Abstract
The eggs of the herbivorous false spider mite Brevipalpus obovatus Donnadieu have a longer incubation period than those of spider mites and are not protected by webs. Brevipalpus obovatus often lays its eggs in the gaps among the hairs on host leaves. We examined the effects of stellate hairs of Viburnum erosum var. punctatum (VEP) leaves on the survival of B. obovatus eggs. Adult B. obovatus and Phytoseius nipponicus Ehara, a generalist predator, were introduced to VEP leaf disks; each B. obovatus egg was inspected daily until hatching. More eggs (63 vs. 42 %) survived on the abaxial surfaces of VEP leaves, where the stellate hairs are more complicated, than on the adaxial surfaces. Predation hazard decreased rapidly with increasing egg age and a substantial portion of the eggs hatched. Phytoseius nipponicus preyed on eggs regardless of egg age when mixed-age eggs were provided. Manipulative experiments with bent stellate hairs showed that the normal hairs reduced the predation risk of B. obovatus eggs by P. nipponicus. Therefore, the predation hazard was considered to decrease since the stellate hairs hindered the search for B. obovatus eggs by the phytoseiid mite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many mites preferentially inhabit lower leaf surfaces (Sudo and Osakabe 2011), where they are sheltered from harsh environments which they experience on upper leaf surfaces including solar ultraviolet-B (UVB) radiation (Ohtsuka and Osakabe 2009; Sakai et al. 2012; Tachi and Osakabe 2012). In contrast, a certain mite species such as an herbivorous tenuipalpid mite, Brevipalpus obovatus Donnadieu (Acari, Tenuipalpidae), exploit not only lower but also upper leaf surfaces (Sudo and Osakabe 2011). A proximal factor causing upper leaf surface use may be tolerance to UVB damage (Fukaya et al. in press). On the other hand, influence of a generalist phytoseiid predator Phytoseius nipponicus Ehara (Acari, Phytoseiidae) on oviposition site choice of B. obovatus females was indicated in our previous study (Sudo and Osakabe in press). Therefore, an issue of the possibility of habitat differentiation in adaxial–abaxial leaf surface distribution in mite taxa and its cost and benefit, especially in relation with their predators and solar UVB radiation, attracts us. However, how the habitat heterogeneity within a single leaf [i.e., upper and lower sides (or adaxial and abaxial surfaces)] affects the prey–predator interaction in foliar mite community is largely unknown.
Common plant leaf microstructures, such as non-glandular trichomes, appear to increase the diversity and abundance of foliar arthropod communities (Johnson 1975; Karban et al. 1995; Lill et al. 2006; Walter 1996). For oviparous animals that do not care for their eggs, the choice of an oviposition site that hides their eggs from predators may be important for reproductive success. Mites that inhabit the leaf surfaces of terrestrial plants frequently lay eggs alongside leaf surface microstructures, such as leaf pubescences or trichomes (Jeppson 1975; Walter 1996; McMurtry and Croft 1997; Walter and Proctor 1999). Unlike glandular trichomes that simultaneously function as a mean of chemical defense against herbivores, non-glandular hairs on leaves solely impede or delay the walking or prey searching by small arthropod predators (Shah 1982; van Haren et al. 1987; Krips et al. 1999; Tian et al. 2012), thereby decreasing predation hazard (predation rate per unit time). Consequently, oviposition alongside leaf surface microstructures appears to be a behavior by which prey species avoid predators (Krips et al. 1999; Roda et al. 2000, 2001).
Conversely, microstructures such as dense, non-glandular trichomes also protect the eggs and larvae of phytoseiid mites (Acari: Phytoseiidae), primary predators of mites and other small arthropods, from intra-guild predation and damage by low humidity (Roda et al. 2000). In addition, trichomes trap air-borne pollen and fungal spores, which are potential alternative foods for generalist phytoseiid mites (van Rijn and Tanigoshi 1999; Kreiter et al. 2002; Roda et al. 2003; Duso et al. 2004; Ferreira et al. 2010; Pina et al. 2012). Therefore, plants with rich leaf surface microstructures inevitably harbor more phytoseiid mites than plants without them (Karban et al. 1995; O’Dowd and Willson 1997; Duso and Vettorazzo 1999; Loughner et al. 2008; Sudo et al. 2010). Moreover, such microstructures are generally more abundant on abaxial leaf surfaces than on adaxial leaf surfaces. Consequently, the abaxial surfaces of microstructure-rich leaves of host plants may increase the abundance of sympatric predators of mite herbivores, while decreasing the predation risk in herbivores by the hindrance of walking and/or prey searching of the predators.
