Abstract
Rhizobia may possess other plant growth-promoting mechanisms besides nitrogen fixation. These mechanisms and the tolerance to different environmental factors, such as metals, may contribute to the use of rhizobia inocula to establish a successful legume-rhizobia symbiosis. Our goal was to characterize a collection of native Portuguese chickpea Mesorhizobium isolates in terms of plant growth-promoting (PGP) traits and tolerance to different metals as well as to investigate whether these characteristics are related to the biogeography of the isolates. The occurrence of six PGP mechanisms and tolerance to five metals were evaluated in 61 chickpea Mesorhizobium isolates previously obtained from distinct provinces in Portugal and assigned to different species clusters. Chickpea microsymbionts show high diversity in terms of PGP traits as well as in their ability to tolerate different metals. All isolates synthesized indoleacetic acid, 50 isolates produced siderophores, 19 isolates solubilized phosphate, 12 isolates displayed acid phosphatase activity, and 22 exhibited cytokinin activity. Most isolates tolerated Zn or Pb but not Ni, Co, or Cu. Several associations between specific PGP mechanisms and the province of origin and species clusters of the isolates were found. Our data suggests that the isolate’s tolerance to metals and ability to solubilize inorganic phosphate and to produce IAA may be responsible for the persistence and distribution of the native Portuguese chickpea Mesorhizobium species. Furthermore, this study revealed several chickpea microsymbionts with potential as PGP rhizobacteria as well as for utilization in phytoremediation strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhizobacteria can alleviate biotic and abiotic stresses on plant growth [1]. Rhizobia are beneficial soil bacteria that form root nodules and fix atmospheric nitrogen when in association with legumes. The use of rhizobia as plant inoculants is an important component of sustainable agriculture since these organisms can simultaneously improve the growth and yield of legume crops and reduce the need for chemical nitrogen fertilizers [2].
Rhizobia typically exhibit other plant growth-promoting mechanism(s) besides nitrogen fixation and help sustain soil health and productivity [3]. In this regard, some rhizobia can solubilize inorganic phosphorous [4], which in acid or alkaline soils is extremely important, once phosphorous becomes unavailable for plant uptake under these soil pH ranges [5]. Phosphate-solubilizing microorganisms can produce some organic acids and enzymes that transform insoluble phosphates into substances that can be easily assimilated by plants [5, 6]. In addition, some rhizobia can release siderophores that chelate soluble iron [7]. This protects the plant against some pathogenic microorganisms [8].
Besides supplying nutrients to plants, rhizobia can regulate the phytohormone levels, namely auxins, cytokinins, and ethylene, which are all implicated in both plant and nodule development. Some rhizobia are able to produce auxins [9] and/or cytokinins [10], and may possess one of the two naturally occurring mechanisms that can modulate plant ethylene levels, i.e., the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase [11, 12] or synthesis of the molecule rhizobitoxine [13]. These mechanisms have beneficial effects on the symbiotic rhizobium-legume process as well as on plant growth itself. For instance, production of indoleacetic acid (IAA) by rhizobia may play a fundamental role in nodulation and competitive ability as well as contribute to the increase of plant stress tolerance [14–18]. Similarly, rhizobia may also synthesize cytokinins. The cytokinin produced by Bradyrhizobium sp. strain ORS285 accelerates nodule formation and alters nodule number and size [19]. Furthermore, engineered Sinorhizobium strains synthesizing more cytokinin could improve the tolerance of alfalfa to severe drought stress, without affecting alfalfa nodulation or nitrogen fixation [20]. As well, the expression of ACC deaminase by some rhizobia can increase the extent of nodulation of legume roots [21, 22]. In addition, ACC deaminase-overexpressing Mesorhizobium strains improve chickpea growth under both control and stressful conditions [23–25].
Since biodiversity contributes to ecosystems stability and productivity, it is important to understand which environmental factors influence rhizobia diversity. Metals may affect growth, total population size, genetic diversity, nodulation, and efficacy of rhizobia in soil [26]. Moreover, metals can disrupt symbiotic rhizobia-legume associations [27]. For example, the presence of high concentrations of metals in soils has substantial deleterious effects on both survival and nitrogen-fixing efficiency of symbiotic rhizobia [28, 29]. It is therefore important to understand the biogeography of rhizobia in terms of stress tolerance in order to select rhizobia inocula adapted to the climate and soil conditions, including soils with metal contamination [30].
Different species belonging to the genus Mesorhizobium [31, 32], which was described for the first time by Jarvis et al. [33], in the Phyllobacteriaceae family, nodulate chickpea. Previous works on chickpea Mesorhizobium isolates have revealed genetic and phenotypic diversity, including a high diversity in symbiotic effectiveness [31, 32] and tolerance to heat [34], salinity [32], and acidity [35, 36].
The potential of rhizobia to tolerate different environmental factors and to facilitate legume growth by several mechanisms other than nitrogen fixation, particularly in damaged soils, may contribute to their persistence in the soils as well as to help legumes grow in those soils. Since chickpea is one of the most important legume crops worldwide, the characterization of native chickpea microsymbionts in terms of plant growth-promoting traits and metal tolerance is imperative. The present study examines plant growth-promoting traits and tolerance to several metals, namely zinc, cobalt, copper, nickel, and lead, in Mesorhizobium spp. isolated from chickpea and investigates whether the plant growth-promoting traits and metal tolerance are related to the species cluster, soil pH, geographical origin, and symbiotic performance of the isolates. Our data suggest that chickpea Mesorhizobium spp. distribution may result from a selective process based on adaptive mechanisms, that allows them to compete and persist in different types of soils.
