Abstract
Plant growth-promoting rhizobacteria are bacteria that improve plant growth and reduce plant pathogen damages. In this study, 100 nodule bacteria were isolated from chickpea, screened for their plant growth-promoting (PGP) traits and then characterised by PCR-RFLP of 16 S rDNA. Results showed that most of the slow-growing isolates fixed nitrogen but those exhibiting fast-growth did not. Fourteen isolates solubilized inorganic phosphorus, 16 strains produced siderophores, and 17 strains produced indole acetic acid. Co-culture experiments identified three strains having an inhibitory effect against Fusarium oxysporum, the primary pathogenic fungus for chickpea in Tunisia. Rhizobia with PGP traits were assigned to Mesorhizobium ciceri, Mesorhizobium mediterraneum, Sinorhizobium meliloti and Agrobacterium tumefaciens. We noted that PGP activities were differentially distributed between M. ciceri and M. mediterraneum. The region of Mateur in northern Tunisia, with clay–silty soil, was the origin of 53% of PGP isolates. Interestingly, we found that S. meliloti and A. tumefaciens strains did not behave as parasitic nodule-bacteria but as PGP rhizobacteria useful for chickpea nutrition and health. In fact, S. meliloti strains could solubilize phosphorus, produce siderophore and auxin. The A. tumefaciens strains could perform the previous PGP traits and inhibit pathogen growth also. Finally, one candidate strain of M. ciceri (LL10)—selected for its highest symbiotic nitrogen fixation and phosphorus solubilization—was used for field experiment. The LL10 inoculation increased grain yield more than three-fold. These finding showed the potential role of rhizobia to be used as biofertilizers and biopesticides, representing low-cost and environment-friendly inputs for sustainable agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensive farming practices that give good yields need chemical fertilizers and pesticides, which are expensive and create environmental problems (Fasusi et al. 2021; Kumar et al. 2022; Nosheen et al. 2021). Extensive use of such products in farming is under debate due to ecological concerns and potentially negative effects on human and animal health. Therefore, there is growing interest in eco-friendly and sustainable farming practices and biological farming systems (Adedeji et al. 2020; Alori and Babalola 2018; Ansari and Mahmood 2019; Fasusi et al. 2021; Kumar et al. 2022; Nosheen et al. 2022).
Plant-associated microbes can produce effects ranging from beneficial to pathogenic. Some of the prominent beneficial interactions of agricultural importance include symbiotic nitrogen fixation, nutrient mobilization, induction of resistance mechanisms against invading pests, biological control of pathogens, and plant growth promotion (Adedeji et al. 2020; Alemneh et al. 2020; Ansari and Mahmood 2019; Fasusi et al. 2021; Kumar et al. 2022). Increased plant growth and crop yield can be obtained following seed or root inoculation with soil bacteria (rhizobia or non-symbiotic bacteria) called plant growth promoting rhizobacteria (PGPR). Studies have shown that only 1–2% of rhizosphere bacteria promote plant growth (Antoun and Kloepper 2001).
Plant growth promoting rhizobia may promote plant growth and yield either indirectly or directly (Adedeji et al. 2020; Fasusi et al. 2021; Kumar et al. 2022; Swift et al. 2019). Direct growth promotion includes (1) biological nitrogen fixation, (2) phosphorus solubilization, (3) improving iron availability by the production of siderophores, (4) production of phytohormones such as auxins, cytokinins, and gibberellins, and (5) lowering of ethylene concentration (Adedeji et al. 2020; Fasusi et al. 2021; Gopalakrishnan et al. 2015; Jha and Saraf 2015; Kumar et al. 2022; Swift et al. 2019). Indirect processes of plant growth promotion by PGPR consist of (1) antibiotic generation, (2) siderophore-mediated competition with harmful organisms for iron, (3) synthesis of fungicidal metabolites, (4) production of enzymes the lyse fungal cells, (5) competition for root colonization sites and (6) stimulation of plant systemic resistance (Adedeji et al. 2020; Ansari and Mahmood 2019; Fasusi et al. 2021; Gopalakrishnan et al. 2015; Jha and Saraf 2015; Kumar et al. 2022; Swift et al. 2019).
Despite the great importance of plant growth-promoting bacteria for agriculture and human health, a limited number of studies have examined PGP properties of rhizobia in Mediterranean Sea countries. Chickpea (Cicer arietinum) is one of the most widely grown legumes globally and has a strong local and international demand. Therefore, the characterization of chickpea rhizobia for their PGP traits is a priority. The purpose of this work was firstly to examine chickpea-rhizobia for their plant growth-promoting activities (nitrogen fixation, phosphate solubilization, phytohormone synthesis, and fungi antagonism). Secondly, rhizobia having multiples PGP traits were characterized and identified taxonomically. Thirdly, field studies examined the potential benefit of field inoculation with selected PGPR strain.