Some spider mite species have specific mechanisms for protecting their eggs from predators, such as spinning threads to construct complicated webs (e.g., Tetranychus spp.; Saito 1985) or constructing web nests and providing maternal care (e.g., Schizotetranychus celarius [Banks]; Saito 1986). Brevipalpus obovatus lacks such protection for its eggs, i.e., it produces no webbing and does not provide maternal care. Its egg stage lasts 9–10 days at 25 °C (Goyal et al. 1985; Ehara and Gotoh 2009), which is relatively long compared to spider mites.
In Kyoto, west-central Japan, B. obovatus occurs on the deciduous shrub Viburnum erosum Thunb. var. punctatum Franch. et Sav. (VEP) from late summer to mid-autumn (i.e., late August–October) during which time they coexist with phytoseiid mites, mainly Phytoseius spp. (Sudo et al. 2010). VEP leaves have non-glandular stellate hairs, and B. obovatus often lays its eggs in gaps among the hairs (Jeppson 1975), on both the adaxial and abaxial leaf surfaces (Sudo and Osakabe 2011). If the stellate hairs and adverse environment of the adaxial (upper) leaf surfaces, such as solar ultraviolet radiation (Ohtsuka and Osakabe 2009; Onzo et al. 2010), hinder predators from accessing prey eggs, B. obovatus eggs might be protected from predation via the mother’s oviposition site choice. A recent study revealed a generalist phytoseiid predator Phytoseius nipponicus Ehara (Acari, Phytoseiidae) preferred to stay on the lower side of a VEP leaf and B. obovatus eggs laid on the leaf upper side were preyed by P. nipponicus less frequently than on the lower side (Sudo and Osakabe in press). On the other hand, it is not clear whether ovipositing among hairs or on adaxial leaf surfaces is advantageous to B. obovatus from the perspective of decreasing predation risk. We investigated this possibility based on sequential observations of egg production by B. obovatus and predation of the eggs by P. nipponicus on the adaxial and abaxial surfaces of VEP leaves.
Materials and methods
Mites
Brevipalpus obovatus was collected from VEP leaves in September 2009 in a secondary broadleaf forest in Iwakura, on the outskirts of Kyoto, Japan (35°5′28″N, 135°46′42″E, 150–160 m a.s.l.). The mites were reared on VEP leaf disks (abaxial surfaces) placed on water-soaked cotton in Petri dishes in a laboratory at 25 °C and a 16-h light (L):8-h dark (D) cycle. The VEP leaves were collected from Iwakura. Bvevipalpus obovatus females that were 40–50 days old (after oviposition by their mothers) were used for the experiments. The females were expected to produce eggs throughout the experimental periods (Goyal et al. 1985).
Phytoseius nipponicus was collected from VEP leaves in Iwakura and reared on VEP leaf disks for 2 days before each experiment. Adults were identified in accordance with Ehara and Amano (2009); we did not distinguish between females and males. Five species of Phytoseiidae have been recorded on VEP in Iwakura (Sudo et al. 2010), in which adults of P. nipponicus were distinguishable under a stereomicroscope (10×–50×) due to pairs of thick dorsal setae on coarse plate (Ehara and Amano 2009).
Host plant leaves
The VEP leaves used for the experiments were collected from four shrubs in Iwakura 2 days before each experiment at the same time as the phytoseiid mites were collected. The leaves were placed in plastic bags, brought to the laboratory, and placed with either the abaxial or adaxial side up on water-soaked cotton in Petri dishes (9 cm in diameter) within 4 h after collection. We used the leaves that had fully developed and were not damaged by leaf-chewing herbivores (leaf miners and leaf beetles). The petiole of each leaf was coated with acrylic emulsion adhesive (#10824, Konishi, Osaka, Japan) to prevent the leaf surface from flooding with water. Leaves collected from the same branch were used for a batch of treatments in each replication. Before the experiments, predaceous mites (Phytoseiidae and Stigmaeidae), insects (aphids, gall midges, and thrips), and rubbish (>1 mm) were removed from the leaves using tweezers. Fungivorous mites (Winterschmidtiidae and Tydeoidea), herbivorous mites (Eriophyidae), pollen, and fungi were left on the leaf surface, which functioned as food sources for P. nipponicus during experiments.
The leaves were photographed together with a 5-mm-diameter marker and the areas of the leaves were measured using the Histogram Function of Photoshop Elements ver. 2 (Adobe Systems Incorporated 2002). The areas of the VEP leaves used in the experiment (mean ± SD) were 13.52 ± 2.55 cm2 (adaxial, predator−), 13.35 ± 1.43 cm2 (adaxial, predator+), 12.34 ± 3.01 cm2 (abaxial, predator−), and 12.47 ± 3.03 cm2 (abaxial, predator+).