Material and Methods
Bacterial Strains
A total of 61 isolates from a collection of native Portuguese chickpea Mesorhizobium spp. previously characterized in terms of plasmid number, symbiotic effectiveness, and tolerance to acidity and salinity [31, 32, 36] were used in this study (Table 1; Fig. 1). Based on the 16S ribosomal RNA (rRNA) gene sequence previously obtained [31, 32], these isolates were assigned to three main species clusters, namely Mesorhizobium ciceri/Mesorhizobium loti (A), Mesorhizobium huakuii/Mesorhizobium amorphae (B), and Mesorhizobium tianshanense/Mesorhizobium mediterraeum/Mesorhizobium temperatum (C) (Supplementary Fig. 1). In addition, six Mesorhizobium type strains, namely M. amorphae ACCC 19665T, M. ciceri UPM-Ca7T, M. mediterraneum UPM-Ca36T, M. huakuii CCBAU2609T, M. tianshanense A-1BST, and M. loti LMG6125T, were used. All strains were routinely grown in Tryptone yeast (TY) broth at 28–30 °C on an orbital shaker at 150 rpm.
Map of Portugal showing the distribution of a total of 61 chickpea mesorhizobia isolates for each province according to their ability to produce siderophores. In each pie chart, the number of isolates and the percentage of isolates able (dark gray) and unable (light gray) to produce siderophores are indicated
Siderophore Production
To estimate siderophore production, we used chrome azurol S (CAS) and hexadecyltrimethylammonium bromide (HDTMA) as indicators [37]. A 10-μl aliquot of an overnight bacterial culture grown in TY liquid medium was spotted onto a CAS agar plate [38] in triplicate and incubated at 30 °C for 1 week. Positive siderophore production was indicated by a color change of the CAS reagent from blue to orange. The formula ((colony + colored zone diameter) / colony diameter) described by Gupta and colleagues [39] was used to distinguish the isolate’s ability to produce siderophores. Index >1 was considered as positive for siderophore production. According to the siderophore production index, four different levels of siderophore production [no production (=1), low (>1 and <2), medium (≥2 and <3), and high (≥3) production] were considered.
Indoleacetic Acid Production
To assess the IAA production by Mesorhizobium spp., a colorimetric method described by Glickmann and Dessaux [40] and modified by Patten and Glick [41] was used. The bacterial inocula were from bacterial cultures grown at 30 °C in minimal liquid medium [42] for 48 h. Aliquots of 250 μl of the bacterial inoculum were used to inoculate 4 ml minimal liquid medium supplemented with tryptophan (0 or 250 μg ml−1) and incubated at 30 °C until the stationary phase was reached (48–72 h). The bacterial cells were removed by centrifugation at 8500×g for 5 min, and 1 ml of the supernatant was mixed with 4 ml Salkowski’s reagent [43]. Following incubation at room temperature for 20–30 min, the absorbance was measured at 535 nm. To calculate the concentration of IAA in each sample, a standard curve ranging from 0.01 to 100 μg ml−1 of pure IAA was used for comparison. According to the amount of IAA produced, four distinct levels of IAA production, low production (<15 μg ml−1), medium production (between 15 and 30 μg ml−1), high production (between 30 and 45 μg ml−1), and very high production (>45 μg ml−1), were considered.
ACC Deaminase Activity
The evaluation of ACC deaminase activity in the chickpea Mesorhizobium cells was performed as described in Brígido et al. [25]. Rhizobium leguminosarum bv. viciae 128C53K was used as a positive control [21] and Mesorhizobium ciceri LMS-1 as a negative control [23].
Organic and Inorganic Phosphate Solubilization
The ability of chickpea Mesorhizobium spp. to solubilize inorganic phosphate solubilization was evaluated according to De Freitas et al. [44]. Briefly, the bacterial inoculum was obtained following overnight growth in TY liquid medium at 30 °C. Ten microliters of bacterial culture was spotted onto solid medium and then incubated for 7–10 days at 30 °C. Phosphate solubilization activity was assayed in triplicate for each isolate. The plates were observed for clear phosphorous-zone formation around the colonies. Inorganic phosphate solubilization activity was estimated by measuring the zone of clearance around each colony and comparison of this zone with the colony diameter. The inorganic phosphate solubilization index = ((colony + clearance zone diameter) / colony diameter) as described by Gupta et al. [39] and was used as the indicator of phosphorous solubilization activity. The cut-off value of phosphate solubilization index is 1, indicating that the bacterial isolate is unable to solubilize phosphorous. According to the phosphate solubilization index, three different levels of inorganic phosphate solubilization were considered: no solubilization (=1), low (>1 and <2), and high (≥2) solubilization.
To investigate if chickpea Mesorhizobium isolates are able to mineralize organic phosphate, the acid phosphatase activity for each Mesorhizobium isolate was determined according to Oliveira et al. [45] with some modifications. Enzyme activity was measured by monitoring the rate of hydrolysis of p-nitrophenyl phosphate (pNPP) into p-nitrophenol. Each Mesorhizobium isolate and strain grew in 5 ml TY liquid medium at 30 °C for 48 h. One milliliter of the bacterial culture was added to 1 ml of acetate buffer (acetic acid/sodium acetate, pH 4.8) and left to equilibrate for 10 min at 30 °C. The absorbance of the cell suspension was measured at 600 nm. An aliquot of 400 μl of cell suspension was transferred to a 1.5-ml Eppendorf tube and 100 μl of 1 mg/ml 4-nitrophenylphosphate was incorporated. The mixture was incubated at 30 °C for 1 h. To stop the reaction, 500 μl of 1 M KOH was added to the mixture. The cells were then pelleted, and the amount of p-nitrophenol produced was quantified by measuring the absorbance at 420 nm. A mixture without bacterial culture was used as a negative control whereas Azospirillum brasilense Sp245 was used as a positive control. The units of specific activity were determined according to the following formula [46]: U/OD600 = 1000(OD420) / (time in minutes * OD600).