Materials and methods
Rhizobial isolation and morphological tests
Rhizobia were isolated from root nodules of chickpea grown at different locations covering all bioclimatic conditions in Tunisia (Sub-humid, Semi-arid, Arid, Saharian). Only healthy and undamaged nodules were sampled. Bacteria were isolated using a standard procedure (Beck et al. 1993). The purity of isolates was checked by repeated streaking on yeast-mannitol agar (YMA) medium supplemented with Congo red (Vincent 1970). The purified rhizobial cultures were stored at 4 °C on YMA slants or in yeast-mannitol broth (Vincent 1970) amended with 25% (v/v) glycerol at − 80 °C for long-term storage. Colony morphology and color were determined by using a hand lens. Cell morphology and size were examined on living cells by phase-contrast microscopy.
A total of 100 strains were isolated to study their PGPR activities.
Nodulation and nitrogen fixation
Nodulation and effectiveness tests of rhizobia on chickpea plants were performed as previously described (Beck et al. 1993). Plastic pots (500 ml) filled with sterilized perlite were used as growth support. Quadruplicate pots were inoculated with 1 ml of a suspension containing approximately 108 bacteria (measured by spectrophotometry at optical density of 600 nm) or left uninocuated (control). A sterilized nitrogen-free solution was supplied aseptically as needed (Vadez et al. 1996). Fertilized plants with complete nutritive solution (without inoculation) were added as positive controls. Non-inoculated and non fertilized plants were added as negative controls. The plants were harvested seven weeks after inoculation. Strain infection ability was verified by nodules number. Symbiotic nitrogen fixation was estimated by measuring shoot dry weight.
Phosphorus solubilization
The method described by Sylvester-Bradley et al. (1982) was used to identify isolates able to solubilize complex phosphates. Bacteria were grown in glucose yeast medium (GY) containing 10 g glucose, 2 g yeast extract and 15 g agar per liter. Two other solutions were prepared separately, one containing 5 g K2HPO4 in 50 ml distilled water and the other containing 10 g CaCl2 in 100 ml distilled water. These solutions were added to one liter of glucose yeast extract (GY) medium just before pouring onto Petri dishes, forming insoluble calcium phosphate and rendering the medium opaque. Bacterial isolates previously grown in GY medium were dropped (10 µl per culture) onto the GY plates and incubated for 7 days at 28 °C. All assays were replicated four times. Isolates that formed visible clearing halos around their colonies were considered phosphate solubilizers. Halo diameters were measured.
Siderophore production
Rhizobial samples were evaluated for their capacity of siderophore production in Petri dishes containing King B (KB) medium (King et al. 1954) supplemented with chrome azurol S [CAS/iron(III)/hexadeciltrimethyl ammonium bromide] as described by Schwyn and Neilands (1987). One drop of a culture grown in KB medium for 48 h at 28 °C was deposited on plates and incubated for at least 7 days. Bacterial colonies that produced siderophores exhibited growth and formed a yellow–orange halo in the blue–green medium. Strains were recorded for siderophore production (+) or no siderophore production (−) in comparison to the control plates.
Auxin production
Production of indole acetic acid (IAA) by rhizobial isolates was tested by inoculation of a bacterial suspension into 10 ml of sucrose minimal salts (SMS) medium (Sheng et al. 2008) (10 g sucrose l−1, 1 g (NH4)2SO4 l− 1, 2 g K2HPO4 l−1, 0.5 g MgSO4⋅7H2O l−1, 0.5 g yeast extract l−1, 0.5 g CaCO3 l−1, 0.1 g NaCl l−1) containing 0.5 mg L-tryptophan ml−1. The culture was incubated at 28 °C for five days under agitation and then centrifuged for 5 min at 10,000g. One ml of supernatant was mixed with 2 ml of Salkowski reagent and 100 µl of orthophosphoric acid (10 mM). After incubation at 25 °C for 30 min, indolic compounds were spectrophotometrically detected at 530 nm. A calibration curve was established using pure IAA (Merck, USA).
Antifungal assay on Fusarium oxysporum
The agar well diffusion method was used. Sixteen rhizobial strains were tested for their antifungal activity against Fusarium oxysporum, the pathogenic fungus of major importance for chickpea in Tunisia. For each fungal strain, three replicate Petri dishes (90 mm diam.) containing PDA medium were inoculated in the center with mycelial plugs (5 mm in diameter). Two days later, rhizobial strains were applied equidistant from one another and 20 mm from the fungal plugs. The plates were incubated at 28 °C until mycelial growth stop or exceed bacterial lines. The antifungal activity was evaluated by measuring the growth inhibition zone against test fungi.