Quantitative and qualitative comparison of the microstructures on the surfaces of VEP leaves
To compare the surface architectures of leaves, three leaves were collected from discrete branches from each of six VEP shrubs (18 leaves in total) in Iwakura on 24 July, 2010. To determine the mean density of stellate hairs, the number of stellate hairs within a unit area (5 mm × 5 mm) was counted on the adaxial and abaxial leaf surfaces under a stereomicroscope. The unit areas were selected to be the longitudinal midpoint of the leaves, avoiding the primary vein. The number of ramifications of all stellate hairs in each unit area was also counted.
Effects of leaf surface and of the presence of Phytoseius nipponicus on fecundity and egg fate of Brevipalpus obovatus
Eight VEP leaves were placed on water-soaked cotton in Petri dishes with the adaxial leaf surface up, and another eight were placed with the abaxial leaf surface up (leaf-surface treatments). The day before the experiments began (day 0), two adult P. nipponicus females were introduced to each of four leaves for each treatment (predator+); no phytoseiids were introduced to the remaining four leaves (predator−). Two sets of treatments (eight leaves) were set up in a plastic box (33.5 × 25 × 5 cm length × width × height) and maintained in a laboratory at 25 °C (relative humidity was between 65 and 85 %) and a 16:8 h light–dark photoperiod. The experiments were conducted in June, July, and October 2010 (4 replicates per month; 12 replicates in total).
At the beginning of the experiment (day 1), three adult B. obovatus females were introduced to each VEP leaf in all treatments. Over the following 10 days, the position of each newly oviposited B. obovatus egg was recorded every 24 h. Egg status (live or dead; died of predation or other causes) was inspected daily until its fate (hatched, preyed upon, or died of other causes) was determined. Brevipalpus obovatus adults were removed from leaves on day 11. If a B. obovatus adult escaped from a leaf disk during the 10 days, it was replaced with an adult female of the same age. The number of predators was also maintained throughout the experiment until the final egg hatching rate was determined. Any P. nipponicus eggs were removed immediately. Larvae of B. obovatus were not removed throughout the experiment.
The position (contact or no contact with stellate hairs on a leaf surface) of every B. obovatus egg was determined under a stereomicroscope (16×). Whether the eggs were alive or dead was based on the presence or absence of egg contents and the status of the eggshell. In preliminary observations, the B. obovatus larva clipped the eggshell and left a round break in the eggshell when the egg hatched, while phytoseiid predators did not clip the eggshells, and no such conspicuous break was left on the eggshell when phytoseiid mites sucked the egg contents. We determined that eggs had hatched when clipping of the eggshell and disappearance of the egg contents (larva) occurred together. If the hatching of a larva was interrupted for any reason for more than 24 h, the egg was recorded as “dead.” Predation was defined as the disappearance of the egg contents in whole or in part without clipping of the eggshell. Data collection was discontinued on day 41 for the first experiment in June. In the experiments performed in July and October, observations ceased when no eggs were found to hatch after 7 consecutive days for all treatments; we made this decision because no eggs had hatched after a 7-day no-hatching interval during the June experiments. Eggs that had not hatched by the end of the experiments were recorded as “dead.”
Brevipalpus obovatus eggs that were flooded in surrounding water before hatching or predation were included only in the analyses of fecundity; they were excluded from the analyses of egg fate (hatching or predation rates). Eggs that died from neither predation nor flooding were included only in the analysis of hatching rate; they were excluded from the comparison of predation rates between leaf surfaces on each leaf.
A generalized linear mixed model (GLMM), assuming a Poisson distribution (log-link), was used to evaluate the effects of leaf surfaces and presence/absence of P. nipponicus on the fecundity of B. obovatus, in which the VEP leaves collected in each of the 3 months (June, July or October) were clustered. We used the GLM with Poisson errors, not the analysis of variance on Gaussian ones, because the fecundity data did not satisfy the homogeneity of variance (Levene’s test, P < 0.001). We formulated all combination among explanatory variables and their two-way interactions into the models, and then selected the appropriate versions based on Akaike’s information criterion (AIC). We constructed the models with the module “glmmML” (in the glmmML package by Broström and Holmberg 2011) of R (version 2.10.1; R Development Core Team 2009). Medians and 95th percentiles were used to represent the age distribution of B. obovatus eggs when they were preyed upon by P. nipponicus on either the adaxial or abaxial leaf surfaces; this was performed because no presupposition for age distribution at egg predation was available.
