured from the stained sections by using the Biowizard 4.2 software.
Expression of results and statistical analysis
For the comparison of data, the ovarian weight changes were expressed on 100 g body weight basis (GSI), diameter of yolky oocytes (SIII) as micrometer, and plasma levels of Vg1 and Vg2 as microgram per milliliter. P values between control and experimental groups were calculated either by Student’s t test (Snedecor and Cochran 1957) or by one-way ANOVA supplemented with Bonferroni’s test at 95 % confidence interval limit using SPSS v 10.0 software to ascertain the significance (P < 0.05) of difference between means.
Results
Purification and partial characterization of catfish vitellogenin
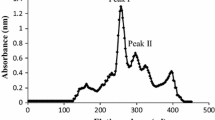
Figure 1a shows the elution profile of crude Vg proteins and control male plasma after gel filtration on Ultrogel AcA 34. Crude Vg produced a major protein peak (peak I) containing ALP (data not shown) eluted just after the void volume (fraction nos. 12–22), whereas no protein peak was eluted at the similar position of the control male plasma. Electrophoresis of plasma samples from control male, vitellogenic female, E2-treated male, and protein of peak I (pooled fractions 12–22) is presented in Fig. 2. In normal vitellogenic female plasma (lane 3), two protein bands (one was more prominent than the other) were present, but absent in normal male plasma (lane 2). These two protein bands were also present in E2-treated catfish plasma (lane 4) and in peak I (arrow heads, lane 5). These protein bands were also stained for carbohydrate and lipid (data not shown). When the Vg proteins of peak I were subjected to adsorption chromatography on HA-Ultrogel, two-bound protein peaks designated as Vg1 and Vg2 were eluted by 50 and 100 mM phosphate buffer (pH 8), respectively (Fig. 1b). As shown in Fig. 3, purified Vg1, Vg2, vitellogenic female plasma, hypophysectomized male catfish serum, and crude egg extract were subjected to double immunodiffusion test using harvested antisera to Vg1 (h-a-Vg1) and Vg2 (h-a-Vg2). Both Vg1 (well no. 4, Fig. 3a) and Vg2 (well no. 4, Fig. 3b) formed one precipitin line against their specific antisera. Crude egg extract (well no. 3, Fig. 3a, b) and vitellogenic female plasma (well no. 5, Fig. 3a, b) also formed precipitin lines against each antiserum, but no precipitin line was formed against hypophysectomized male serum (well no. 6) and alternate Vg (well no. 2). On native PAGE (4–15 %), both Vg1 and Vg2 resolved into single protein band in each case having an apparent molecular mass of 375 and 450 kDa, respectively (Fig. 4a). Besides, immunoblot analysis revealed specific immunodetection of two anti-Vg proteins with dissimilar molecular mass; Vg1 (lane 2′, Fig. 4b) and Vg2 (lane 3′, Fig. 4c) cross-reacted strongly with h-a-Vg1 and h-a-Vg2, respectively, but weekly to alternate harvested anti-serum (Fig. 4b, c). Apparently, h-a-Vg1 and h-a-Vg2 cross-reacted with alternate Vg because they recognize common or identical epitopes on the alternate Vg, albeit with differential intensity.
a Elution profile of crude Vg (55 % ammonium sulfate precipitated proteins of E2-treated male plasma) and control male plasma of catfish after gel filtration on Ultrogel AcA 34 column (73 × 2.6 cm). Five-milliliter fractions were collected in each tube with 0.05 M Tris–HCl buffer (pH 8) containing 2 % KCl at a flow rate of 25 ml h−1. The optical density of each fraction was monitored at 280 nm. Fractions (12–22) of peak I were pooled and used for adsorption chromatography. b Elution profile of 30 mg Vg protein (peak 1 of Ultrogel AcA 34) on HA-Ultrogel column (12 × 1.5 cm) equilibrated with 10 mM phosphate buffer, pH 8. Protein was eluted with 50 mM (Vg1) and 100 mM (Vg2) phosphate buffer, pH 8
Plasma samples of control male (3 μl, lane 2), vitellogenic female (2 μl, lane 3), E2-treated male (1 μl, lane 4) catfish, and eluted protein of peak I (10 μg, lane 5) were separated by 4–15 % native PAGE and stained for proteins with Coomassie Brilliant Blue R250. At extreme left (lane 1), migration position of high molecular weight marker proteins (Pharmacia) is shown. Arrowheads indicate migration position of putative Vgs
Precipitin reactions of hypophysectomized male catfish serum, vitellogenic female plasma, egg extract, and purified Vgs (Vg1 and Vg2) using harvested antisera (see the “Materials and methods” section) targeted to purified Vg1 (h-a-Vg1) (a) and Vg2 (h-a-Vg2) (b). Egg extract, vitellogenic female plasma, and hypophysectomized male catfish serum were added to wells 3, 5, and 6, respectively, while wells 1, 2, and 4 received h-a-Vg1, Vg2, and Vg1 in a and h-a-Vg2, Vg1, and Vg2 in b, respectively
Native PAGE (4–15 % gel) analysis of HMW markers (lane 1), Vg1 (10 μg, lane 2), and Vg2 (10 μg, lane 3) (a); Western blot analysis of male plasma (3 μl, lane MP′), purified Vg1 (10 μg, lane 2′), and Vg2 (10 μg, lane 3′) with harvested a-Vg1, h-a-Vg1 (b); and male plasma (3 μl, lane MP″), Vg1 (10 μg, lane 2″) and Vg2 (10 μg, lane 3″) with harvested a-Vg2, h-a-Vg2 (c). After the run, one set was stained for protein with Coomassie Brilliant Blue R250 (Fig. 2a) and those of b and c were electroblotted onto nitrocellulose membrane separately and Western blot analysis was performed as described in the text. Note that both Vg1 and Vg2 gave single peptide band, and in Western blot analysis, h-a-Vg1 strongly reacted with Vg1 and very poorly with Vg2, whereas, h-a-Vg2 strongly reacted with Vg2 and weakly with Vg1
These two purified Vgs were different in their carbohydrate, lipid, total phosphorus, and ALP content. The carbohydrate contents of Vg1 and Vg2 were 190.4 ± 19.3 and 320 ± 3.5 μg mg−1 protein, respectively. The total lipid content of Vg1 was 73.6 ± 1.2 μg mg−1 protein and 48.3 ± 2.03 μg mg−1 protein for Vg2 (P < 0.005). Total phosphorus and ALP contents of Vg1 were 7.67 ± 0.44 and 6.17 ± 0.44 μg mg−1 protein, whereas Vg2 contained 11 ± 0.58 and 10.2 ± 1.04 μg mg−1 protein, respectively.