Cytokinin Production
To screen for cytokinin production by Mesorhizobium spp., a cucumber cotyledon bioassay was used according to Hussain and Hasnain [47] with some modifications. Briefly, the cucumber seeds were surface sterilized with 0.1% HgCl2 for 3 min and washed six times with sterile distilled water. The seeds were germinated in sterile Petri plates containing double layers of filter papers soaked in sterile distilled water and placed in the dark at room temperature for 5 days. After germination, the cotyledons were excised and transferred to separate sterile Petri plates with double layers of filter papers soaked in sterile distilled water.
Mesorhizobium isolates were streaked on half of a Petri plate containing modified minimal medium [24]. The plates were incubated for 5 days at 30 °C. Ten etiolated cucumber cotyledons per plate were placed close (±2–3 mm) to the bacterial culture and incubated in the dark for 24 h. After incubation, the plates were kept in the light (intensity 30 μmol m−2 s−2) for 4 h at 25 °C. Plates without bacterial culture were used as a negative control, and the chlorophyll content from cotyledons grown with 1 ml of standard cytokinins solution (1.0 μM N-6-benzyladenine) was considered as a positive control. Chlorophyll was extracted from cotyledons grown in each plate with cold acetone and quantified by measurement of the absorbance at 665 nm. The chlorophyll concentration compared to the negative control (D/D 0) was determined. Enhanced chlorophyll formation (>1) was considered as evidence of positive cytokinin activity.
Heavy Metal Tolerance
Mesorhizobium spp. tolerance to Ni, Co, Cu, Zn, and Pb was evaluated based on their growth in 96-well microplates filled with 200 μl per well of Yeast extract mannitol (YEM) broth supplemented with NiCl2, CoCl2, ZnCl2, or CuCl2 at final concentrations 0.25, 0.5, 1, 3, 5, and 10 mM and Pb(CH3COO)2 at final concentrations of 0.05, 0.1, 0.5, 1, 2, and 5 mM. For each isolate, an initial inoculum with an OD565 nm = 0.05 was inoculated into the 96-well microplate. The microplates were incubated with shaking at 28 °C for 4 days. After incubation, the absorbances of the microplates were read at 565 nm using a microplate reader (Multiskan spectrum, Thermo Scientific). Strains were considered tolerant when they showed at least 50% of their growth under control conditions (YEM liquid medium without addition of heavy metals).
Statistical Analysis
Statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, USA). To evaluate the goodness of fit of data to the normal distribution, the one-sample Kolmogorov-Smirnov test for continuous sample distributions was performed. In order to explore the relationship between continuous dependent variables and categorical independent variables, the Kruskal-Wallis one-way nonparametric analysis of variance was conducted. To identify categories that differ significantly from others, three different post hoc tests (Tamhane, Dunnett T3, and Games-Howell) were used. To detect structure in the relationships between categorical variables, the correspondence analysis (CA) was conducted as an exploratory data analysis technique. To investigate whether metal tolerance and specific plant growth-promoting abilities were related to the pH of the origin soil of the isolates, these pH values were divided into three classes (soils with pH values below 6.5 were considered acid soils, whereas neutral soils include soils with pH values ranging from 6.5 to 7.4 and alkaline soils represented soils with pH values above 7.4; data in Supplementary Table 2).
Relationships between categorical variables were determined using the chi-square test of association. Results are presented as the test statistic (χ 2), degrees of freedom (d.f.), and probability of equal or greater deviation (P). Nonparametric correlations between continuous variables were determined using Spearman’s rank order correlation coefficient.
Results
Chickpea Rhizobia with Potential Plant Growth-Promoting Features
From all 67 isolates and type strains tested, 50 isolates produced siderophores (Figs. 1 and 2; Supplementary Table 1). The prevalence of this trait was related to the province of origin and the phylogeny of the isolates. An association between an isolate’s ability to produce siderophores and its province of origin was found (χ 2 = 19.938; d.f. = 10; P < 0.05). For instance, all isolates collected from Trás-os-Montes e Alto Douro, Beira Litoral, Algarve, and Madeira produced siderophores, whereas few isolates from Beira Baixa, Alto Alentejo, Baixo Alentejo, and none from Ribatejo showed this plant growth-promoting trait (Fig. 1). Likewise, an association between the levels of siderophore production and species clusters was found (χ 2 = 14.255; d.f. = 6; P < 0.05). Isolates belonging to the M. huakuii/M. amorphae cluster produced no or low amount of siderophores, whereas the majority of the isolates belonging to the M. ciceri/M. loti cluster produced medium or high amount of siderophores. Similar to what was observed with the chickpea Mesorhizobium isolates, among the six Mesorhizobium type strains, only two strains were unable to produce siderophores, namely M. amorphae ACCC 19665T and M. huakuii CCBAU 2609T. In contrast, no association/relationship between the levels of siderophore production and the soil pH classes was found.