The predation hazard (i.e. the instantaneous rate of predation) for B. obovatus eggs of each age (day) was defined as the proportion of eggs preyed upon in the following 24 h out of the eggs that had survived each egg age. The hazard was calculated based on the egg survival data between days 2 and 11 of the experiment (i.e., the period during which B. obovatus adults were present); the eggs that survived after day 11 were treated as censored samples and only the survival data until day 11 was used for calculation. Local polynomial regression, a method of nonparametric regression, was used to represent the dynamics of predation hazard on each leaf surface. The degree of the polynomials and the span (smoothing parameter) were set at 2 and 1, respectively. For each egg, the predation rate within 24 h of oviposition was not included in the regression because of uncertainty regarding the initial number of oviposited eggs. Only the data for leaves with predators were used for the regression. The module “loess” of R ver. 2.10.1 was used for regressing predation hazards.
Effects of stellate hair manipulation on egg predation risk
A manipulative experiment was conducted to evaluate how stellate hairs protected B. obovatus eggs from P. nipponicus. Four VEP leaves were placed on water-soaked cotton in Petri dishes with the abaxial side up. On the first day of the experiment, 10 adult B. obovatus females were introduced to each VEP leaf. The position of every egg laid by the females was recorded every day, and the females were removed after 3 days.
All B. obovatus eggs that had contact with both a vein and a stellate hair on the VEP leaf surface were studied. The eggs were divided randomly into two groups. In the first group, the eggs were moved temporarily using fine-point brushes. And then, the stellate hairs that had direct contact with the eggs were bent at their basal parts using tweezers in order to increase the exposure of eggs touching the hairs to the predators; thus the hairs fell sideways but not removed. Then the eggs were immediately replaced to the basal part of the stellate hair from the opposite direction where the hair was bent (the hair-bending treatment). In the second group (control), the eggs were temporarily picked up using fine-point brushes with no modification of the stellate hairs touching eggs. Instead, we bent a stellate hair that was the second nearest neighbor of the stellate hair touching each egg to imitate chemical cues for predators, if they exist.
Five adult P. nipponicus were introduced to each leaf immediately after removing the B. obovatus females. The eggs were checked every day for 3 days after introducing the phytoseiid mites.
Results
Quantitative and qualitative comparison of the microstructure of VEP leaf surfaces
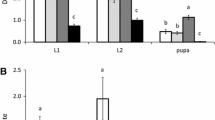
The VEP leaves have stellate hairs on both the adaxial and abaxial surfaces, and stellate hairs and thick straight hairs also occur on the veins on the abaxial surface (Fig. 1a, b). The adaxial leaf surfaces have more stellate hairs than the abaxial leaf surfaces, with 133.5 ± 9.34 and 104.1 ± 9.15 (mean ± SE) hairs (as hair tufts) per 5 mm × 5 mm area on the adaxial and abaxial surfaces, respectively (Welch’s two-sample t test, t = 2.2474, df = 33.986, P < 0.05). On the other hand, the abaxial leaf surfaces have more ramifications (as the total in the unit area) than the adaxial leaf surfaces, with 403.7 ± 44.0 and 240.5 ± 21.2 (mean ± SE) ramifications on the abaxial and adaxial surfaces, respectively (Welch’s two-sample t test, t = −3.3423, df = 25.524, P < 0.01). The mode and median for the number of ramifications of the stellate hairs were one and two on the adaxial leaf surfaces, respectively, and three and four on the abaxial leaf surfaces, indicating that the abaxial surfaces of VEP leaves have larger, more complicated stellate hairs than the adaxial leaf surfaces (Wilcoxon’s rank-sum test, W = 651855.5, P < 2.2 × 10−16; Fig. 1c).
a, b Stellate hairs on the surface of a VEP leaf (scanning electron microscope image), showing the a adaxial and b abaxial surfaces of the primary vein axil of the same leaf. The scale bar in each figure is 500 μm. c The number of ramifications of stellate hairs on the adaxial (open bars) and abaxial (shaded bars) surfaces of VEP leaves as the mean frequency with the standard deviation in 18 quadrats (5 mm × 5 mm) from 18 leaves
Effects of leaf surface and the presence of Phytoseius nipponicus on the fecundity and egg fate of Brevipalpus obovatus
Fecundity and egg fates of Brevipalpus obovatus
The fecundity of B. obovatus on abaxial leaf surfaces of VEP leaves was greater than that on adaxial leaf surfaces, and the presence of P. nipponicus reduced fecundity (Table 1). The number of eggs produced by three females over 10 days (mean ± SE, across the three time periods) was 12.0 ± 1.75 and 5.42 ± 0.723 on adaxial leaf surfaces in the predator− and predator+ treatments, respectively, and 15.2 ± 3.07 and 9.50 ± 1.28 on the abaxial leaf surfaces. Almost all eggs (501 of 505 eggs in total) had contact with stellate hairs on leaf surfaces. Model selection supported the GLMM containing the effect of leaf surface, predator presence and their two-way interaction, suggesting that the reduction in the fecundity of B. obovatus due to the presence of P. nipponicus was marginally mitigated on abaxial leaf surfaces compared to adaxial leaf surfaces (Table 1).