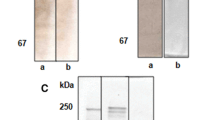
SDS-PAGE analysis under reducing condition (Fig. 5) revealed that both Vg1 (lane 1, Fig. 5a) and Vg2 (lane 3, Fig. 5b) resolved into two major bands of 95 and 67 kDa along with minor bands. Western blot analysis indicated that these two major bands were immunostained with h-a-Vg1 (lane 2, Fig. 5a) and h-a-Vg2 (lane 4, Fig. 5b) antiserum.
SDS-PAGE (0.1 %) analysis (stacking 4 %, resolving 12.5 %) of 20 μg each of purified Vg1 (a: lane 1) and Vg2 (b: lane 3) under denatured and reduced conditions and Western blot analysis of Vg1 with h-a-Vg1 (a: lane 2) and Vg2 with h-a-Vg2 (b: lane 4). Both Vgs resolved into two similar major bands (95 and 67 kDa) along with minor bands
ELISA for catfish Vg1 and Vg2
Competitive ELISAs for Vg1 and Vg2 were developed using their respective harvested antisera (h-a-Vg1/h-a-Vg2). In the condition used in the present study, the working ranges of ELISAs were 100–800 ng ml−1 using h-a-Vg1 for Vg1 (Fig. 6a) and 40–800 ng ml−1 using h-a-Vg2 for Vg2 (Fig. 6c). In the condition used in the present study, the assay produced consistently the routine calibration curve that ranges from 12.5 to 1600 ng/ml in diluted samples, with approximate linearity between 25 and 800 ng/ml (85–20 % binding). The specificity of Vg1 ELISA using h-a-Vg1 and Vg2 ELISA using h-a-Vg2 was monitored by comparing the slopes of six different standard curves in each assay (data not shown). A good parallelism among the standard curves was observed by ANCOVA (F obs = 0.33 < F 0.05 = 3.99 with d.f. 5, 35 for Vg1 and F obs = 0.15 < F 0.05 = 3.99 with d.f. 5, 35 for Vg2) by comparing the results from different plates. The intra- and inter-assay coefficients of variance (CV) were 1.7 (n = 6) and 2.41 (n = 7) for Vg1 ELISA and 1.28 (n = 6) and 2.0 (n = 6) for Vg2 ELISA, respectively. While Vg2 did not show any significant cross-reaction in Vg1 ELISA, Vg1 had ≤20 % cross-reaction in Vg2 ELISA (Fig. 6). Accordingly, for measuring Vg2 in plasma samples, values obtained from Vg2 standard curve was corrected by reducing 20 % to avoid over estimation. The ability of the antisera (h-a-Vg1 and h-a-Vg2) to recognize respective native Vg (Vg1 and Vg2) was assessed by comparing binding displacement curves for standard preparation and increasing serial dilutions (1:2 to 1:1000) of plasma from vitellogenic female, E2-treated male, and normal male catfish (Fig. 6b, d). Linearization of the displacement curves by logit/log transformation (Fig. 6a–d) revealed that serial dilutions of plasma from vitellogenic female (F obs = 1.43 < F 0.05 = 12.83 with d.f. 1, 11 in Vg1 ELISA and F obs = 13.4 < 14.69 with d.f. 1, 8 in Vg2 ELISA) and E2-treated male (F obs = 6.46 < F 0.05 = 12.83 with d.f. 1, 10 in Vg1 ELISA and F obs = 10.55 < 14.69 with d.f. 1, 8 in Vg2 ELISA) had slopes which were not statistically different from the standard preparation in both Vg ELISAs and thus demonstrates that the antibodies recognize in the same manner the purified and plasma Vgs. In contrast, plasma from normal male catfish did not show any significant displacement at dilutions 1:2 and above in each assay. For subsequent assay, plasma samples were diluted to at least 1:50 to avoid possible non-specific effects of other plasma proteins.