Molecular characterisation
PCR amplification of 16S rRNA genes using primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGCTTAAGGTGATCCAG-CC-3′) (Weisburg et al. 1991) was carried on cells treated with proteinase-K. Digestion was carried out by six endonucleases HaeIII, HinfI, MspI, NdeII, CfoI and RsaI (Ben Romdhane et al. 2007b). Rhizobial species were inferred by comparing the 16 S rDNA RFLP profiles observed with those from a printed database of designed restriction sites in the 16 S rRNA genes of rhizobia (Laguerre et al. 1997).
Field experiment
The field inoculation using M. ciceri (LL10)—selected mainly for its highest symbiotic nitrogen fixation and phosphorus solubilization—was performed in Menzel Ibrahim farm, located in the district of Klebia (Nabeul, North East of Tunisia, Semi-arid condition). The soil of this field is deficient in nutrients (phosphorus, iron) necessary for plant growth and health. The main soil characteristics of this field were: Clay (%), 42; Silt (%), 30; Sand (%), 28; CE (ms cm−1), 0.4; pH (1/2.5), 8.2; N (ppm), 2.4; Fe (ppm), 1.5; P2O5 (ppm), 22; K2O (ppm), 70. Four treaments were imposed and were distributed in randomized blocks in the field: Non-inoculated control (NIC), Nitrogen–Phosphate–Potassium (NPK) Fertilized, Nitrogen (N) fertilized, and LL10-inoculated treatment. The field was cultivated with chickpea cv. Amdoun I (kabuli genotype), which is the most widely cultivated cultivar in Tunisia. The treatments were 15 m × 8 m in size and contained 60 rows. The sowing density was 25 seeds/m2. A margin of 1 m separated the inoculated and the non-inoculated blocks. The field experiment was carried under rain-fed conditions between March 20th and July 10th 2017. The inoculant strain of M. ciceri LL10 was grown to late exponential phase in YEM (Beck et al. 1993) and diluted with well water to 108 bacteria/ml and then used to inoculate two-leaf stage seedlings. Inoculation was done on April 10th. Weeds were removed manually. Thirty plants were randomly collected from each treatment and used for nodule counting, shoot dry weight at flowering stage, and grain yield analysis.
Statistical analysis
In laboratory experiments, each treatment for various PGP activities was replicated four times. In field assay, harvest consisted on ninety plants for each treatment. The data of different PGP activities and plant growth/yield were expressed as the mean value. One-way analysis of variance (ANOVA) was performed to assess the significant differences between strains in having PGP activities and to analyse the effect of rhizobial inoculation on plant-shoot dry weight, nodules number, pod number and grain yield (Armstrong and Hilton 2010). Significant differences between means were compared using Fisher’s protected LSD test at P = 0.05 by using STATISTICA version 8.0 software (StatSoft, Tulsa, Okla) (Weiß 2007).
Results
Rhizobial collection and morphological characteristics
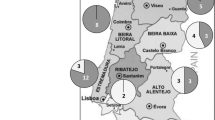
A total of 100 strains, isolated from different locations representing all bioclimatic stages in Tunisia, were obtained to study their PGPR activities. We obtained two types of bacterial isolates differing in their morphology and growth rates on YMA medium: fast-growing bacteria (FGB) with transparent to creamy colonies obtained after 3 days incubation at 28 °C, and moderately slow-growing bacteria (SGB) with light red colonies observed after 5 days. We noticed that FGB were mostly isolated from the central and southern regions of Tunisia that have arid or Saharan climates. Conversely, SGB were exclusively isolated from northern Tunisia which has a semi-arid or sub-humid climate.
Nitrogen fixation
Symbiotic nitrogen-fixing potential is an important feature for PGPR, especially rhizobia. Among the isolates tested, all SGB (56% of isolates), originating from the north regions with clayey soils and humid climates, were able to nodulate chickpea but with variable degrees of effectiveness (Tables 1, 2; Fig. 1A). Conversely, most FGB, isolated from the south with sandy soils and arid climates (Table 1, S1) could nodulate chickpea but not fix nitrogen. In addition, a few fast-growing isolates could not nodulate their host plant. Our results showed that strains LL6, LL7 and LL10 were the most effective nitrogen fixing rhizobia with chickpea.