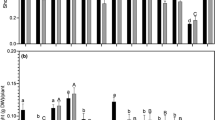
Percentage of isolates displaying different plant growth-promoting traits: inorganic phosphate solubilization, acid phosphatase, and siderophore and cytokinin production. Percentage of isolates possessing and lacking each plant growth-promoting trait is indicated by black and gray bars, respectively
Despite the fact that only 19 of 67 isolates and strains showed the ability to solubilize inorganic phosphorous (Fig. 2), a significant relationship between an isolate’s ability to solubilize inorganic phosphorous and species clusters was found (χ 2 = 22.305; d.f. = 2; P < 0.001). The majority of the isolates able to solubilize inorganic phosphorous belong to the cluster M. ciceri/M. loti, whereas no isolate belonging to the cluster M. huakuii/M. amorphae was able to solubilize inorganic phosphorous. Also the Mesorhizobium type strains, M. amorphae ACCC 19665T and M. huakuii CCBAU 2609T, were incapable of solubilizing inorganic phosphorous. A CA reinforced the previous observation, revealing that isolates belonging to the clusters M. huakuii/M. amorphae were unable to solubilize inorganic phosphorous while the isolates assigned to the clusters M. ciceri/M. loti and M. tianshanense/M. mediterraneum/M. temperatum were high and low inorganic phosphorous solubilizers, respectively (Fig. 3). Interestingly, all isolates unable to produce siderophores also failed to solubilize inorganic phosphorous. An association between the levels of inorganic phosphorous solubilization and siderophore production was found (χ 2 = 33.867; d.f. = 6; P < 0.001). The majority of the isolates with no or poor siderophore production do not solubilize inorganic phosphorous, while most of the isolates with medium or high siderophore production were able to solubilize phosphorous, suggesting a possible interaction between these traits. In addition, all isolates able to solubilize inorganic phosphorous were from neutral or alkaline soils and none were from acidic soils, suggesting that soil pH may act as a selective pressure. The amount of solubilized phosphate correlated with phosphate, nitrogen, or potassium content of the soils of the isolates’ origin (r = 0.425, P ≤ 0.001; r = 0.380, P < 0.005; r = 0.404, P ≤ 0.001, respectively). On the other hand, no association between an isolate’s ability to solubilize inorganic phosphate and its symbiotic effectiveness was found.
Similar to what was observed with inorganic phosphate solubilization, the ability to solubilize organic phosphate by acid phosphatases was rare in chickpea Mesorhizobium isolates. Only 11 chickpea Mesorhizobium isolates and the type strain M. huakuii CCBAU 2609T displayed acid phosphatase-specific activity (Fig. 2), ranging from 0.011 to 0.569 (Table 2, Supplementary Table 1). Only three of these isolates possess both acid phosphatase activity and ability to solubilize inorganic phosphate. Among the isolates displaying acid phosphatase activity, seven belong to the M. tianshanense/M. mediterraneum/M. temperatum species cluster and two belong to M. ciceri/M. loti and M. huakuii/M. amorphae species clusters. The average of acid phosphatase-specific activity from isolates belonging to the M. ciceri/M. loti and M. huakuii/M. amorphae species clusters is significantly different from the average of acid phosphatase-specific activity from isolates assigned to M. tianshanense/M. mediterraneum/M. temperatum species cluster.
The ability to synthesize cytokinins was not associated with a particular species group, province of origin, or the symbiotic effectiveness of the isolates. Of the 50 isolates tested for cytokinin production, 22 increased chlorophyll concentration in the cucumber cotyledon bioassay, indicating their ability to synthesize cytokinins (Table 3, Fig. 2). The two most efficient cytokinin producers, isolates BR-15-Bragança and C-7-Coimbra, came from two different provinces and belong to the M. ciceri/M. loti and M. huakuii/M. amorphae species clusters, respectively. These two isolates increased chlorophyll concentrations by 20% in the cucumber bioassay but showed relatively low symbiotic effectiveness (Table 1).
All isolates synthesized IAA (Table 2) when grown in the presence of tryptophan, but the production rates varied between the province of origin and with the species groups. Significant differences between provinces of origin regarding their ability to synthesize IAA (χ 2 = 46.171; d.f. = 30; P < 0.05) were found. For instance, the three provinces with the highest IAA production averages (Beira Alta, Estremadura, Beira Litoral) were found to be significantly different from the province with the lowest growth average (Douro Litoral). M. ciceri/M. loti isolates showed the highest IAA production average, while the M. tianshanense/M. mediterraneum/M. temperatum isolates showed the lowest IAA production average. Similarly, the IAA production average between species clusters was also significantly different (χ 2 = 12.621; d.f. = 6; P < 0.05). Isolates from the M. tianshanense/M. mediterraneum/M. temperatum cluster showed a low level of IAA production, whereas the majority of isolates belonging to M. huakuii/M. amorphae or to the M. ciceri/M. loti species cluster presented a high level and very high level of IAA production, respectively.
Almost half of the isolates (32/67) displayed high or very high IAA production, with 12 of them being able to synthesize more than 45 μg ml−1 of IAA in the presence of Trp. Curiously, positive correlations between the IAA production and the isolates’ abilities to solubilize inorganic phosphate (r = 0.327, P < 0.01) or to tolerate pH 5 (data from [36]) (r = 0.403, P ≤ 0.001) were found. For instance, moderately acidophilic isolates showed the highest IAA production, a situation significantly different from that displayed by the acid-sensitive isolates. However, no significant relationship between the IAA production or its levels and the symbiotic effectiveness or pH class of the origin soil was found. None of the 61 isolates deaminated ACC under free-living conditions.