Homologous ELISA for catfish Vg1 and Vg2 using antisera preincubated with hypophysectomized catfish serum (s) and then harvested with alternate Vg (h). Logit–log transform of binding curves of Vg1 standard using (h-a-Vg1) and cross-reaction of Vg2 with h-a-Vg1 (a) serial dilutions of plasma of normal male, vitellogenic female, and E2-treated male catfish using h-a-Vg1 (b) and Vg2 standard using h-a-Vg2 and cross-reaction of Vg1 with h-a-Vg2 (c) and serial dilution of plasma of normal male, vitellogenic female, and E2-treated male catfish using h-a-Vg2 (d). All points are the mean of triplicate determinations
E2 induction of Vg synthesis in male catfish and seasonal changes in plasma Vgs and ovarian growth in female catfish
Both Vg1 and Vg2 ELISAs were verified by measuring both Vgs in plasma samples of E2-treated male catfish and females collected during different reproductive phases of its annual ovarian cycle (Figs. 7 and 8). Five injections of E2 on alternate days (Fig. 7) induced synthesis of both Vgs (Vg1 22,400 ± 7400 μg ml−1 and Vg2 4000 ± 1000 μg ml−1 plasma) in male catfish during late preparatory period (April). The plasma Vg1 level was significantly (P < 0.05) more than that of Vg2, and the ratio was about 5.6:1. In saline injected controls, both Vgs (Vg1 21 ± 4 μg ml−1) and (Vg2 16 ± 3 μg ml−1) were detected in small amount.
In the female catfish, during preparatory period (March), the concentrations of both Vgs were low (Vg1 32.8 ± 2.3 μg ml−1 and Vg2 0.6 ± 0.02 μg ml−1). During this period, the GSI was low (0.24 ± 0.11) and ovary contained mostly SI non yolky oocytes (diameter 51.8 ± 7.2 μm) and a few yolk vesicle stage (SII) (Fig. 9a). During the prespawning season (May), both Vg levels (Vg1 1370.4 ± 211.8 μg ml−1 and Vg2 657 ± 9.0 μg ml−1) increased significantly (P < 0.005) coinciding with a significant increase (P < 0.05) in GSI (9.2 ± 1.2) presumably due to the formation of vitellogenic (SII) and fully formed yolky oocytes (SIII) (average diameter of SIII oocyte 469 ± 62 μm) (Figs. 8 and 9b). The ratio of Vg1 to Vg2 was about 2:1. During the prespawning period, it is generally observed that vitellogenesis was completed in this catfish and then wait for spawning during July–August when there is heavy rainfall. In India, this catfish like other freshwater fishes breeds once a year during the summer monsoon (Ghosh and Nath 2005; Sundararaj and Vasal 1976 and references therein). Therefore, in the spawning period (Aug), GSI (5.78 ± 2.16) significantly (P < 0.05) reduced than that in the prespawning period. In this period, the ovary contained mostly SIII oocytes having the average diameter of 490 ± 24 μm (Figs. 8 and 9c). Plasma Vg1 (297.6 ± 58.4 μg ml−1) levels decreased significantly (P < 0.05) than the prespawning values, whereas a sharp increase (P < 0.005) in Vg2 concentrations (5859 ± 280 μg ml−1) was observed and the Vg1/Vg2 ratio was about 1:20. Thereafter, both Vg1 and Vg2 were not detectable in plasma, and ovary became regressed containing only SI oocytes (Fig. 9d) during post-spawning period (Dec).
Photomicrograph of transverse section of the ovary during different phases of the breeding cycle. a Preparatory period (March) showing SI non-yolky oocytes and yolk vesicle (YV) stage (SII) oocytes. b The presence of SII with cortical alveoli (CA) at the periphery of cytoplasm and fully form (SIII) yolky oocytes during prespawning period (May), c SIII oocytes during the spawning period (August), and d SI non-yolky oocytes during the post-spawning period (December) (200 μm)
Discussion
In the present study, we successfully isolated and purified two forms of Vg from the plasma of E2-treated catfish using a two-step procedure, which involved gel filtration on Ultrogel AcA-34 followed by adsorption chromatography on HA-Ultrogel. A similar procedure was used to purify two forms of Vg in medaka (Shimizu et al. 2002) and mrigal (Maitra et al. 2007). A double chromatography procedure was adopted to purify Vgs in many fish species (Takemura and Kim 2001; Hiramatsu et al. 2002a; Ohkubo et al. 2003; Roy et al. 2004; Fujiwara et al. 2005; Sawaguchi et al. 2005, 2006; Ndiaye et al. 2006; Braathen et al. 2009; see Maitra et al. 2007 for earlier references).
Both catfish Vgs (Vg1 and Vg2) satisfy the following characteristics of several criteria proposed for teleost Vg (see Mommsen and Walsh 1988; Specker and Sullivan 1994) for their identification as Vg. In this study, both Vgs were of high molecular masses, induced by E2 injections in male- and female-specific and contained lipid, carbohydrate, and phosphorus indicating glycolipophosphoprotein in nature. Further, antibodies raised against each Vg immunostained not only the respective Vg (see Fig. 4) but also the cross-reacted with crude egg yolk extract and the plasma from vitellogenic female and E2-treated male catfish in Ouchterlony immunodiffusion test (see Fig. 3).