PGPR activities. A Symbiotic nitrogen fixation efficiency assay for chickpea rhizobia. A, non-inoculated and non-fertilized plants; B, fertilized plants (without inoculation) with complete nutritive solution (Vadez et al. 1996); C,D,E (are LL6, LL10 and LL7, respectively), Plants inoculated with efficient rhizobia; F,G (are LL21 and LL56, respectively), Plants inoculated with non-efficient rhizobia. B Clearing zone around the colonies indicating the ability of this isolates to solubilize phosphate in GYP agar medium. C Siderophores production test-ability of rhizobial isolates. D Development of pink color in PGPR cultures indicating IAA production. 1, 2, 3, 4 and 5 are LL21, LL25, LL3, LL58 and LL24, respectively. E Growth inhibition of Fusarium oxysporum on nutrient agar medium by chickpea root-nodules bacteria (LL20)
Phosphorus solubilization
Soil is a storehouse of several forms of phosphates including inorganic and organic phosphates. In our study, rhizobial strains that can nodulate chickpea were tested for their inorganic phosphorus solubilization ability on Petri dishes. We found fourteen strains having the ability to solubilize phosphate as revealed by the clearing zone of solubilization halo around the colony (Fig. 1B). The diameter of the halo was measured for each strain and, and the data obtained were analyzed statistically (Table 2). The P-solubilizing strains were divided into three groups. The first group contained three bacteria producing a small-diameter halo (halo < 10 mm). This group contains the least active P-solubilizers, which are LL8, LL12 and LL74. The second group contained ten bacteria with a moderately high phosphatase activity (halo sizes 10 to 20 mm). The third group includes two bacteria with high phosphatase activity (halo ≥ 20 mm) which are LL3 and LL10.
Siderophore production
Sixteen rhizobial strains were able to synthesize siderophores under iron-deficient conditions when tested on CAS media plates (Fig. 1 C). Two isolates (LL13 and LL20) showed low siderophore production (halo diameters 1 to 10 mm) (Table 2). Twelve isolates (LL1, LL9, LL10, LL14, LL23, LL24, LL28, LL55, LL56, LL72, LL74 and LL75) exhibited moderate abilities to synthesize siderophores (10 to 20 mm). Finally, two isolates (LL25 and LL58) exhibited high siderophore production (halos ≥ 20 mm).
Auxin production
Seventeen of the isolates produced IAA as indicated by the change in reagent color from uncolored to pink (Fig. 1D; Table 2). Four isolates (LL21, LL25, LL56 and LL75) gave weak reactions with Salkowski reagent, which indicated low IAA production. Four isolates (LL1, LL5, LL6 and LL7) appeared to be moderate IAA producers (10 to 50 µg/ml). Four isolates (LL3, LL4, LL58 and LL72) produced AIA at concentrations of 50 to ≤ 100 µg/ml. Finally, five isolates (LL8, LL11, LL24, LL55 and LL74) yielded exceptionally high levels of IAA (> 100 µg/ml).
Antifungal assay
Sixteen rhizobial strains were tested for antifungal activity against Fusarium oxysporum (Table 2). Three strains (LL20, LL29 and LL72) inhibited in vitro fungal growth by 43% (Fig. 1E). These rhizobial strains inhibited mycelial growth, and no overgrowth was observed even after eight days of incubation.
Molecular characterizatio
Chickpea isolates exhibiting at least one type of PGPR activity (without considering symbiotic nitrogen fixation activity only) were subjected to molecular characterisation for identification. PCR/RFLP of 16 S rRNA genes showed that chickpea-rhizobia were genetically diverse and belonged to four species (Table 2): M. ciceri (12 strains), M. mediterraneum (10 strains), S. meliloti (3 strains), and Agrobacterium tumefaciens (3 strains). M. ciceri and M. mediterraneum were isolated from northern and central regions of Tunisia having sub-humid and semi-arid climates. However, S. meliloti strains were isolated from southern Tunisia which has an arid climate. The A. tumefaciens strains were isolated from the semi-arid region of Enfidha.
Field inoculation
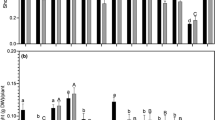
Strain LL10 isolated from the region of Mastouri in Mateur was used for field inoculation. Our results showed that inoculation significantly increased nodule number and agronomic parameters with chickpea cv. Amdoun I. Inoculation increased nodules number (with pink color) by 400%, shoot dry weight by 200%, and pod number and grain yield by more than 200% and 300%, respectively (Fig. 2). Interestingly, inoculation with this strain produced grain yields greater than those obtained with NPK-fertilized plants. Finally, non-inoculated plants showed poor nodulation and limited grain yield (Fig. 2).