Evaluation of Mesorhizobium spp. Tolerance to Heavy Metals
Despite the fact that Mesorhizobium spp. growth was largely inhibited by the presence of heavy metals, the isolate’s ability to tolerate different metals was related to the province of origin. Only tolerance to zinc was associated with a particular species group (χ 2 = 34.913; d.f. = 6; P < 0.001) or with the pH of the origin soil (χ 2 = 27.598; d.f. = 10; P < 0.005). In this case, isolates from acid or neutral soils were able to grow in the presence of zinc, whereas isolates from alkaline soils were unable to grow in the presence of this metal. Similarly, the CA revealed an association between the isolate’s ability to tolerate zinc and the species clusters of the isolates (Fig. 4). For instance, almost all isolates from the M. tianshanense/M. mediterraneum/M. temperatum species cluster did not tolerate zinc, whereas the majority of the isolates belonging to the M. huakuii/M. amorphae and the M. ciceri/M. loti species clusters showed tolerance to this metal.
Associations between the isolate’s ability to tolerate different metals and the province of origin of the isolates (χ 2 = 74.576; d.f. = 50; P < 0.05 for zinc; χ 2 = 42.999; d.f. = 20; P < 0.001 for nickel; χ 2 = 58.586; d.f. = 30; P < 0.001 for copper) were found. In fact, several CA associations between some provinces of origin and isolate’s growth in the presence of zinc, nickel, or copper were found (Fig. 5 for the case of Zn). For instance, isolates from Ribatejo, Baixo Alentejo, and Estremadura provinces were unable to grow in the presence of zinc, whereas all isolates from Madeira, Beira Baixa, Beira Alta, Beira Litoral, and Douro Litoral grew well in the presence of zinc. Moreover, all isolates from Baixo Alentejo could grow in the presence of nickel but were unable to grow in the presence of copper or zinc; all isolates from Beira Alta grew in the presence of zinc and lead but not in the presence of nickel or copper; and no isolate from Algarve was able to grow in the presence of nickel, copper, or cobalt but was in the presence of lead (Table 4). Negative correlations between the isolate’s tolerance to Zn and the pH value of the origin soil of the isolates were found (r = −0.449, P < 0.001). In contrast, positive correlations between the isolate’s tolerance to Zn and the acid tolerance (r = 0.502, P < 0.001) and IAA production (r = 0.387, P < 0.001) were recognized. In addition, an association between the isolate’s ability to solubilize inorganic phosphate and isolate’s tolerance to Zn was found (χ 2 = 27.272; d.f. = 8; P < 0.001).
Some isolates were able to grow in the presence of high concentrations of specific metals. For instance, the highest maximum concentration tolerated by some chickpea Mesorhizobium isolates was 5 mM for zinc, 3 mM for cobalt, 0.5 mM for nickel, and 1 mM for both copper and lead (Fig. 6). Despite the fact that the highest concentration tolerated was with zinc, the metal more broadly tolerated by chickpea Mesorhizobium isolates was lead (Table 4). Moreover, chickpea microsymbionts exhibited different levels of tolerance to the different heavy metals tested. For example, more than 73.1% of the isolates show growth inhibition in the presence of nickel, cobalt, or copper, whereas only 35.8% of the isolates were unable to grow in the presence of zinc. On the other hand, the majority (68.7%) of the isolates could grow in the presence of ≥0.5 mM lead (Fig. 6). Overall, these results indicate that chickpea microsymbionts possess different levels of tolerance for different types of metals.
Mesorhizobium isolates showed high diversity in terms of the number of metals tolerated and this ability was associated with both the province of origin (χ 2 = 58.559; d.f. = 40; P < 0.05) and the species cluster (χ 2 = 27.272; d.f. = 8; P < 0.001) of the isolates . All isolates were tolerant to at least one metal, with the majority of the isolates able to tolerate two or more different metals. In fact, four isolates showed tolerance to all of the metals tested, namely PII-3—Porto, CB-30—Castelo Branco, 78—Elvas, and 93—Évora isolates. Furthermore, almost all isolates assigned to the M. huakuii/M. amorphae and M. ciceri/M. loti clusters showed tolerance to two or more metals, while isolates belonging to M. tianshanense/M. mediterraneum/M. temperatum cluster exhibited tolerance to only one metal. Likewise, all isolates from Beira Litoral, Beira Alta, Beira Baixa, and Douro Litoral tolerated two or more different metals, whereas the majority of isolates from Algarve, Estremadura, and Trás-os-Montes e Alto Douro were tolerant to one or two different metals. No association between an isolate’s tolerance to different metals and the symbiotic effectiveness of the isolates or specific plant growth-promoting trait was found.
Discussion
The present study indicates that the majority of the chickpea Mesorhizobium isolates possess two or more plant growth-promoting mechanisms, with indoleacetic acid and siderophore production the most common traits. In addition, most Mesorhizobium spp. tolerate Zn and Pb but are sensitive to Ni, Co, and Cu. Several associations reveal that the prevalence of specific PGP traits and metal tolerance are related to the province of origin and/or the species of the isolates.
The high prevalence of siderophore production in the collection of mesorhizobia species reported here may reflect the sampling site. Some rhizobial species produce siderophores, such as Rhizobium tropici, R. leguminosarum bv. viciae, R. leguminosarum bv. trifolii, and Sinorhizobium meliloti [48, 49], but this trait is rare in rhizobia [50]. The association found between an isolate’s ability to produce siderophores and its province of the origin suggests that the origin soil may have acted to help select for this ability. These results are in agreement with Vargas et al. [50] who observed that plant growth-promoting trait frequency, including siderophore production, in clover rhizobia, was related to the sampling site. One possible explanation for the common presence of this plant growth-promoting trait in chickpea Mesorhizobium isolates may be due to the iron deficiency in soils in some provinces of origin, thereby conferring plants associated with these strains a competitive advantage in those soils. In addition, the association found between the levels of siderophore production and the species clusters suggests that this plant growth-promoting trait may be more species specific than strain specific.