Apparently, the native molecular mass of catfish Vg1 and Vg2 are 375 and 450 kDa, respectively, and are comparable to other teleost Vgs. Two Vgs identical to each other in their native forms were also purified in barfin flounder (Verasper moseri) (VgA 530 kDa, VgB 550 kDa) (Matsubara et al. 1999), white perch (M. americana) (Female-specific plasma protein: FSPP1 and FSPP2 532 kDa) (Hiramatsu et al. 2002a), and mrigal (C. mrigala) (HAI 500 kDa, HAII 550 kDa) (Maitra et al. 2007). Two Vgs with similar molecular weights were also reported in mummichog, Fundulus heteroclitus (LaFleur et al. 1995a; LaFleur et al. 1995b); haddock, M. aeglefinus (Reith et al. 2001); and mosquito fish, G. affinis (Sawaguchi et al. 2005). In contrast, two heteromolecular forms of Vg in native and denatured and reduced conditions have been reported in tilapia species (Ding et al. 1989; Kishida and Specker 1993, Takemura and Kim 2001; Buerano et al. 1995), medaka (Shimizu et al. 2002) and Japanese common goby (Ohkubo et al. 2003). Based on isoelectric focusing and other biochemical analyses, fish Vg has been demonstrated as charged heterogeneity in murrel, Channa punctatus (Sehgal and Goswami 2005) and redhorse (Maltais and Roy 2009).
In this study, SDS-PAGE of Vg1 and Vg2 under reducing conditions resolved into two major protein bands and a number of minor bands in each case, of which 95 and 67 kDa bands have been considered as major Vg subunits (see Fig. 5). The resulting multiple bands may be due to dissociation of major Vgs into their parent subunits or due to proteolytic cleavage facilitated by SDS-induced unfolding of protein chains as reported earlier in other fish species (Roy et al. 2004; Maitra et al. 2007 and the references therein). The apparent molecular masses of major Vg subunits (95 and 67 kDa) in catfish are identical to Vg monomers (97 and 67 kDa) in Asian catfish (Garnayak et al. 2013) and also comparable to mrigal Vgs (HAI 75 kDa, HAII 85 kDa) earlier reported by us (Maitra et al. 2007). However, catfish Vg subunits are significantly smaller than monomeric Vg ranging from 130 to 180 kDa, reported in other fish species (Shimizu et al. 2002; Hiramatsu et al. 2002a; Sun, Pankhurst, and Watts 2003; Roy et al. 2004 and the references therein), suggesting that fish Vgs are heterogeneous in nature (see Sawaguchi et al. 2005, 2006).
In addition to variation in their native but not subunit molecular mass, Vg1 and Vg2 differ considerably with respect to lipid, carbohydrate and phosphorus content. Compared to Vg2, Vg1 is lipid rich but less phosphorylated. Further, the phosphorus contents of Vg1 and Vg2 either in lipidated (total phosphorus) or in delipidated (ALP) conditions are almost similar, indicating that most of the phosphorus is protein bound and not associated with phospholipids. Compared to earlier reports (Silversand and Haux, 1995; Fremont and Riazi 1988; Maitra et al. 2007), present data demonstrate that catfish Vgs are apparently poor in lipid content but rich in degree of glycosylation. Such high carbohydrate content of both Vg1 and Vg2 is being reported for the first time and requires further investigation in future.
Ouchterlony double immune diffusion analysis demonstrate that both h-a-Vg1 and h-a-Vg2 formed single precipitin line each against purified Vg1 and Vg2, respectively, but not the other form of Vg indicating that both purified Vgs are immunologically distinct. While precipitin line obtained with Vg2 revealed partial identity (spur formation) to crude egg extract and vitellogenic female plasma (Fig. 3b), Vg1 showed partial identity to egg extract but not female plasma (possibly due to unequal synthesis of two Vgs in plasma; Vg1 > > Vg2) indicating that both purified Vgs are immunologically distinct, present in circulation of females undergoing vitellogenesis, and contribute to the formation of yolk. However, in Western blot analysis, each Vg-specific harvested antibody recognized faintly the alternate Vg indicating that the preincubation with alternate Vg might not be 100 % efficient to remove all antibodies directed against common epitopes. Previously, two immunologically different Vg molecules have been described in tilapia, Oreochromis niloticus (Chan et al. 1991; Buerano et al. 1995); nonetheless, Ndiaye et al. (2006) reported in O. niloticus that polyclonal antibodies raised against VtG1 and VtG2 recognized both VtGs because the antisera were not preincubated with alternate VtG. Therefore, our results along with those of Ndiaye et al. (2006) suggest that both Vgs may share some common epitopes. Teleost Vgs have been classified into two groups, VgA and VgB, based on similarities in their primary structures, constituent yolk protein domains, and physiological functions (Hiramatsu et al. 2002b; Sawaguchi et al. 2006); presently, no such data is available for catfish Vgs, restricting us to classify catfish Vg1 and Vg2 further. Interestingly, Garnayak et al. (2013) recently reported identification and purification of a single Vg with a native molecular mass of 482 kDa from the plasma of E2-treated catfish. However, based on variation in relative molecular mass of native Vg1 and Vg2, subunit molecular mass, distinct antigenicity, and above all the presence of both in the vitellogenic female plasma and their participation in the formation of yolk proteins catfish Vg1 and Vg2 are, thus, considered as separate catfish Vgs and not the degradation product of any of the purified Vgs.