On predator− leaves, most B. obovatus eggs hatched regardless of the leaf surface (Fig. 2); the hatching rate was 0.935 (n = 138; 95 % CI, 0.880–0.970) and 0.938 (n = 177; 95 % CI, 0.891–0.969) on adaxial and abaxial leaf surfaces, respectively. On predator+ leaves, the respective hatching rates were reduced to 0.423 (n = 52; 95 % CI, 0.287–0.568) and 0.634 (n = 112; 95 % CI, 0.538–0.723) (Fig. 2). The predation rate, excluding the eggs that died from neither predation nor flooding, was significantly higher on adaxial leaf surfaces (0.50; n = 44) than on abaxial leaf surfaces (0.32; n = 104) (2 × 2 Fisher’s exact test, P = 0.042).
Dynamics of predation risk on Brevipalpus obovatus eggs
Egg predation events in each experiment occurred continuously from day 3 to day 13 after the introduction of B. obovatus (Fig. 3). The eggs required 11.41 ± 0.203 and 12.02 ± 0.277 days to hatch on the leaves in the predator− and predator+ treatment groups, respectively (Fig. 4). Though the cause was unknown, the incubation periods of predator-present eggs were significantly longer than ones of predator-absent treatment (Wilcoxon rank sum test, W = 11680.5, P = 0.025).
The median and 95th percentile of egg age at predation was 2.0 and 5.95 days on adaxial surfaces and 2.0 and 8.80 days on abaxial surfaces, respectively. This suggests that predation on B. obovatus eggs by P. nipponicus mostly occurred in early stages of egg development (Fig. 4). Consequently, the predation hazard (potential predation rate each day) for surviving eggs decreased with increasing age and reached zero before the start of hatching on both adaxial and abaxial leaf surfaces (Fig. 5).
Predation hazard per day (i.e., potential predation rate for the following 24 h) of Brevipalpus obovatus eggs at each age (days) on a adaxial and b abaxial leaf surfaces. The hazard lines were determined using local polynomial regression (degree of polynomial = 2, span = 1.0). The predation rate within 24 h of oviposition (day zero) was not used for the regression. Only the data for leaves with predators were used
Effects of stellate hair manipulation on egg predation risk
The proportion of B. obovatus eggs consumed by P. nipponicus over 3 days was 0.94 (n = 47; 95 % binomial CI, 0.82–0.99) for eggs attached to bent stellate hairs and 0.64 for eggs attached to normal stellate hairs (control; n = 45; 95 % CI, 0.49–0.78). Both of the treatments in this manipulative experiment showed significantly higher predation rates than one in mentioned above (0.32, n = 104) (P < 0.01, paired Fisher’s exact tests with Bonferroni correction), Thus, manipulation on B. obovatus eggs might increase predation risk. Nevertheless, the difference in egg consumption between hair-bending treatments was significant (Fisher’s exact test, P < 0.001), indicating that the stellate hairs of VEP leaves reduce the predation risk of B. obovatus eggs by P. nipponicus.
Discussion
Because unguarded eggs may be decimated by predation, a refuge is advantageous to the propagation of herbivorous species on host plants on which generalist predators occur. On VEP leaves, stellate hairs are suggested to protect B. obovatus eggs from P. nipponicus, a phytoseiid predator. Brevipalpus obovatus eggs survived for more than 6 days (approximately half of their incubation periods observed in this experiment) were scarcely preyed by P. nipponicus. Phytoseius nipponicus constantly preyed on B. obovatus eggs while B. obovatus females produced new eggs, suggesting that some particular factor other than the activity of P. nipponicus reduced risk of aged B. obovatus eggs being preyed. Therefore, the temporal saturation in egg predation during this period was not caused by reduced predator effort. Possible mechanisms reducing the predation risk during the late egg period of B. obovatus include augmentation of the egg defense system itself, such as physical and chemical defense, and the hindering effects of stellate hairs. However, there was no obvious effect of the age of eggs on the reduction of predation risk during the late egg period (see electronic supplementary material). A protective effect of stellate hairs on B. obovatus eggs hindering predation by P. nipponicus was directly demonstrated in the manipulative experiments comparing predation risk between eggs attached to bent and straight stellate hairs.