Effect of field inoculation with PGPR strain LL10 on a Nodule numbers, b shoot dry weights, c pod numbers, d and grain yield of chickpea cv. Amdoun I. T0, Non-inoculated control; NPK, Nitrogen–Phosphate–Potassium Fertilized treatment; NF, Nitrogen fertilization; LL10, inoculated with M. ciceri strain LL10; Each value corresponds to the mean of 90 replicates. Standard errors were shown in the histograms with bars and lowercase letters indicate significant difference (P < 0.05) according to Fisher's protected LSD
Discussion
The rhizosphere is a dynamic and complex habitat where various microorganisms interact with plants for beneficial, neutral, or deleterious effects (Barea 2015). Rhizobia represent a major group of beneficial rhizobacteria interacting with leguminous plants. As such, rhizobial bacteria are thought to hold great promise for enhancing agricultural productivity and sustainability.
Nitrogen fixation in chickpea is performed by its specific rhizobia in the genus Mesorhizobium: M. ciceri, M. mediterraneum (Nour et al. 1995) and M. muleiense (Zhang et al. 2012). However, many species, that are the natural symbionts of other legumes, can also nodulate chickpea effectively. These strains might have obtained symbiotic genes from M. ciceri and M. mediterraneum through horizontal gene transfer, since they share identical symbiotic genes (Gunnabo et al. 2021). In our study, most of chickpea-Mesorhizobium strains were able to nodulate and fix nitrogen efficiently (Fig. 3). This is consistent with our previous studies on chickpea-rhizobia (Ben Romdhane et al. 2007b, 2009). However, not all of S. meliloti strains were able to fix nitrogen. This inefficient nodulation was recorded in the Saharan and arid climates in Tunisia by S. meliloti bv. medicagenis (Ben Romdhane et al. 2007a).
Phosphate solubilization is considered as one of the major direct growth-promoting mechanisms of PGPRs (Fasusi et al. 2021). Soils usually contain high amounts of inorganic P compounds, but much of it is in the form of insoluble precipitates with metal elements depending on the soil pH and thus unavailable to plants (Fasusi et al. 2021; Korbu et al. 2020). This was the case of Menzel Ibrahim field used in this study which was poor in assimilated phosphorus mainly influenced by the soil richness in clay and it’s basic pH. Our study showed that only 14% of rhizobial strains were able to solubilize P (Fig. 3). These results are in agreement with previous studies obtained in Spain, India and Portugal (Ahmad et al. 2008; Brígido et al. 2017; Peix et al. 2001; Sridevi and Mallaiah 2009). By screening 446 rhizobial strains nodulating various legume-plant species in Iran, Alikhani et al. (2006) found that 44% of them could solubilize inorganic phosphate. However, Saïdi et al. (2013) reported that none of rhizobia isolated from root-nodules of Vicia faba in Tunisia could solubilize complex phosphate on GY medium.
Another important trait of PGPRs, that influences plant growth is the production of siderophores. These are known to have a high affinity for Fe3+ and to promote plant health by making it available to plants but not phytopathogens (Kumar et al. 2022). In the present investigation, the siderophores production by chickpea nodules bacteria is strain dependent; since only few strains from each identified species have shown this capacity (Fig. 3). These selected strains could be particularly interesting for soil with low availability of assimilated iron, as the case of Menzel Ibrahim field, due to complexation phenomenon. In corroboration with our finding, Brígido et al. (2017) showed that cheakpea nodulating bacteria are potential siderophores producing strains with an interesting role in low iron-content soils. However, none of the rhizobial strain that nodulate chickpea in India was found able to produce siderophore (Ahmad et al. 2008; Joseph et al. 2007).
A fourth important direct growth enhancement mechanism is IAA production. IAA, a type of auxin, is involved in many physiological processes in plant development such as cell elongation and division, tissue differentiation, root initiation (Gravel et al. 2007), nodule formation (Gopalakrishnan et al. 2015) and N2 fixation (Alemneh et al. 2020; Defez et al. 2019). In our study, we found that 17% of tested strains were able to synthesize IAA in vitro up to 120 µg/ml (Fig. 3). The enhancement of root ramification by auxins is interesting in low fertile and water deficient soils, allowing plants to increase their mineral nutrient/water availability. In India, two different investigations found respectively that 85.7% (30/35) and 83.3% (5/6) of M. ciceri strains tested were able to produce IAA (Ahmad et al. 2008; Joseph et al. 2007). In Portugal, all isolates synthesized IAA but the production rates varied between the province of origin and the species group (Brígido et al. 2017). We noticed that some of our strains produce a higher amount of IAA than those in Portugal and India (Ahmad et al. 2008; Brígido et al. 2017; Verma et al. 2013).
Biocontrol is a biotic interaction by which an organism reduces the growth of undesired organisms. Various rhizobial strains have been shown to have biocontrol properties (Ansari and Mahmood 2019; Gopalakrishnan et al. 2015). In our study, three isolates (LL20, LL29 and LL72) were shown to inhibit the growth of Fusarium oxysporum by 43% (Fig. 3) and were assigned to M. mediterraneum and A. tumefaciens. Recently, Bouabdellah and Laouana (2014) reported an inhibitory action of chickpea-rhizobia against Fusarium sp.