The focus of our research was to develop separate ELISA for each Vg so as to quantify Vg1 and Vg2 in the plasma of female catfish at different physiological status to know whether these two Vgs are produced in females in relation to the ovarian growth and after E2 treatment in male catfish. The results obtained with respect to working ranges of standard curves in both Vg1 and Vg2 ELISAs clearly indicate that after preincubation of each Vg-antisera with alternate Vg the working ranges of each assay reduced (see Fig. 6), which further suggest that both Vgs share some common epitopes. Data of the present study demonstrate that while Vg1 ELISA is relatively free from significant Vg2 cross-reaction, Vg1 shows ≤20 % cross-reaction in Vg2 ELISA requiring a correction by 20 % to avoid over-estimation of Vg2 in plasma samples. Given that Vg1 concentration (either negligible in amount or moderate or very high) may have significant bias, especially when the Vg1 displacement is not parallel to the Vg2 standard, application of 20 % correction to all such cases may have its own limitation. Even though we concede to such inherent limitation of multiple Vg ELISAs with overlapping immunoreactivity, we believe that repeated absorption of h-a-Vg2 with Vg1 resulting in ≤20 % cross-reaction that too non-parallel to the standard and application of correction factor might have allowed us to avoid the risk of overestimation and helped us to reach the most realistic values under present situation. Currently, work is in progress to identify unique domains and to raise specific antisera to each of Vg1 and Vg2. ELISAs for either one or two forms of Vg have been developed earlier to establish a correlation between the plasma levels of Vg and ovarian growth in many fish species (Takemura and Kim 2001; Ohkubo et al. 2003; Ndiaye et al. 2006; Maitra et al. 2007; see Specker and Sullivan 1994 for earlier references). The working ranges of both catfish Vg ELISAs (25–800 ng ml−1) are comparable to those reported in other fish species (Takemura and Kim 2001; Ohkubo et al. 2003; Maitra et al. 2007 and the references therein). The parallelism observed between each dilution curve of purified Vg and plasma dilution curves of vitellogenic females and E2-treated males in both ELISAs demonstrated that each antibody recognized corresponding circulating Vg in a similar fashion and did not show any significant cross-reaction with the plasma of normal male. However, the low levels of Vg1 (21 ± 4 μg ml−1) and Vg2 (16 ± 3 μg ml−1) detected in males during late preparatory period (April) may be non-specific as demonstrated in Vg ELISAs of other fish species (Bon et al. 1997; Roy et al. 2004 and references therein). The other possible reason for anti-Vg antisera cross-reacted with non-Vg plasma protein may be due to the presence of very low-density lipoprotein that resemble Vg as suggested by many other authors (see Roy et al. 2004 and references therein). Another possibility is that since fish were fed with vegetable based diet and it may contain phytoestrogen, which can stimulate Vg synthesis as reported in teleost (Hiramatsu et al. 2005).
Using both Vg ELISAs, we measured the plasma concentrations of two Vgs in E2-treated male catfish and field-collected females in relation to ovarian growth during different reproductive phases of its annual breeding cycle. In females, the concentrations of Vg1 and Vg2 showed a correlation with the ovarian development. During preparatory period, both Vg1 and Vg2 appeared in the plasma but the concentrations of Vg1 were higher than that of Vg2, which leads to the formation of stage II oocytes (early vitellogenic stage). In the prespawning period (May–June), there was a rapid conversion of SII to SIII (late vitellogenic stage) oocytes along with a high plasma Vg1 levels (∼1.3 mg ml−1). This concentration of Vg1 in plasma was very similar to those reported in other fishes by many investigators (Masconi et al. 1998; Lomax et al. 1998; Ohkubo et al. 2003; Maitra et al. 2007). In contrast, plasma Vg2 concentrations (∼0.66 mg ml−1) were significantly increased during prespawning period compared to those of preparatory period but were lower than Vg1 concentrations (Vg1/Vg2 was 2:1). A similar situation was also observed when male catfish were injected with E2 (Vg1/Vg2 was 5.6:1). The present findings are in accordance with those reported in other fish species (Kishida and Specker 1993; Takemura and Kim 2001; Ohkubo et al. 2003; Fujiwara et al. 2005). During the spawning period, the GSI reduced significantly with a simultaneous decrease in the concentration of Vg1 which suggests that no further formation of yolky oocytes occurs in this period. Interestingly, there was a tremendous rise in plasma Vg2 concentrations (∼6 mg ml−1), and the ratio of Vg1 to Vg2 was about 1:20, which indicates a phase shift in the synthesis of Vg that may be necessary to facilitate some other physiological functions. Further research is necessary to clarify the regulation of synthesis of two Vgs in the catfish. During the post-spawning period, the GSI reduced sharply and ovary became regressed containing only oogonia and SI oocytes. Vg1 and Vg2 were not detectable in plasma, which supports the observations made in many other fishes (Sundararaj and Vasal 1976; van Bohemen et al. 1981; Maitra et al. 2007).
As noted above, it is clear that a dual Vg system exists in the catfish and their proportional concentrations in the plasma differ during different reproductive phases of the annual breeding cycle. This is in accordance with the findings reported for tilapia, Japanese common goby and mosquito fish (Kishida and Specker 1993; Ohkubo et al. 2003; Sawaguchi et al. 2005). Similar multiple Vg system has been demonstrated in many marine and brackish water teleosts emphasizing their specific physiological roles during oogenesis (Matsubara et al. 1999; Reith et al. 2001; Hiramatsu et al. 2002a, b; Sawaguchi et al. 2005, 2006). In contrast, although dual or multiple Vg system has been demonstrated in fish spawning demersal (adhesive) eggs (Shimizu et al. 2002; Sawaguchi et al. 2005), there is no such information regarding their specific individual physiological role during reproduction. The catfish C. batrachus spawns demersal eggs and as noted above, disproportionate quantities of Vg1 and Vg2 in plasma of female catfish during prespawning and spawning periods may possibly suggest that Vg1 and not Vg2 is the major Vg that contributes in the formation of yolkly oocytes. Unequal accumulation of different forms of Vg by growing oocytes has been reported in some fish species during vitellogenesis and post vitellogenesis, which is regulated by mechanisms for receptor mediated uptake of Vg from circulation (Matsubara et al. 1999; Lafleur et al. 2005; Amano et al. 2008; Sawaguchi et al. 2008; see Reading et al. 2011 and references therein).