Although the ability of inorganic phosphate solubilization among chickpea Mesorhizobium isolates is a feature not as common as siderophore production, its presence is associated not only to soil pH but also to the species clusters. The ability to solubilize inorganic phosphate has been found in several species of rhizobia, such as R. leguminosarum, Ensifer meliloti, and Bradyrhizobium sp. [4, 51], including two species that nodulate chickpea, M. ciceri and M. mediterraneum [52]. In the present study, 28.4% of the isolates tested exhibit this ability, a frequency higher than that reported by others using M. ciceri and R. leguminosarum isolates [53, 54]. In contrast, among the plant growth-promoting traits assessed for 252 clover rhizobia isolates, phosphate solubilization was the most common plant growth-promoting characteristic, being present in 42% of the isolates [50]. These differences between surveys may be due to the soil characteristics of the sampling sites, mainly the soil pH as the phosphorous availability is affected by the soil pH, becoming less available in alkaline soils or when it forms complexes with iron and aluminum oxides in acidic soils [55]. Therefore, it is expected to find a higher proportion of phosphate solubilizer isolates in neutral and alkaline soils. Indeed, in our survey, all isolates able to solubilize inorganic phosphate are from neutral or alkaline soils. According to Neila and coworkers [56], the phosphate solubilization activity used by rhizobia may constitute an adaptive mechanism against the phosphorous deficiency in soils as in the case of alkaline soils. In our previous work [36], it is observed that isolates from M. ciceri/M. loti species cluster are associated with neutral soils, whereas isolates from M. mediterraneum/M. temperatum and M. tianshanense species clusters are associated with alkaline soils. Curiously, most of the isolates belonging to the M. ciceri/M. loti cluster have the ability to solubilize inorganic phosphate, whereas the isolates from the other two clusters do not have this ability, suggesting that the ability to solubilize inorganic phosphate may be species related. Moreover, the association between the levels of inorganic phosphorous solubilization and siderophore production found in this study corroborates the previous findings by several studies reporting that phosphate-solubilizing microorganisms exhibit multifunctional properties, including release of siderophores (e.g., [57–61]). Although it is uncertain whether these two mechanisms are interconnected, the potential role of siderophores in increasing the availability of inorganic phosphate seems plausible [62].
In this study, only a few Mesorhizobium isolates possess the ability to solubilize organic phosphate through acid phosphatases, most being from alkaline soils. The absence of this feature in most isolates, especially those from acid soils, was unexpected. Typically, acid phosphatases dominate in acid soils, while alkaline phosphatases are more abundant in neutral and alkaline soils [63]. This result may be due to the fact that very few isolates possess the ability to solubilize both organic and inorganic phosphate, which differs from other reports [64].
In this study, all chickpea microsymbionts revealed the ability to synthesize IAA, differing greatly in the amount produced. A number of soil bacteria from plant rhizosphere produce cytokinins and IAA [65, 66]. Also rhizobial strains can produce both IAA [50, 67] and cytokinins [14, 68] in variable amounts. These results are in agreement with other reports where more than 80% of the clover rhizobia isolates and Mesorhizobium isolates are able to synthesize IAA [50, 53], but contrast with what was observed for lentil rhizobia [54]. On the other hand, only 22 of the 50 isolates tested produce cytokinins. Moreover, the amount of relative chlorophyll concentration observed is lower compared to other reports [47], whose rhizobacteria strains show relative chlorophyll concentrations above 1.22.
Although both IAA and cytokinins play a role in the symbiotic rhizobium-legume interaction, no relationship or association between the production of either IAA or cytokinins and the symbiotic effectiveness of the isolates was found in this study. For example, lack of auxin synthesis can negatively affect nitrogen fixation, whereas (up to a point) auxin overproduction can increase nodulation efficiency [69, 70]. Alternatively, cytokinins produced by rhizobia may provide a mechanism to counteract the negative effects caused by the different types of stresses [20]. In addition, some researchers have noted a direct relationship between cytokinin concentrations and nodulation [71, 72] and between IAA production by rhizobia and nodulation and competitive ability [14–16].
Remarkably, an association between the level of IAA produced and an isolate’s tolerance to acidity was found (Fig. 7). These results suggest that the pathways for IAA production may be part of a bacterial stress response. In fact, it was previously observed that the expression of the key gene in IAA production, ipdC gene, is enhanced by carbon limitation and acidic pH in A. brasilense Sp245 [73, 74]. In Pseudomonas putida GR12-2 and Pseudomonas agglomerans, the ipdC gene expression is regulated by RpoS [75, 76], which is known to regulate transcription of genes in response to stress conditions and starvation. This result may also explain the associations found between the amount of IAA produced and the species cluster or the province of origin of the isolates. Actually, our previous results [36] show that an isolate’s tolerance to acidity is related with species clusters as well as with the pH of the origin soil. Similar to inorganic phosphate solubilization and the level of siderophore production, IAA production seems to be a species-specific plant growth-promoting feature allowing these organisms to survive and persist in specific types of soils, mainly acidic and neutral soils. On the other hand, our results also indicate a positive correlation between the amount of IAA produced and the amount of inorganic phosphate solubilized (r = 0.327, P < 0.05). This result agrees with what was reported previously by Bianco and Defez [18], where an IAA-overproducing E. meliloti strain shows a greater ability to solubilize phosphate.
