Abstract
A collection of 104 isolates from root-nodules of Vicia faba was submitted to 16S rRNA PCR–RFLP typing. A representative sample was further submitted to sequence analysis of 16S rRNA. Isolates were assigned to 12 genera. All the nodulating isolates (45 %) were closely related to Rhizobium leguminosarum USDA2370T (99.34 %). The remaining isolates, including potential human pathogens, failed to nodulate their original host. They were checked for presence of symbiotic genes, P-solubilization, phytohormone and siderophore production, and then tested for their growth promoting abilities. Results indicated that 9 strains could induce significant increase (41–71 %) in shoot dry yield of faba bean. A Pseudomonas strain was further assessed in on-farm trial in combination with a selected rhizobial strain. This work indicated that nodule-associated bacteria could be a valuable pool for selection of effective plant growth promoting isolates. Nevertheless, the possible involvement of nodules in increasing risks related to pathogenic bacteria should not be neglected and needs to be investigated further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although root nodules represent a minor fraction of the rhizosphere, they are a wealthy interface for microbial resources and molecular connections. It was admitted for longtime that root-nodule occupation was restricted to rhizobia. However, an increasing number of α- β- and γ-Proteobacteria have been isolated from root nodules of a wide range of legumes and reported as nodulating bacteria (Moulin et al. 2001; Sy et al. 2001; Rivas et al. 2002; van Berkum and Eardly 2002; Vandamme et al. 2002; Valverde et al. 2005; Lin et al. 2008) or nodule-associated bacteria (Sturz et al. 1997; Gao et al. 2001; Zakhia et al. 2006; Kan et al. 2007). The non-symbiotic nodule endophytes have been poorly studied compared to symbiotic bacteria. Rhizobia were also frequently isolated from roots of non-legume plants and consequently could also be included as non-symbiotic endophytes (Antoun and Prévost 2005). Generally, endophytes are defined as microorganisms able to successfully colonize tissues of higher plants and cause unapparent and asymptomatic infections. They may establish neutral, detrimental or beneficial interactions with plants. Rhizobacteria that exert beneficial effects on plant development are termed plant growth-promoting rhizobacteria (PGPR). Although the mechanisms by which they promote plant growth are not yet fully understood, multiple modes of action were revealed to be responsible for growth promotion activities. They include direct and indirect mechanisms. Direct effects result from their ability to fix nitrogen, to increase nutrient uptake through solubilization of minerals, or to produce siderophores and phytohormones (Kennedy et al. 2004; Glick et al. 2007). Indirect effects are expressed when PGPR act like biocontrol agents inducing resistance against plant pathogens, reducing diseases, stimulating other beneficial symbioses, or degrading xenobiotics in contaminated soils (Jacobsen 1997). However, the biological significance and the agronomic implications of nodule endophytism are still not well understood. It was reported that nodule endophytic bacteria might evolve into symbiotic bacteria by acquiring symbiotic genes from rhizobia by lateral gene transfer (Taghavi et al. 2005). It was also reported that an endophytic Agrobacterium strain may behave like a PGPR or plant growth deleterious rhizobacteria (PGDR) according to the antagonistic interaction with the rhizobial strain (Chihaoui et al. 2012; Salem et al. 2012). Other studies outlined the potential involvement of legume nodules in increasing risks related to human pathogens (Muresu et al. 2010).
This study aimed to characterize the diversity of bacteria associated with root-nodules of Vicia faba plants collected from different locations in Tunisia and to assess their ability for promoting plant growth in order to select potential PGPR that perform higher plant growth promotion for use as biofertilizers.