The tremendous rise in Vg2 concentrations in plasma as noted in the present study can possibly be explained in the following way. One possible reason is that vitellogenesis generally completes with the formation of yolky oocytes at the end of the prespawning period (i. e., June), and hence, no more Vg will be internalized into oocytes and there may be a phase shift in the synthesis of Vg during spawning period causing an increase in the concentrations of Vg2. This increase in Vg2 levels may possibly provide signal in an unknown manner to facilitate maturation, ovulation and spawning of oocytes because of the fact that luteinizing hormone (LH) (maturational hormone) peak occurs in plasma after the Vg2 peak (unpublished data). Further, in vitro culture of fully formed yolky oocytes (SIII) with purified Vg resulted in the germinal vesicle breakdown (GVBD) of oocytes (Bhattacharya and Nath in preparation). However, to confirm the above explanation and assign different physiological roles for catfish Vg1 and Vg2 further research is necessary to identify the specific yolk protein products of each Vg and discover their proteolytic fate and utilization during oocyte maturation and subsequent development of embryos.
In conclusion, our results demonstrate that (i) two forms of Vg (Vg1 and Vg2) were purified from the plasma of E2-treated catfish and development of ELISA for each Vg using their respective harvested antibodies to quantify respective Vg in the catfish. (ii) A correlation has been demonstrated between the plasma Vg (Vg1/Vg2) concentrations and the ovarian growth during different reproductive phases of the annual breeding cycle of the catfish, and a possible phase shift in the synthesis of Vg1 and Vg2 was reflected during the cycle. (iii) The high plasma concentrations of Vg2 during the spawning period may possibly speak about its other physiological role besides its contribution to the formation of yolk, which can be confirmed only when the different yolk protein products derived from both Vgs will be analyzed during vitellogenesis, oocyte maturation and embryonic development.
References
Amano H, Fujita T, Hiramatsu N, Kagawa H, Matsubara T, Sullivan CV, Hara A (2008) Multiple vitellogenin-derived yolk proteins in gray mullet (Mugil cephalus): disparate proteolytic patterns associated with ovarian follicle maturation. Mol Reprod Dev 75:1307–1317
Bartlett GR (1959) Phosphorous assay in column chromatography. J Biol Chem 234:466–468
Bon E, Barbe U, Nunez-Rodriguez J, Cuisset B, Pellissero C, Sumpter JP, Lee Menn F (1997) Plasma vitellogenin levels during the annual reproductive cycle of female rainbow trout, (Oncorhynchus mykiss): establishment and validation of an ELISA. Comp Biochem Physiol 117B:75–84
Braathen M, Mdegela RH, Correia D, Rundberget T, Myburgh J, Botha C, Skaare JU, Sandvik M (2009) Vitellogenin in African sharptooth catfish (Clarias gariepinus): purification, characterization, and ELISA development. J Toxicol Environ Health, Part A 72:173–183
Buerano CC, Inaba K, Natividad FF, Morisawa M (1995) Vitellogenins of Oreochromis niloticus: identification, isolation, and biochemical and immunochemical characterization. J Exp Zool 273:59–69
Chan SL, Tan C, Pang MK, Lam TJ (1991) Vitellogenin purification and development of an assay for vitellogenin receptor in oocyte membranes of the tilapia Oreochromis niloticus, Linnaeus 1766. J Exp Zool 257:96–109
Debnath S (2011) Clarias batrachus, the medicinal fish: an excellent candidate for aquaculture & employment generation. International Conference on Asia Agriculture and Animal, IPCBEE, IACSIT Press, Singapoore vol. 13
Ding JL, Hee PL, Lam TJ (1989) Two forms of vitellogenin in the plasma and gonads of male Oreochromis aureus. Comp Biochem Physiol 93B:363–370
Folch J, Lees M, Stanley GSH (1957) A simple method for the isolation and purification of total lipid from animal tissue. J Biol Chem 226:497–509
Finn RN, Kristoffersen BA (2007) Vertebrate vitellogenin gene duplication in relation to the “3R hypothesis”: correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS One 2(1):e169. doi:10.1371/journal.pone.0000169
Fremont L, Riazi A (1988) Biochemical analysis of vitellogenin from rainbow trout (Salmo gairdneri): fatty acid composition of phospholipids. Reprod Nutr Develop 28(4A):939–952
Fujiwara Y, Fukada H, Shimizu M, Hara A (2005) Gilthead seabream (Sparus aurata) vitellogenin: purification, partial characterization, and validation of an enzyme-linked immunosorbent assay (ELISA). Gen Comp Endocrinol 143:267–277
Garnayak SK, Mohanty J, Rao TV, Sahoo SK, Sahoo SK (2013) Vitellogenin in Asian catfish, Clarias batrachus: purification, partial characterization and quantification during the reproductive cycle by ELISA. Aquaculture 392-395:148–155