The lack of detectable ACC deaminase activity under free-living conditions agrees with our previous results using 18 chickpea Mesorhizobium isolates [12]. Furthermore, the Mesorhizobium sp. strain MAFF303099 only expresses ACC deaminase inside the nodules, under the transcriptional regulation of the NifA2 protein [77], similarly in M. ciceri UPM-Ca7T, the acdS gene is transcribed under symbiotic conditions [12]. As far as we know, no report reveals this ability in any Mesorhizobium spp. under free-living conditions.
The relatively high tolerance of Mesorhizobium spp., isolated from chickpea, to metals may reflect the rhizobial population’s tolerance to specific metals present in their origin soils, suggesting that an isolate’s tolerance to different metals may be determined by the isolate’s geographical origin. In this study, chickpea microsymbionts show a higher tolerance to zinc, cobalt, and nickel and a lower tolerance to copper and lead compared to other studies conducted with Ensifer medicae, R. leguminosarum biovar trifolii isolates, and R. leguminosarum biovar viciae [78, 79]. Associations between an isolate’s ability to tolerate specific metals and their origin were detected. This is consistent with previous studies [78, 79] where it is shown that the metal tolerances of rhizobial populations are related to the origin soils. Here, the number of metals tolerated was associated with the phylogeny of the isolates. Moreover, the isolate’s tolerance to Zn is associated with the inorganic phosphate solubilization ability and is correlated with the pH value of the origin soil of the isolates, acid tolerance, and IAA production. Herein, acid-sensitive isolates do not tolerate zinc, while acid-tolerant isolates display tolerance to different concentrations of zinc, which may be explained by the fact that zinc becomes more available in soils with low pH and less available in more basic soils. These results are not unexpected since the soil pH controls the bioavailability of metals in soils [80], and phosphate solubilization and siderophore and acid production are mechanisms, adopted by bacteria toward metals in soil, involved in mobilizing metals [81]. Furthermore, all chickpea Mesorhizobium isolates showed tolerance to at least one of the metals tested, which agrees with previous reports conducted with rhizobial species isolated from nodules of pea, clover, alfalfa, and chickpea [78, 79, 82, 83]. Altogether, these results may be also an explanation for the species cluster distribution found previously by Brígido and Oliveira [36].
Interestingly, a positive correlation (r = 0.277, P < 0.05) is found between the number of plasmids carried by the various isolates and the number of metals tolerated. This result suggests that the genes encoding metal resistance may be present on plasmids. Similar results were previously reported by Lakzian et al. [84].
Overall, our data reinforces the hypothesis that the association found between the soil pH and the species group may be due to the fact that these species were selected by adaptive mechanisms, such as phosphate solubilization, siderophore production, IAA production, and tolerance to acidity and to different metals, that allow them to compete and persist in different type of soils. For instance, most of the isolates belonging to the M. huakuii/M. amorphae species cluster obtained from Beira Alta, Beira Litoral, Trás-os-Montes e Alto Douro, Beira Baixa, Estremadura, and Madeira provinces shared the ability to produce medium or high amounts of IAA and tolerance to acidity, zinc, and lead. This also explains the association found between the species clusters and the province of origin of the isolates (χ 2 = 49.628; d.f. = 20; P < 0.001) and the relationship between the number of PGP traits and the phylogeny of the isolates (χ 2 = 25.205; d.f. = 8; P < 0.001).
In this study, no relationship between the presence of several plant growth-promoting mechanisms and the symbiotic effectiveness of the isolates was found. However, the impact of these plant growth-promoting features may become important when the symbiotic rhizobium-legume association occurs under stressful conditions.
Several studies showed the co-existence of plant growth-promoting traits with heavy metal resistance. For example, Wani and Khan [85] isolated a rhizobium strain RL9 possessing not only high tolerance to several heavy metals but also plant growth-promoting traits, such as production of IAA and siderophores. Further, Joseph and coworkers [86] found that the rhizobacteria associated with chickpea tolerance to multiple heavy metals also exhibited at least two plant growth-promoting activities. Although no association between the number of metals tolerated and the number of potential plant growth-promoting mechanisms is found, most of the isolates possessing two or more plant growth-promoting activities tolerate two or more distinct metals. It is possible that an effective symbiotic Mesorhizobium-chickpea association in metal-contaminated soils depends not only on the metal tolerance exhibited by the rhizobial partner but also on its ability to counteract the negative effects of metals in the plants. Indeed, previous reports suggest that several plant growth-promoting mechanisms from soil bacteria can help the plant to alleviate metal toxicity; these traits include siderophore production [87], ACC deaminase [88, 89], and IAA production [90].
This study shows that many native chickpea Mesorhizobium isolates display multifaceted plant growth-promoting abilities, as well as tolerance to several different metals. These properties may increase their potential in promoting legume growth by several mechanisms other than nitrogen fixation, making them a suitable choice for multiple agricultural applications. Furthermore, this work shows that several plant growth-promoting traits in chickpea Mesorhizobium spp. are related or determined by their origin site or species group. Thus, the data obtained provide important background information for further ecological and phylogenetic studies. Additionally, this study provides extensive information on plant growth-promoting traits and metal tolerance of chickpea rhizobia that can be useful in the selection of the most adapted rhizobial inoculants to different challenging field conditions.