Molecular characterization showed that chickpea-isolates having PGPR activities belonged to four species: M. ciceri, M. mediterraneum, S. meliloti and A. tumefaciens. The first two species are specific rhizobia for chickpea. A. tumefaciens strains are pathogenic bacteria but sometimes, when missing their oncogenic DNA, can behave as endophytic bacteria. Different non-nodulating species belonging to genus Burkholderia, Pantoea, Pseudomonas, and Stenotrophomonas were isolated from chickpea-nodules (Benjelloun et al. 2019). M. ciceri and M. mediterraneum have been previously reported to have PGPR activities (Gopalakrishnan et al. 2015) however; S. meliloti and A. tumefaciens strains isolated from chickpea root-nodules have been identified for the first time, in our study, having PGP traits. These bacteria should be further studied for the stability of their PGP activities.
In our study, we found a differential distribution of rhizobia showing PGP traits according to their geographic origin (Fig. 4). The region of Mateur in northern Tunisia, with clay-silty soil, was the origin of 53% of PGP isolates; however, the smallest number (11%) was found in the regions of Sousse and Mograne, with sandy-clay and silty-clay soils, respectively. In fact, microbial communities are controlled by biotic and abiotic factors (e.g., soil type, plant host genotype, climate variability, anthropogenic activities) which are not totally understood (Igiehon and Babalola 2018; Raymond et al. 2021). Similar results were found with nitrogen-fixing rhizobia nodulating chickpea in Tunisia (Ben romdhane et al. 2007b, 2009), in Ethiopia (Gunnabo et al. 2021) and chickpea-rhizobia showing different plant growth-promoting traits in Portugal (Brígido et al. 2017). Recently, Greenlon et al. (2019) found a gradient in chickpea-Mesorhizobium diversity from north to south, and by soil type, showing that bacteria dominating each region are probably adapted to the environmental conditions in these regions. The capacity of rhizobacteria to manifest PGP activities and their diversity can be affected by host-plant characteristics, management practices and micro-environmental conditions (Vives-Peris et al. 2020).
Moreover, our results showed that most of M. ciceri isolates that had PGP traits were able to solubilize phosphorus (8/12) and produce auxin (7/12) whereas most of those belonging to M. mediterraneum were able to produce siderophores (7/10). However, results obtained from Portugal showed that isolates unable to make siderophores also failed to solubilize inorganic phosphorus (Brígido et al. 2017).
The application of PGPR for plant growth and control of fungal pathogens in greenhouse systems shows important expectations due to the stable environmental conditions. Obtaining consistent results in the field where there is heterogeneity of abiotic and biotic factors and competition with indigenous microorganisms is challenging (Alemneh et al. 2020; Raymond et al. 2021). In our study, field inoculation with the selected strain LL10 in Menzel Ibrahim field, which was low in assimilated phosphorus and iron, induced an increase in efficient nodulation (with pink color), plant growth and grain-yield production. However, the mechanisms by which rhizobacteria enhance nodulation of inoculant and soil rhizobia remain evasive (Alemneh et al. 2020). Our results corroborate with those of Saïdi et al. (2013) for field inoculation of faba bean with Rhizobium sp. strain FB206. A significant increase in straw yield and chickpea-grain yield was also obtained in India (Verma et al. 2013). The application of microbial biofertilizers improved the growth, crop yield and soil fertility (Kumar et al. 2022; Nosheen et al. 2022). Our strains had also proved to be good plant growth-promoting bacteria with cereals in crop rotation systems (subjective observations by farmers without statistical analysis). Similar results were found by inoculating M. mediterraneum strain on chickpea and barley under growth chamber conditions in Spain (Peix et al. 2001). The effectiveness of biofertilizers in increasing the production of cereals and pulses was recently documented by Khanna et al. (2019).