Ghosh J, Nath P (2005) Seasonal effects of melatonin on ovary and plasma gonadotropin and vitellogenin levels in intact and pinealectomized catfish, Clarias batrachus (Linn). Indian J Exp Biol 43:224–232
Hiramatsu N, Cheek AO, Sullivan CV, Matsubara T, Hara A (2005) Vitellogenesis and endocrine disruption. In: Mommsen TP, Moon T (eds) Biochemistry and molecular biology of fishes. Elsevier Science Press, Amsterdam, pp. 431–471
Hiramatsu N, Matsubara T, Hara A, Donato DM, Hiramatsu K, Denslow ND, Sullivan CV (2002a) Identification, purification and classification of multiple forms of vitellogenin from white perch (Morone americana). Fish Physiol Biochem 26:355–370
Hiramatsu N, Matsubara T, Weber GM, Sullivan CV, Hara A (2002b) Vitellogenesis in aquatic animals. Fish Sci 68:694–699
Kishida M, Specker JL (1993) Vitellogenin in tilapia (Oreochromis mossambicus): induction of two forms by estradiol, quantification in the plasma and characterization in oocyte extract. Fish Physiol Biochem 12:171–182
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
LaFleur GJ Jr, Byrne BM, Kanungo J, Nelson L, Greenberg RM, Wallace RA (1995b) Fundulus heteroclitus vitellogenin: the deduced primary structure of a piscine precursor to noncrystalline, liquid-phase yolk protein. J Mol Evolution 41:505–521
LaFleur GJ Jr, Byrne BM, Haux C, Greenburg RM, Wallace RA (1995a) Liver-derived cDNAs: vitellogenins and vitelline envelope protein precursors (choriogenins). In: Goetz WF, Thomas P (eds) Reproductive physiology of fish. The University of Texas at Austin, Texas, pp. 505–521
LaFleur GJ Jr, Raldua D, Fabra M, Carnevali O, Denslow N, Wallace RA, Cerda J (2005) Derivation of major yolk proteins from parental vitellogenins and alternative processing during oocyte maturation in Fundulus heteroclitus. Biol Reprod 73:815–824
Lomax DP, Roubal WT, Moore JD, Johnson LL (1998) An enzyme-linked immunosorbent assay (ELISA) for measuring vitellogenin in English sole (Pleuronectes vetulus): development, validation and cross-reactivity with other pleuronectids. Comp Biochem Physiol 121B:425–436
Lowry OH, Rosebrough NJ, Farr AL, Randel RJ (1951) Protein measurement with the Folin reagent. J Biol Chem 193:265–272
Maitra S, Sahu R, Trehan N, Garg SK, Nath P (2007) Circannual variation in plasma vitellogenin and gonadotropin II levels in relation to annual ovarian cycle in female mrigal, Cirrhinus mrigala. Aquaculture 265:370–384
Maltais D, Roy RL (2009) Purification and partial characterization of vitellogenin from shorthead redhorse (Moxostoma macrolepidotum) and copper redhorse (Moxostoma hubbsi) and detection in plasma and mucus with a heterologous antibody. Fish Physiol Biochem 35:241–254
Masconi G, Carnevali O, Carletta R, Nabissi M, Polzonetti-Magni AM (1998) Gilthead seabream (Sparus aurata) vitellogenin: purification, partial characterization, and validation of an enzyme-linked immunosorbent assay (ELISA). Gen Comp Endocrinol 110:252–261
Matsubara T, Nagae M, Ohkubo N, Andoh T, Sawaguchi S, Hiramatsu N, Sullivan CV, Hara A (2003) Multiple vitellogenins and their unique roles in marine teleosts. Fish Physiol Biochem 28:295–299
Matsubara T, Ohkubo N, Andoh T, Sullivan CV, Hara A (1999) Two form of vitellogenin yielding two distinct lipovitellins, play vital roles during oocyte maturation early development of barfin flounder, Verasper moseri, a marine teleost that spawns pelagic eggs. Dev Biol 213:18–32
Mommsen TP, Walsh PJ (1988) Vitellogenesis and oocyte assembly. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic, New York, pp. 347–406
Nath P (1999) Some aspects of teleost vitellogenesis. In: Saksena DN (ed) Ichthyology: recent advances. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, pp. 249–259
Nath P, Maitra S (2001) Role of two plasma vitellogenins from Indian major carp (Cirrhinus mrigala) in catfish (Clarias batrachus) vitellogenesis. Gen Comp Endocrinol 124:30–44
Nath P, Sundararaj BI (1981) Isolation and identification of female specific serum lipophosphoprotein (vitellogenin) in the catfish, Heteropneustes fossilis (Bloch). Gen Comp Endocrinol 43:184–190
Nath P, Sahu R, Sk K, Bhattacharya D (2007) Vitellogenesis with special emphasis on Indian fishes. Fish Physiol Biochem 33:359–366
Ndiaye P, Forgue J, Lamothe V, Cauty C, Tacon P, Lafon P, Davail B, Fostier A, Le Menn F, Nunez J (2006) Tilapia (Oreochromis niloticus) vitellogenins: development of homologous and heterologous ELISAs and analysis of vitellogenin pathway through the ovarian follicle. J Exp Zool 305A:576–593
Ohkubo N, Mochida K, Adachi S, Hara A, Hotta K, Nakamura Y, Matsubara T (2003) Development of enzyme-linked immunosorbent assays for two forms of vitellogenin in Japanese common goby (Acanthogobius flavimanus). Gen Comp Endocrinol 131:353–364