Materials and methods
Nodule bacteria isolation
Local V. faba plants growing in 20 different sites commonly cultivated with this crop were collected. The collection area covered different bioclimatic regions from the north to the south of Tunisia. The roots were thoroughly washed and five nodules representing size and color variability were sampled in each site from a single plant. Nodules were surface sterilized in ethanol 95° for 15 s and HgCl2 0.1 % for 2 min, thoroughly washed with sterile water and then aseptically crushed onto YEM plates according to Vincent (1970). After 4 days of incubation at 28 °C, a single colony representing the major phenotype from each nodule extract was picked and purified by single-colony streaking on YEM plates. To test the efficiency of the surface-sterilization protocol, aliquots of the sterile distilled water used in the final rinse were plated onto YEM plates and incubated at 28 °C for 5 days. Whole sterilized nodules were also checked for surface sterilization by incubation in YEM broth (Vincent 1970).
PCR–RFLP and sequencing analysis
PCR–RFLP of the 16S rRNA genes was conducted for the 104 isolates using the restriction endonucleases MspI and NdeII according to Mhamdi et al. (2002). Representative isolates of the different ribotypes were selected for nearly full-length sequencing of the 16S rRNA gene according to Saïdi et al. (2011). Sequences were checked manually and assembled by the CAP program (http://pbil.univ-lyon1.fr/cap3.php). The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to search for sequence similarities in the DNA databases. Sequences were aligned using the ClustalW2 software (http://www.ebi.ac.uk/Tools/clustalw2/). Phylogenetic trees were constructed using the phylogenetic inference package MEGA5 by the Neighbor-Joining method. The trees were bootstrapped with 1,000 replicates to assess the confidence limits of the branching. The evolutionary distance was computed using the Kimura 2-parameter method.
Symbiotic tests
The nodule formation was checked for all isolates by inoculating faba bean (V. faba) and Lentil (Lens culinaris) seedlings grown on pots containing sterile gravel with approximately 108 cfu as described by Vincent (1970). Nodule formation was checked 40 days after inoculation. The nodulating isolates were typed by PCR–RFLP of the nodC gene and the intergenic spacer nifD-K using HaeIII as previously reported (Saïdi et al. 2009). Effectiveness on faba bean was tested on pots filled with sterile sand using a sample of 10 isolates representing the different nifD-K and nodC types as previously reported (Mhamdi et al. 2002). After two months, plants were harvested and shoot dry weights were recorded. Data were submitted to mean comparison using the LSD test (p < 0.05) by the ANOVA program of the STATISTICA software. The non-nodulating isolates were tested for the presence of nifH-like genes using primers nifHf and nifHi according to Mhamdi et al. (2002) and by decreasing the hybridization temperature to 55 °C. A subsample of 18 isolates representing the different ribotypes found among the non-nodulating isolates was retained for further analysis.
Production of indolic compounds
Production of indolic compounds by the selected isolated was analyzed using the PC-colorimetric technique of Pilet and Chollet (1970). TY medium supplemented with 2.5 mM of tryptophan (Trp) was used for preparing bacterial cultures (Glickmann and Dessaux 1995). The stationary phase culture was centrifuged at 6,000 rpm for 30 min and the supernatant was mixed with the Salkowski reagent (1:1 v/v). After incubation in darkness for 30 min at room temperature, color changes were measured at 530 nm. The production of indolic compounds was recognized by the presence of red coloring. Varied concentrations of pure indole-3-acetic acid were used as standard curve.
Phosphate solubilization assay
Selected isolates were screened for their phosphate solubilization ability according to Beneduzi et al. (2008). Three replicates were considered for each strain. The formation of a clear zone around the bacterial colony was considered as positive result.
Siderophore production assay
Siderophore production by the selected isolates was tested qualitatively using Chrome Azurol S (CAS) agar as described by Alexander and Zuberer (1991). The bacterial cultures were spread on the CAS agar plates with two replications each. Orange halos around colonies after overnight incubation indicated the production of siderophores.
Salt tolerance
In order to select for isolates adapted to saline and arid regions, salt tolerance of isolates was carried out as previously described (Mnasri et al. 2007). Bacterial strains were grown on 1 M NaCl YEM broth medium. The ability to grow was checked visually after 5 days of incubation at 28 °C.