Studies have reported that the walking and foraging capacities of phytoseiid mites are determined by the interaction between the trichome density on the leaf surface and body width of the phytoseiid mites (Krips et al. 1999; Kreiter et al. 2002). The body length and width of B. obovatus adult females are 290 and 170 μm, respectively (Ehara and Gotoh 2009), while those of P. nipponicus are 360 and 230 μm, respectively (Ehara 1962). At oviposition sites, B. obovatus may be able to exploit gaps that are narrower than the minimum gap size that P. nipponicus can enter. Therefore, a reduction in the predation rate with egg age should result as the accessible eggs are removed from the leaf surfaces by predation, making it more difficult for the predators to find the remaining (older) eggs.
Phytoseius nipponicus may have more difficulty penetrating B. obovatus eggs laid among the dense stellate hairs on the abaxial surfaces of VEP leaves. In fact, the hatchability of B. obovatus eggs on VEP leaves with predators was higher on abaxial leaf surfaces than on adaxial leaf surfaces. Stellate hairs on the abaxial surfaces of VEP leaves had more ramifications than ones on the adaxial surfaces, and more eggs survived on the abaxial than on the adaxial surfaces after the whole incubation periods. It suggests stellate hairs on abaxial leaf surfaces of VEP protected B. obovatus eggs more effectively than on the abaxial, though considerable amount of B. obovatus eggs survived under stellate hairs on VEP leaves on both adaxial and abaxial surfaces. On VEP leaves, B. obovatus laid most eggs in contact with the stellate hairs. Such an oviposition-site choice is suggested to be beneficial in reducing predation risk on eggs, though it remains unknown whether an adult B. obovatus can assess the accessibility of a phytoseiid predator to each oviposition site (e.g. the width of the gap between stellate hairs). Stellate hairs on VEP leaf surfaces hinder the predation of P. nipponicus on B. obovatus eggs, whereas pubescences on plant leaves are in general considered to provide alternative foods for population of generalist phytoseiid mites by trapping pollen and/or fungal spores (Kreiter et al. 2002; Roda et al. 2003; Duso et al. 2004) and retaining small fungivorous mites such as Winterschmidtiidae (Sudo et al. 2010). From the viewpoint of integrated pest management, the balance between these direct and indirect effects of foliar microstructures on predator guild on a plant should be addressed in future study.
The protective function of the leaf surface microstructure was greater on abaxial leaf surfaces. Nevertheless, Sudo and Osakabe (2011) reported that a substantial portion of B. obovatus eggs (35 %) and motile individuals (13 %) remained on the adaxial leaf surfaces of VEP, whereas only 1.9 % of the phytoseiid mite population was found on the adaxial leaf surfaces. In our previous study, P. nipponicus showed positive geotaxis and a preference for the abaxial surface over the adaxial surface of VEP leaves, whereas B. obovatus had negative geotaxis and a preference for surface environments on the adaxial leaf surfaces of VEP (Sudo and Osakabe in press). In addition, B. obovatus adults actively changed their leaf-surface distribution to avoid the predator. In the present study, oviposition rates of B. obovatus on adaxial and abaxial leaf discs in the presence of predators decreased by 1/2 and 2/3, respectively, in comparison with predator-free surfaces. On the other hand, in the previous study using an experimental setup that made both VEP leaf surfaces accessible to the mites, the daily fecundity of B. obovatus on predator-present leaves was approximately 5/6 that on predator-free leaves (Sudo and Osakabe in press). It is considered the spatial segregation reduced opportunities for encounters between the predator and the prey, and hence the indirect negative effect of predator presence on prey fecundity would be alleviated on wild VEP plants in comparison with a leaf disc.
Besides, adaxial (upper) leaf surfaces likely subject small arthropods to harsher conditions, involving high temperatures, desiccation (Gutschick 1999), rainfall (Jeppson 1975), and solar ultraviolet radiation (Ohtsuka and Osakabe 2009; Onzo et al. 2010) compared to abaxial (lower) leaf surfaces. This may be why phytoseiid mites were seldom found on the adaxial leaf surfaces of the host plants. All of the threat of phytoseiid predators on each leaf surface, the availability of shelters such as stellate hairs or domatia, and the environmental (abiotic) factors are suspected as determinants of the oviposition site choice by B. obovatus adult females on their host plant leaves. Our ongoing study will clarify the effects of temperature and solar UVB radiation on B. obovatus egg development and hatchability on the upper leaf surface of VEP.