Oubina A, Gascon J, Barcelo D (1996) Determination of the cross-reactivities of immunoassays: effect of common cross-reactants for chlorpyrifos-ethyl in water matrices using magnetic particle-based ELISA. Environ Sci Technol 30:513–516
Ouchterlony O (1953) Antigen-antibody reaction in gels IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand 32:231–240
Patino R, Sullivan CV (2002) Ovarian follicle growth, maturation and ovulation in teleost fish. Fish Physiol Biochem 26:57–70
Reading BJ, Hiramatsu N, Sullivan CV (2011) Disparate binding of three types of vitellogenin to multiple forms of vitellogenin receptor in white perch. Biol Reprod 84:392–399
Reith M, Munholland J, Kelly J, Finn RN, Fyhn HJ (2001) Lipovitellins derived from two forms of vitellogenin are differentially processed during oocyte maturation in haddock (Melanogrammus aeglefinus). J Exp Zool 291:58–67
Roy RL, Morin Y, Courtenay SC, Robichaud P (2004) Purification of vitellogenin from smooth flounder (Pleuronectes putnami) and measurement in plasma by homologous ELISA. Comp Biochem Physiol 139B:235–244
Sawaguchi S, Kagawa H, Ohkubo N, Hiramatsu N, Sullivan CV, Matsubara T (2006) Molecular characterization of three forms of vitellogenin and their yolk protein products during oocyte growth and maturation in red seabream (Pagrus major), a marine teleost spawning pelagic eggs. Mol Reprod Dev 73:719–736
Sawaguchi S, Koya Y, Yoshizaki N, Ohkubo N, Andoh T, Hiramatsu N, Sullivan CV, Hara A, Matsubara T (2005) Multiple vitellogenins (Vgs) in mosquitofish (Gambusia affinis): identification and characterization of three functional vg genes and their circulating and yolk protein products. Biol Reprod 72:1045–1060
Sawaguchi S, Ohkubo N, Amano H, Hiramatsu N, Hara A, Sullivan CV, Matsubara T (2008) Controlled accumulation of multiple vitellogenins into oocytes during vitellogenesis in the barfin flounder, Verasper moseri. Cybium: Int J Ichthyol 32(suppl 2):262
Sehgal N, Goswami SV (2005) Vitellogenin exists as charge isomers in the Indian freshwater murrel, Channa punctatus (Bloch). Gen Comp Endocrinol 141:12–21
Selman K, Wallace RA (1989) Cellular aspects of oocyte growth in teleosts. Zool Sci Tokyo 6:211–231
Shimizu M, Fujiwara Y, Fukada H, Hara A (2002) Purification and identification of a second form of vitellogenin from ascites of medaka (Oryzias latipes) treated with estrogen. J Exp Zool 293:726–735
Silversand C, Haux C (1995) Fatty acid composition of vitellogenin from four teleost species. J Comp Physiol B 164:593–599
Snedecor W, Cochran G (1957) Statistical methods. Iowa University Press, Ames, IA, pp. 481–488
Specker JL, Sullivan CV (1994) Vitellogenesis in fish: status and perspectives. In: Davey KG, Peter RE, Tobe SS (eds) Perspectives in comparative endocrinology. National Research Council of Canada, Ottawa, pp. 304–315
Sun B, Pankhurst NW, Watts M (2003) Development of an enzyme-linked immunosorbent assay (ELISA) for vitellogenin measurement in greenback flounder. J Fish Biol 64:1399–1412
Sundararaj BI, Vasal S (1976) Photoperiod and temperature control in the regulation of reproduction in the female catfish, Heteropneustes fossilis. J Fish Res Board Can 33:959–973
Takemura A, Kim BH (2001) Effects of estradiol-17β treatment on in vitro and in vivo synthesis of two distinct vitellogenins in tilapia. Comp Biochem Physiol 129A:641–651
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. PNAS. U S A 76:4350–4354
Umbreit WN, Burris RH, Stauffer JF (1958) Manometric techniques, 3rd edn. Burgess Publishing Company, Minneapolis, pp. 272–279
van Bohemen CG, Lambert JGD, Peute J (1981) Annual changes in plasma and liver in relation to vitellogenesis in the female rainbow trout, Salmo gairdneri. Gen Comp Endocrinol 44:94–107
Wallace RA, Selman K (1981) Cellular dynamic aspect of oocyte growth in teleosts. Amer Zool 21:325–343
Wang H, Yan T, Tan JTT, Gong (2000) A zebrafish vitellogenin gene (vg3) encodes a novel vitellogenin without a phosvitin domain and may represent a primitive vertebrate vitellogenin gene. Gene 256:303–310
Acknowledgment
This research was supported in part by grants from ICAR (F. No. 4(20)2002-ASR-I) and DST (F. No. SR/SO/AS-31/2003), New Delhi sanctioned to Dr. P. Nath and by the UGC sponsored CAS program to Department of Zoology, Visva-Bharati University, Santiniketan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments conducted on the live fish were performed following all the rules and regulations of the Institutional Animal Ethics Committee of Visva-Bharati University.
Rights and permissions
About this article
Cite this article
Mahapatra, S., Kabita, S., Bhattacharya, D. et al. Purification and development of ELISAs for two forms of vitellogenin in Indian walking catfish, Clarias batrachus (L.). Fish Physiol Biochem 43, 477–491 (2017). https://doi.org/10.1007/s10695-016-0304-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0304-5