Controlled inoculation trial
Representatives of non-nodulating isolates were checked for their PGPR potentialities in a glasshouse trial. The isolates that featured taxa known as human pathogens were excluded from this experiment. Seeds of V. faba var. minor were grown in plastic pots (Ø 30 cm) containing soil samples from an experimental field of the Centre of Biotechnology of Borj-Cedria. YEM broth containing flasks were inoculated with a YEM slant pre-culture suspension and incubated on a rotary shaker at 150 rev min−1 and 28 °C. At mid-exponential growth phase, the equivalent of 20 μl of broth culture for OD620nm = 1 was transferred to 250 ml flasks containing 100 ml of YEM broth and grown for 48 h. Emerging seedlings were inoculated with a fresh suspension of the bacterial strain (approximately 109 cfu). Nine plants were considered for each treatment (three plants per pot). Uninoculated plants were included as control. After 2 months, plants were harvested and shoot dry weights were recorded. Data were submitted to mean comparison as described above.
On-field inoculation trial
A small-scale field inoculation trial was performed in the region of Takilsa (36°47′12.98″N, 10°37′41.83″E) using a selected rhizobial strain (FB206) and a selected nodule endophyte (FB201b). The field was chosen for its proximity and also for the willingness of the farmer to cooperate to the experiment. The experimental design consisted of four treatments: uninoculated, inoculated with FB206, inoculated with FB201b and co-inoculated with both strains. Treatment plots were 10/10 m and were separated by a margin of 3 m. The sowing density was 20 seeds m−2. The inoculants were grown separately to late exponential phase in YEM medium and diluted with well water (1/20) and then used to inoculate two-leaf stage seedlings by manually applying about 5 ml on each plant along the seedling line using a watering can. The experiment was conducted under irrigation and without further fertilization, or any other amendment. Weeds were removed manually. Seventy days after inoculation, 30 plants were randomly collected from each treatment and used for the determination of shoot dry weight and grain yield. Data were submitted to mean comparison as described above.
Results
Diversity of V. faba root-nodule isolates
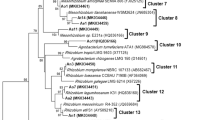
A collection of 104 strains isolated from surface-sterilized root-nodules of V. faba was firstly investigated with PCR–RFLP of 16S rRNA genes. Although the use of two restriction enzymes does not allow full determination of diversity, it allowed as a preliminary screening a quite accurate estimation of 22 ribotypes that were sampled for further analysis. Sequence analysis of nearly full-length 16S rRNA genes of representative isolates revealed a high genetic diversity (Table 1; Fig. S1). The three representative isolates of the dominant ribotype A1 (45 % of total isolates) showed 99.34 % sequence identity with the type strain of Rhizobium leguminosarum (USDA 2370T). When re-examined for nodulation, all members of ribotype A1 induced nodules on faba bean and lentil. However, all the remaining isolates, failed to induce nodules on both hosts. Non-symbiotic rhizobia lacking symbiotic genes (ribotypes A2, D, E, F, C, B1 and B2) were also frequently isolated (14 strains), their closest relatives were respectively, Rhizobium grahamii, Rhizobium huautlense, Rhizobium albertimagni (formerly Agrobacterium albertimagni), Rhizobium pusense, Rhizobium nepotum (formerly Agrobacterium tumefaciens genomovar G14) and Ensifer meliloti. These are followed by isolates of ribotype N (9 isolates) and ribotypes L1/2/3 (9 isolates), respectively affiliated to Pseudomonas and Enterobacter. Isolates affiliated to Pantoea (ribotypes L4 and Q), Bacillus (ribotypes O, T and G) and Rahnella (ribotype I) were respectively represented by 7, 6 and 5 members. The remaining isolates within the ribotypes H, P, R and S were affiliated to Staphylococcus, Serratia, Stenotrophomonas and Xanthomonas.