Our investigation showed that 26% of native chickpea rhizobial isolates had multifaceted plant growth-promoting activities; two rhizobial strains (LL20 and LL72) had four PGP traits; twelve strains had three PGP traits; twelve other strains had two PGP traits; and finally, two strains (LL4 and LL21) had one PGP trait. Moreover, this work revealed that PGP traits in chickpea Mesorhizobium spp. were related to their region of origin and species identity. Additionally, the inoculated strain LL10 gave a significant increase in plant growth and health, leading to higher grain yield in Tunisian soils. These rhizobial strains could be used for multiple agricultural fertilizations depending on farmer’s needs to avoid soil and water contamination by chemical fertilizers and pesticides.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adedeji AA, Häggblom MM, Babalola OO (2020) Sustainable agriculture in Africa: plant growth promoting rhizobacteria (PGPR) to the rescue. Sci Afr. https://doi.org/10.1016/j.sciaf.2020.e00492
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Alemneh AA, Zhou Y, Ryder MH, Denton MD (2020) Mechanisms in plant growth promoting rhizobacteria that enhance legume–rhizobial symbioses. J Appl Microbiol 129:1133–1156
Alikhani HA, Saleh-Rastin N, Antoun H (2006) Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 287:35–41
Alori ET, Babalola OO (2018) Microbial inoculants for improving crop quality and human health in Africa. Front Microbiol 9:2213
Ansari RA, Mahmood I (2019) Plant Health Under Biotic Stress. Volume 2, Microbial Interactions. Springer, Singapore
Antoun H, Kloepper JW (2001) Plant growth promoting rhizobacteria. In: Brenner S, Miller JH (eds) Encyclopedia of genetics. Academic Inc, New York, pp 1477–1480
Armstrong RA, Hilton AC (2010) Statistical analysis in microbiology: statnotes. Wiley, New Jersey
Barea JM (2015) Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. J Soil Sci Plant Nutr 15:261–282
Beck DP, Materon LA, Afandi F (1993) Practical Rhizobium-legume technology manual, Technical manual no. 19. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria
Ben Romdhane S, Aouani ME, Mhamdi R (2007a) Inefficient nodulation of chickpea (Cicer arietinum L.) in the arid and Saharan climates in Tunisia by Sinorhizobium meliloti biovar medicaginis. Ann Microbiol 57:15–20
Ben Romdhane S, Tajini F, Trabelsi M, Aouani ME (2007b) Competition for nodule formation between introduced strains of Mesorhizobium ciceri and the native populations of rhizobia nodulating chickpea (Cicer arietinum) in Tunisia. World J Microbiol Biotechnol 23:1195–1201
Ben Romdhane S, Aouani ME, Trabelsi M, De Lajudie P (2009) The diversity of rhizobia nodulating chickpea (Cicer arietinum) under water deficiency as a source of more efficient inoculants. Soil Biol Biochem 41:2568–2572
Benjelloun I, Thami-Alami I, Douira A, Udupa SM (2019) Phenotypic and genotypic diversity among symbiotic and non-symbiotic bacteria present in chickpea nodules in Morocco. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01885
Bouabdellah F, Laouana S (2014) Etude de la solubilisation du phosphate et effet d’antagonisme des bactéries isolées de la légumineuse Cicer arietinum. Dissertation, University of Constantine 1, Algeria
Brígido C, Glick BR, Oliveira S (2017) Survey of plant growth-promoting mechanisms in native portuguese chickpea Mesorhizobium isolates. Microb Ecol 73:900–915
Defez R, Andreozzi A, Romano S, Pocsfalvi G, Fiume I, Esposito R, Angelini C, Bianco C (2019) Bacterial IAA-delivery into medicago root nodules triggers a balanced stimulation of C and N metabolism leading to a biomass increase. Microorganisms 7:403
Fasusi OA, Cruz C, Babalola OO (2021) Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture. https://doi.org/10.3390/agriculture11020163
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole 3 acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Greenlon A, Chang PL, Damtew ZM, Muleta A, Carrasquilla-Garcia N, Kim D, Nguyen HP, Suryawanshi V, Krieg CP, Yadav SK, Patel JS, Mukherjee A, Udupa S, Benjelloun I, Thami-Alami I, Yasin M, Patil B, Singh S, Sarma BK, von Wettberg EJB, Kahraman A, Bukun B, Assefa F, Tesfaye K, Fikre A, Cook DR (2019) Global-level population genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria. PNAS 116(30):15200–15209
Gunnabo AH, van Heerwaarden J, Geurts R, Wolde-meskel E, Degefu T, Giller KE (2021) Phylogeography and symbiotic effectiveness of rhizobia nodulating chickpea (Cicer arietinum L.) in Ethiopia. Microb Ecol 81:703–716
Igiehon NO, Babalola OO (2018) Rizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. Int J Environ Res Public Health 15:574–586