References
Adobe Systems Incorporated (2002) Adobe Photoshop Elements. version 2.0. San Jose, California
Broström G, Holmberg H (2011) glmmML: Generalized linear models with clustering. version 0.81-8
Duso C, Vettorazzo E (1999) Mite population dynamics on different grape varieties with or without phytoseiids released (Acari: Phytoseiidae). Exp Appl Acarol 23:741–763
Duso C, Malagnini V, Paganelli A, Aldegheri L, Bottini M, Otto S (2004) Pollen availability and phytoseiid abundance (Acari: Phytoseiidae) on natural and secondary hedgerows. Biocontrol 49:397–415
Ehara S (1962) Notes on some predatory mites (Phytoseiidae and Stigmaeidae). Jpn J Appl Entomol Zool 6:53–60
Ehara S, Amano H (2009) Phytoseius (Dubininellus) nipponicus Ehara. In: Ehara S, Gotoh T (eds) Colored guide to the plant mites of Japan. Zenkoku Noson Kyoiku Kyokai, Tokyo (in Japanese), pp 96–97
Ehara S, Gotoh T (2009) Brevipalpus obovatus. In Ehara S, Gotoh T (eds.) Colored Guide to the Plant Mites of Japan, p. 152. Zenkoku Noson Kyoiku Kyokai, Tokyo (in Japanese)
Ferreira JAM, Pallini A, Oliveira CL, Sabelis MW, Janssen A (2010) Leaf domatia do not affect population dynamics of the predatory mite Iphiseiodes zuluagai. Basic Appl Ecol 11:144–152. doi:10.1016/j.baae.2009.10.008
Fukaya M, Uesugi R, Ohashi H, Sakai Y, Sudo M, Kasai A, Kishimoto H, Osakabe M (in press) Tolerance to solar ultraviolet-B radiation in the citrus red mite, an upper surface user of host plant leaves. Photochem Photobiol doi: 10.1111/php.12001
Goyal M, Sadana GL, Sharma NK (1985) Influence of temperature on the development of Brevipalpus obovatus (Acarina: Tenuipalpidae). Entomon 10:125–129
Gutschick VP (1999) Biotic and abiotic consequences of differences in leaf structure. New Phytol 143:3–18. doi:10.1046/j.1469-8137.1999.00423.x
Jeppson LR (1975) Chapter 2. Population ecology. In: Jeppson LR, Keifer HH, Baker EW (eds) Mites injurious to economic plants, pp. 17–46. University of California Press, Berkeley, California
Johnson HB (1975) Plant pubescence: an ecological perspective. Bot Rev 41:233–258. doi:10.1007/BF02860838
Karban R, English-Loeb G, Walker MA, Thaler J (1995) Abundance of phytoseiid mites on Vitis species: effects of leaf hairs, domatia, prey abundance and plant phylogeny. Exp Appl Acarol 19:189–197
Kreiter S, Tixier MS, Croft BA, Auger P, Barret D (2002) Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ Entomol 31:648–660. doi:10.1603/0046-225X-31.4.648
Krips OE, Kleijn PW, Willems PEL, Gols GJZ, Dicke M (1999) Leaf hairs influence searching efficiency and predation rate of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 23:119–131. doi:10.1023/A:1006098410165
Lill JT, Marquis RJ, Forkner RE, Le Corff J, Holmberg N, Barber NA (2006) Leaf pubescent affects distribution and abundance of generalist slug caterpillars (Lepidoptera: Limacodidae). Environ Entomol 35:797–806. doi:10.1603/0046-225X-35.3.797
Loughner R, Goldman K, Loeb G, Nyrop J (2008) Influence of leaf trichomes on predatory mite (Typhlodromus pyri) abundance in grape varieties. Exp Appl Acarol 45:111–122. doi:10.1007/s10493-008-9183-5
McMurtry JA, Croft BA (1997) Life styles of phytoseiid mites and their roles as biological control agents. Ann Rev Entomol 42:291–321. doi:10.1146/annurev.ento.42.1.291
O’Dowd DJ, Willson MF (1997) Leaf domatia and the distribution and abundance of foliar mites in broadleaf deciduous forest in Wisconsin. Am Midl Nat 137:337–348
Ohtsuka K, Osakabe Mh (2009) Deleterious effects of UV-B radiation on herbivorous spider mites: they can avoid it by remaining on lower leaf surfaces. Environ Entomol 38:920–929. doi:10.1603/022.038.0346
Onzo A, Sabelis MW, Hanna R (2010) Effects of ultraviolet radiation on predatory mites and the role of refuges in plant structures. Environ Entomol 39:695–701