The non-nodulating bacteria were checked for the presence of symbiotic genes. They failed to amplify nifH or nodC genes with the primers used even under low stringency conditions except for isolates assigned to Rahnella that showed a nifH-like amplification product.
PGPR abilities
The non-symbiotic isolates were checked for their in vitro abilities to produce indolic compounds, to solubilize phosphates and to produce siderophores. They were then used to inoculate faba bean plants grown in non-sterile soil under glasshouse conditions. The results are given in Table 2. The shoot dry yield of several inoculated plants increased significantly (41–71 %) comparing to the uninoculated treatment. To confirm these results further, Pseudomonas sp. strain FB201b, showing positive results for all screening assays, was retained for a small scale field inoculation trial. Similarly, nodulating representatives of the different nodC and nifD-K PCR–RFLP groups were evaluated for their symbiotic effectiveness on faba bean under laboratory conditions (data not shown). Strain FB206, inducing the highest shoot dry weight, was also retained for field inoculation. Separate inoculations with strains FB206 or BF201b highly increased shoot dry weight and grain yield, the latter increased by more than thrice (Table 3). Co-inoculation with both strains also significantly increased grain yield comparing to inoculation with the rhizobial strain alone.
Discussion
Faba bean (V. faba var. minor) and fava bean (V. faba var. major) are considered major legume crops in Tunisia and had been extensively cultivated as green manure as well as for feed and food. They are extensively used in co-cropping and intercropping systems with different cultures where they are believed to enhance soil fertility through their symbiotic potentialities. These crops are usually well nodulated in Tunisian soils; however, there are no studies about the diversity and the effectiveness of the nodulating rhizobia. In this study a special interest has been focused on microbial diversity of V. faba nodule isolates with the aim to select efficient strains for inoculation. A unique isolate representing the major colony-morphology phenotype was retained for each nodule analyzed. Only 45 % of isolates were able to re-nodulate their original host. They were all closely related to R. leguminosarum. However, a high proportion of isolates (55 %) could not nodulate V. faba and either L. culinaris. The most frequent were non-symbiotic rhizobia, mainly taxa previously known as agrobacteria (25 %), and taxa affiliated to Pseudomonas (16 %) and Enterobacter (16 %), which have been frequently isolated from root-nodules of various legumes (de Lajudie et al. 1999; Ibanez et al. 2009; Ramezanpour 2011). In most cases, isolates were affiliated to type strains with high 16S sequence identity, except for isolates of ribotypes I, O and R, and two isolates from ribotype B1, whose closest relative species were respectively, Rahnella aquatilis, Bacillus muralis, Stenotrophomonas maltophilia and Shinella kummerowiae, with less than 99 % of sequence similarity (Table 1). These strains could probably constitute new species but further analysis such as housekeeping gene sequencing and DNA-DNA hybridization have to be performed.
The prevalence of non-nodulating isolates in nodule extracts may be explained by different alternatives, including loss of symbiotic genes, opportunistic colonization by rhizospheric bacteria or surface contamination of nodules. However, since they survived after chemical surface sterilization of nodules and represented the major phenotype recovered from each nodule they will be considered as putative endophytes. Microscopic observations are required to definitively confirm the endophytic character of these isolates and clarify their localization inside nodule tissues. This had been shown at least for nodule isolates related to the former Agrobacterium (Mhamdi et al. 2005). Compared to similar studies on other legumes in Tunisia, such as Sulla coronaria, Cicer arietinum (unpublished data) Phaseolus vulgaris (Mhamdi et al. 2002) that showed that endophytic colonization of nodules does not exceed 25 %; in the present study we found that 55 % of nodule isolates were putative endophytes. This high frequency of endophytes in V. faba root-nodules prompted the study of their effect on plant growth. However, the strains related to the species Serratia odorifera, causing serious disease including pneumonia and bacteremia (Lee et al. 2006), Staphylococcus saprophyticus, associated with urinary tract infection (Raz et al. 2005), S. maltophilia, an emerging pathogen for nosocomial infections and meningitis (Adamek et al. 2011), Enterobacter amnigenus, that can cause opportunistic bacterial infection in human (Capdevila et al. 1998) and Enterobacter cancerogenus, that can cause wound infections and septicemia (Abbott and Janda 1997) will not be further considered in this work because of their potential human disease properties. In all cases, it would be necessary to check the pathogenicity of all strains prior to recommend their future use.