Jha CK, Saraf M (2015) Plant growth promoting rhizobacteria (PGPR): a review. E3. J Agric Res Develop 5:108–119
Joseph B, Patra RR, Lawrence R (2007) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Prod 2:141–152
Khanna R, Pawar J, Gupta S, Verma H, Trivedi H, Kumar P, Kumar R (2019) Efficiency of biofertilizers in increasing the production potential of cereals and pulses: a review. J pharmacogn phytochem 8(2):183–188
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Korbu L, Tafes B, Kassa G, Mola T, Fikre A (2020) Unlocking the genetic potential of chickpea through improved crop management practices in Ethiopia. Agron Sustain Dev 40:1–20
Kumar S, Diksha, Sindhu SS, Kumar R (2022) Biofertilizers: an ecofriendly technology for nutrient recycling and environmental sustainability. CRMICR. https://doi.org/10.1016/j.crmicr.2021.100094
Laguerre G, van Berkum P, Amarger N, Prévost D (1997) Genetic diversity of rhizobial symbionts isolated from legume species within the genera Astragalus, Oxytropis and Onobrychis. Appl Environ Microbiol 63:4748–4758
Nosheen S, Ajmal I, Song Y (2021) Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability. https://doi.org/10.3390/su13041868
Nosheen A, Yasmin H, Naz R, Keyani R, Mumtaz S, Hussain SB, Hassan MN, Alzahrani OM, Noureldeen A, Darwish H (2022) Phosphate solubilizing bacteria enhanced growth, oil yield, antioxidant properties and biodiesel quality of Kasumbha. Saudi J Biol Sci 29:43–52
Nour SM, Cleyet-Maret JC, Normand P, Fernandez MP (1995) Genomic heterogeneity of strains nodulating chikpeas (Cicer arietinum L.) and description of Rhizobium mediterraneum sp. nov. Int J Syst Bacteriol 45:640–648
Peix A, Rivas-Boyero AA, Mateos PF, Rodríguez-Barrueco C (2001) Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol Biochem 33:103–110
Raymond NS, Gomez-Munoz B, van der Bom FJT, Nybroe O, Jensen LS, Muller-Stover DS, Oberson A, Richardson AE (2021) Phosphate-solubilising microorganisms for improved crop productivity: a critical assessment. New Phytol 229:1268–1277
Saïdi S, Chebil S, Gtari M, Mhamdi R (2013) Characterization of root-nodule bacteria isolated from Vicia faba and selection of plant growth promoting isolates. World J Microbiol Biotechnol 29:1099–1106
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156:1164–1170
Sridevi M, Mallaiah KV (2009) Phosphate solubilization by Rhizobium strains. Indian J Microbiol 49:98–102
Swift R, Denton MD, Melino VJ (2019) Plant probiotics for nutrient acquisition by agriculturally important grasses: A comprehensive review of the science and the application. Annu Plant Rev 2:1–47
Sylvester-Bradley R, Asakawa N, La Torraca S, Magalhaes FMM (1982) Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramineas e leguminosas forrageiras na Amazônia. Acta Amaz 12:15–22
Vadez V, Rodier F, Payré H, Drevon JJ (1996) Nodule permeability to O2 and nitrogenase-linked respiration in bean genotypes varing in the tolerance of N2 fixation to P deficiency. Plant Physiol Biochem 34:871–878
Verma JP, Yadav J, Tiwari KN, Kumar A (2013) Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng 51:282–286
Vincent JM (1970) A manual for the practical study of root nodule bacteria, IBP handbook no 15. Blackwell, Oxford
Vives-Peris V, de Ollas C, Gomez–Cadenas A, Perez–Clemente RM (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39:3–17
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Weiβ CH (2007) statSoft, Inc., Tulsa, OK: STATISTICA, version 8, AStA, vol 91, pp 339–341
Zhang JJ, Liu TY, Chen WF, Wang ET, Sui XH, Zhang XX, Li Y, Li Y, Chen WX (2012) Mesorhizobium muleiense sp. nov., nodulating with Cicer arietinum L. Int J Syst Evol Microbiol 62:2737–2742
Acknowledgements
The authors thanks Dr. Olubukola Oluranti Babalola’s, PhD Microbiology (North West University, Department of Natural and Agricultural Sciences, South Africa), for her quick editing.
Funding
This work was supported by the Tunisian Ministry of Higher Education & Scientific Research (MHESR) under Grant 2015–2018 (Improvement of Legume Production) for the Laboratory of Legumes, Centre of Biotechnology of Borj-Cédria.
Author information
Authors and Affiliations
Contributions
Samir Ben romdhane designed and performed the experiments. Data analysis and manuscript writing were also done by the same author. Moncef Mrabet, Philippe De lajudie and Jeffry J. Fuhrmann edited the manuscript critically and very carefully. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ben Romdhane, S., De Lajudie, P., Fuhrmann, J.J. et al. Potential role of rhizobia to enhance chickpea-growth and yield in low fertility-soils of Tunisia. Antonie van Leeuwenhoek 115, 921–932 (2022). https://doi.org/10.1007/s10482-022-01745-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-022-01745-5