Pina T, Argolo PS, Urbaneja A, Jacas JA (2012) Effect of pollen quality on the efficacy of two different life-style predatory mites against Tetranychus urticae in citrus. Biol Control 61:176–183. doi:10.1016/j.biocontrol.2012.02.003
R Development Core Team (2009) R: A language and environment for statistical computing, version 2.10.1. R Foundation for Statistical Computing, Vienna
Roda A, Nyrop J, Dicke M, English-Loeb G (2000) Trichomes and spider-mite webbing protect predatory mite eggs from intraguild predation. Oecologia 125:428–435. doi:10.1007/s004420000462
Roda A, Nyrop J, English-Loeb G, Dicke M (2001) Leaf pubescence and two-spotted spider mite webbing influence phytoseiid behavior and population density. Oecologia 129:551–560. doi:10.1007/s004420100762
Roda A, Nyrop J, English-loeb G (2003) Leaf pubescence mediates the abundance of non-prey food and the density of the predatory mite Typhlodromus pyri. Exp Appl Acarol 29:193–211
Saito Y (1985) Life types of spider mites. In: Helle W, Sabelis MW (eds.) Spider Mites. Their Biology, Natural Enemies and Control, Vol. 1A, pp. 253–264. Elsevier, Amsterdam
Saito Y (1986) Prey kills predator: counter-attack success of a spider mite against its specific phytoseiid predator. Exp Appl Acarol 2:47–62. doi:10.1007/BF01193354
Sakai Y, Sudo M, Osakabe M (2012) Seasonal changes in the deleterious effects of solar ultraviolet-B radiation on eggs of the twospotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Appl Entomol Zool 47:67–73
Shah MA (1982) The influence of plant surfaces on the searching behavior of coccinellid larvae. Entomol Exp Appl 31:377–380. doi:10.1007/BF02996700
Sudo M, Osakabe M (2011) Do plant mites commonly prefer the underside of leaves? Exp Appl Acarol 55:25–38. doi:10.1007/s10493-011-9454-4
Sudo M, Osakabe M (in press) Geotaxis and leaf-surface preferences mitigate negative effects of a predatory mite on an herbivorous mite. Exp Appl Acarol doi: 10.1007/s10493-012-9622-1
Sudo M, Nishida S, Itioka T (2010) Seasonal fluctuations in foliar mite populations on Viburnum erosum Thunb. var. punctatum Franch. et Sav. (Adoxaceae) and sympatric shrubs in temperate secondary forests in western Japan. Appl Entomol Zool 45:405–415. doi:10.1303/aez.2010.405
Tachi F, Osakabe M (2012) Vulnerability and behavioral response to ultraviolet radiation in the components of a foliar mite prey-predator system. Naturwissenschaften 99:1031–1038. doi:10.1007/s00114-012-0984-3
Tian D, Tooker J, Peiffer M, Chung SH, Felton GW (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236:1053–1066. doi:201210.1007/s00425-012-1651-9
van Haren RJF, Steenhuis MM, Sabelis MW, de Ponti OMB (1987) Tomato stem trichomes and dispersal success of Phytoseiulus persimilis relative to its prey Tetranychus urticae. Exp Appl Acarol 3:115–121. doi:10.1007/BF01270473
van Rijn PCJ, Tanigoshi LK (1999) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802
Walter DE (1996) Living on leaves: mites, tomenta, and leaf domatia. Ann Rev Entomol 41:101–114. doi:10.1146/annurev.en.41.010196.000533
Walter DE, Proctor HC (1999) Mites: ecology, evolution, and behaviour. CABI Publishing, Wallingford
Acknowledgments
We thank Prof. H. Amano and Dr. S. Yano of Kyoto University for valuable suggestions. This study was partially supported by Grants-in-Aid for Scientific Research (B) Nos. 22380036 to MO from the Ministry of Education, Culture, Sports, Science and Technology, Japan and Grant-in-Aid for JSPS Fellows Nos. 23.2696 to SM from Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sudo, M., Osakabe, M. Stellate hairs on leaves of a deciduous shrub Viburnum erosum var. punctatum (Adoxaceae) effectively protect Brevipalpus obovatus (Acari: Tenuipalpidae) eggs from the predator Phytoseius nipponicus (Acari: Phytoseiidae). Exp Appl Acarol 60, 299–311 (2013). https://doi.org/10.1007/s10493-012-9648-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9648-4