Screening for PGPR abilities showed that production of indolic compounds by nodule endophytes varied greatly among different species and strains. The highest producers were strains affiliated to Pantoea. However, in presence of Trp, a metabolic precursor of indolic compounds used by most soil bacteria, the largest productions were found among strains affiliated to the Agrobacterium/Rhizobium group, then Rahnella sp., Shinella sp. and Pantoea sp. The lowest producers of indolic compounds that seemed unaffected by the addition of Trp were strains affiliated to Bacillus. Only strains affiliated to R. nepotum and Pseudomonas marginalis were positive producers of siderophores. The most efficient phosphate solubilizing strains were affiliated to Pseudomonas genus whose strains are well known as efficient phosphate solubilizers (Tilak et al. 2005). Strains affiliated to Rahnella, Bacillus and Pantoea spp. were also phosphate-solubilizing bacteria, but with less efficiency. For the remaining strains, the halo was discreet or absent, their phosphate solubilization ability has to be verified according to the direct measurement of phosphate solubilization in NBRI-BPB broth assay (Mehta and Nautiyal 2001).
The glasshouse trial indicated that 9 strains induced significant increase (41–71 %) in shoot dry yield. These strains were respectively affiliated to Rhizobium (5 isolates), Ensifer, Rahnella, Pantoea and Pseudomonas species. However, there was no clear correlation between the PGPR abilities observed in vitro and shoot dry yield. Nevertheless, isolates with low production of indolic compounds (<10 μg/ml) usually did not produce significant increase in plant growth. The on-farm trial confirmed the high effectiveness of Pseudomonas sp. strain FB201b when inoculated alone or in combination with the selected rhizobial strain FB206. Plants in the control treatment were all highly nodulated, which would indicate the high competitive ability of the selected rhizobial strain against the resident bacterial population. The positive effect observed with the rhizobial strain could be also due to indirect effects on rhizosphere microbial communities. Trabelsi et al. (2012) showed that inoculation of common bean with rhizobia may induce proliferation of bacterial communities with PGPR potentialities. The extent of these changes marked also the second crop season in more ways than just furnishing residual nutrients, but also through stimulating plant growth and enhancing disease resistance. The way that rhizobia enhance proliferation of other microbial communities is not clear and should be investigated further. It seems that nodules may play a crucial role as a niche for proliferation and dissemination seeing their ability to be colonized by different bacterial taxa. This issue raises many questions about the possible involvement of legume nodules in increasing risks related to plant and human pathogens. If this is true, agronomic practices based on co-cropping and inter-cropping with legumes, which had been prompted for a long time, should be reconsidered mainly in soils contaminated with pathogens. Further work should be done to shed more light on this issue.
Conclusion
Bacteria isolated from root-nodules of V. faba grown in different Tunisian soils were highly diverse and could be affiliated to 12 genera, including R. leguminosarum and a high proportion of putative nodule endophytes. Some of these endophytes showed PGPR abilities, indicating that legume nodules may be a valuable source for selecting effective microorganisms. However, other endophytes featured taxa known as human pathogens, raising the concern of enhancing the hazards posed by pathogens.
References
Abbott SL, Janda JM (1997) Enterobacter cancerogenus (“Enterobacter taylorae”) infections associated with severe trauma or crush injuries. Am J Clin Pathol 107:359–361
Adamek M, Overhage J, Bathe S, Winter J, Fischer R, Schwartz T (2011) Genotyping of environmental and clinical Stenotrophomonas maltophilia isolates and their pathogenic potential. PLoS ONE 6(11):e27615. doi:10.1371/journal.pone.0027615
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fert Soils 2:39–45
Antoun H, Prévost D (2005) Ecology of plant growth Promoting Rhizobacteria. ZA Siddiqui (ed.) PGPR: Biocontrol and Biofertilization. Springer, Dordrecht, pp 1–38
Beneduzi A, Peres D, Vargas LK, Bodanese-Zanettini MH, Passaglia LMP (2008) Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing bacilli isolated from rice fields in South Brazil. Appl Soil Ecol 39:311–320
Capdevila JA, Bisbe V, Gasser I, Zuazu J, Olivé T, Fernández F, Pahissa Berga A (1998) Enterobacter amnigenus. An unusual human pathogen. Enferm Infecc Microbiol Clin 16:364–366
Chihaoui SA, Mhadhbi H, Mhamdi R (2012) The antibiosis of nodule-endophytic agrobacteria and its potential effect on nodule functioning of Phaseolus vulgaris. Arch Microbiol 194:1013–1021
de Lajudie P, Willems A, Nick G, Mohamed TS, Torck U, Filali-Maltouf A, Kersters K, Dreyfus B, Lindstrom K, Gillis M (1999) Agrobacterium bv.1 strains isolated from nodules of tropical legumes. Syst Appl Microbiol 22:119–132
Gao J, Terefework Z, Chen W, Lindstrom K (2001) Genetic diversity of rhizobia isolated from Astragalus adsurgens growing in different geographical regions of China. J Biotechnol 91:155–168
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. App Environ Microbiol 61:793–796
Ibanez F, Angelini J, Taurian T, Tonelli ML, Fabra A (2009) Endophytic occupation of peanut root nodules by opportunistic Gammaproteobacteria. Syst Appl Microbiol 32:49–55
Jacobsen CS (1997) Plant protection and rhizosphere colonization of barley by seed inoculated herbicide degrading Burkholderia (Pseudomonas) cepacia DBO1 (pRO101) in 2, 4-D contaminated soil. Plant Soil 189:139–144
Kan FL, Chen ZY, Wang ET, Tian CF, Sui XH, Chen WX (2007) Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes grown in Qinghai–Tibet Plateau and in other zones of China. Arch Microbiol 188:103–115
Kennedy IR, Choudhury ATM, Mihaly A, Kecske′s L (2004) Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion be better exploited? Soil Biol Biochem 36:1229–1244
Lee J, Carey J, Perlman DC (2006) Pneumonia and bacteremia due to Serratia odorifera. J Infection 53:212–214
Lin DX, Wang ET, Tang H, Han TX, He YR, Guan SH, Chen WX (2008) Shinella kummerowiae sp. nov., a symbiotic bacterium isolated from root nodules of the herbal legume Kummerowia stipulacea. Int J Syst Evol Microbiol 58:1409–1413
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Mhamdi R, Laguerre G, Aouani ME, Mars M, Amarger N (2002) Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol Ecol 41:77–84
Mhamdi R, Mrabet M, Laguerre G, Tiwari R, Aouani ME (2005) Colonization of Phaseolus vulgaris nodules by Agrobacterium-like strains. Can J Microbiol 51:105–111
Mnasri B, Aouani ME, Mhamdi R (2007) Nodulation and growth of common bean (Phaseolus vulgaris) under water deficiency. Soil Biol Biochem 39:1744–1750
Moulin L, Munive A, Dreyfus B, Boivin-Masson C (2001) Nodulation of legumes by members of beta-class of Proteobacteria. Nature 411:948–950
Muresu R, Maddau G, Delogu G, Cappuccinelli P, Squartini A (2010) Bacteria colonizing root nodules of wild legumes exhibit virulence-associated properties of mammalian pathogens. Antonie Van Leeuwenhoek 97:143–153
Pilet PE, Chollet R (1970) Sur le dosage colorimétrique de l’acide indolylacétique. C R Acad Sci Ser D 271:1675–1678
Ramezanpour MR (2011) Biochemical characteristics and genetic diversity of fluorescent pseudomonads isolated from rice rhizosphere in north of Iran. Am Eurasian J Agric Environ Sci 10:180–185
Raz R, Colodner R, Kunin CM (2005) Who are you-Staphylococcus saprophyticus? Clin Infect Dis 40:896–898
Rivas R, Velazquez E, Willems A, Vizcaino N, Subbarao NS, Mateos PF, Gillis M, Dazzo FB, Molina EM (2002) A new species of Devosia that forms a unique nitrogen fixing root symbiosis with aquatic legume Neptunia natans (l.f) Druce. Appl Environ Microbiol 68:5217–5222
Saïdi S, Zribi K, Badri Y, Aouani ME (2009) Genetic characterization and symbiotic proprieties of native sinorhizobia trapped by Medicago sativa nodules on Tunisian soils. Aust J Soil Res 47:321–327
Saïdi S, Mnasri B, Mhamdi R (2011) Diversity of nodule endophytic agrobacteria-like strains associated to different grain legumes in Tunisia. Syst Appl Microbiol 34:524–530
Salem S, Saidi S, Chihaoui SA, Mhamdi R (2012) Inoculation of Phaseolus vulgaris, Medicago laciniata and Medicago polymorpha with Agrobacterium sp. strain 10C2 may enhance nodulation and shoot dry weight but does not affect host range specificity. Ann Microbiol 62:1811–1817
Sturz AV, Christie BR, Matheson BG, Nowak J (1997) Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol Fert Soils 25:13–19
Sy A, Giraud E, Jourand P, Garcia N, Willems A, de Lajudie P, Prin Y, Neyra M, Gillis M, Boivin-Masson C, Dreyfus B (2001) Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J Bacteriol 183:214–220
Taghavi S, Barac T, Greenberg B, Borremans B, Vangronsveld J, van der Lelie D (2005) Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl Environ Microbiol 71:8500–8505
Tilak KVBR, Ranganayaki N, Pal KK, De R, Saxena AK, Nautiyal CS, Mittal S, Tripathi AK, Johri BN (2005) Diversity of plant growth and soil health supporting bacteria. Curr Sci 89:136–150
Trabelsi D, Ben Ammar H, Mengoni A, Mhamdi R (2012) Appraisal of the crop-rotation effect of rhizobial inoculation on potato cropping systems in relation to soil bacterial communities. Soil Biol Biochem 54:1–6
Valverde A, Velazquez E, Fernandez-Santos F, Vizcaıno N, Rivas R, Mateosn PF, Martınez-Molina E, Igual JM, Willems A (2005) Phyllobacterium trifolii sp. nov., nodulating Trifolium and Lupinus in Spanish soils. Int J Syst Evol Microbiol 55:1985–1989
van Berkum P, Eardly BD (2002) The aquatic budding bacterium Blastobacter denitrificans is a nitrogen fixing symbiont of Aeschynomene indica. Appl Environ Microb 68:1132–1136
Vandamme P, Henry D, Coenye T, Nzula S, Vancanneyt M, LiPuma JJ, Speert DP, Govan JR, Mahenthiralingam E (2002) Burkholderia anthina sp. nov. and Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound results of new molecular diagnostic tools. FEMS Immunol Med Microbiol 33(2):143–149
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. IBP Handbook, vol 15. Blackwell, Oxford
Zakhia F, Jeder H, Willems A, Gillis M, Dreyfus B, de Lajudie P (2006) Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb Ecol 51:375–393
Acknowledgments
The authors wish to thank Mr. Bilel Hchicha (PDG of SOTAM) for assistance in field inoculation trial and Mr. Nabil Lassouad for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saïdi, S., Chebil, S., Gtari, M. et al. Characterization of root-nodule bacteria isolated from Vicia faba and selection of plant growth promoting isolates. World J Microbiol Biotechnol 29, 1099–1106 (2013). https://doi.org/10.1007/s11274-013-1278-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1278-4