References
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica ID963401, 15 pages
Zahran HH (2006) Nitrogen (N2) fixation in vegetable legumes: biotechnological perspectives. In: Ray RC, Ward OP (eds) Microbial biotechnology in horticulture, vol 1. Science, Enfield, pp 49–82
Esitken A, Ercisli S, Karlidag H et al (2005) Potential use of plant growth promoting rhizobacteria (PGPR) in organic apricot production. In: Libek A, Kaufmane E, Sasnauskas A (eds) Proceedings of the international scientific conference of environmentally friendly fruit growing, Polli, Estonia, pp. 90–97
Chabot R, Antoun H, Cescas M (1996) Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar phaseoli. Plant Soil 184:311–321. doi:10.1007/BF00010460
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339. doi:10.1016/S0734-9750(99)00014-2
Rodriguez H, Fraga R, Gonzalez T et al (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21. doi:10.1007/s11104-006-9056-9
Johnston AWB (2004) Mechanisms and regulation of iron uptake in the rhizobia. In: Crosa JH, Payne SM (eds) Iron transport in bacteria: molecular genetics, biochemistry, microbial pathogenesis and ecology. ASM Press, Washington, pp 469–488
Avis TJ, Gravel V, Antoun H et al (2008) Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol Biochem 40:1733–1740. doi:10.1016/j.soilbio.2008.02.013
Hayat R, Ali S, Amara U et al (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598. doi:10.1007/s13213-010-0117-1
Cooper JB, Long SR (1994) Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell 6:215–225. doi:10.1105/tpc.6.2.215
Ma WB, Sebestianova SB, Sebestian J et al (2003) Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Anton Leeuw Int J Gen Mol Microbiol 83:285–291. doi:10.1023/A:1023360919140
Nascimento FX, Brígido C, Glick BR et al (2012) ACC deaminase genes are conserved among Mesorhizobium species able to nodulate the same host plant. FEMS Microbiol Lett 336:26–37. doi:10.1111/j.1574-6968.2012.02648.x
Yuhashi KI, Ichikawa N, Ezura H et al (2000) Rhizobitoxine production by Bradyrhizobium elkanii enhances nodulation and competitiveness on Macroptilium atropurpureum. Appl Environ Microbiol 66:2658–2663. doi:10.1128/AEM.66.6.2658-2663.2000
Boiero L, Perrig D, Masciarelli O et al (2007) Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol 74:874–880. doi:10.1007/s00253-006-0731-9
Ali B, Hayat S, Hasan SA, Ahmad A (2008) IAA and 4-Cl-IAA increases the growth and nitrogen fixation in mung bean. Commun Soil Sci Plant Anal 39:2695–2705. doi:10.1080/00103620802358839
Camerini S, Senatore B, Lonardo E et al (2008) Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch Microbiol 190:67–77. doi:10.1007/s00203-008-0365-7
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107. doi:10.1093/jxb/erp140
Bianco C, Defez R (2010) Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl Environ Microbiol 76:4626–4632. doi:10.1128/AEM.02756-09
Podlesakova K, Fardoux J, Patrel et al (2013) Rhizobial synthesized cytokinins contribute to but are not essential for the symbiotic interaction between photosynthetic Bradyrhizobia and Aeschynomene legumes. Mol Plant Microbe Interact 26:1232–1238. doi:10.1094/MPMI-03-13-0076-R
Xu J, Li LX, Luo L (2012) Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl Environ Microbiol 78:8056–8061. doi:10.1128/AEM.01276-12
Ma WB, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69:4396–4402. doi:10.1128/AEM.69.8.4396-4402.2003
Ma WB, Charles TC, Glick BR (2004) Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Sinorhizobium meliloti increases its ability to nodulate alfalfa. Appl Environ Microbiol 70:5891–5897. doi:10.1128/AEM.70.10.5891-5897.2004
Nascimento F, Brígido C, Alho L et al (2012) Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant Soil 353:221–230. doi:10.1007/s11104-011-1025-2
Nascimento FX, Brígido C, Glick BR et al (2012) Mesorhizobium ciceri LMS-1 expressing an exogenous 1-aminocyclopropane-1-carboxylate (ACC) deaminase increases its nodulation abilities and chickpea plant resistance to soil constraints. Lett Appl Microbiol 55:15–21. doi:10.1111/j.1472-765X.2012.03251.x
Brígido C, Nascimento FX, Duan J et al (2013) Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol Lett 349:46–53. doi:10.1111/1574-6968.12294
Brígido C, Glick BR (2015) Phytoremediation using rhizobia. In: Ansari AA, Gill SS, Gill R, Lanza RG, Newman L (eds) Phytoremediation: management of environmental contaminants, vol 2. Springer, New York, pp 95–114
Zheng ZW, Fang W, Lee HY, Yang ZY (2005) Responses of Azorhizobium caulinodans to cadmium stress. FEMS Microbiol Ecol 54:455–461. doi:10.1016/j.femsec.2005.05.006
Broos K, Uyttebroek M, Mertens J et al (2004) A survey of symbiotic nitrogen fixation by white clover grown on metal contaminated soils. Soil Biol Biochem 36:633–640. doi:10.1016/j.soilbio.2003.11.007
Younis M (2007) Responses of Lablab purpureus-rhizobium symbiosis to heavy metals in pot and field experiments. World J Agric Sci 3:111–122, Accession # 25165156
Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crop Res 65:249–258. doi:10.1016/S0378-4290(99)00090-8
Alexandre A, Brígido C, Laranjo M, Rodrigues S, Oliveira S (2009) A survey of chickpea rhizobia diversity in Portugal reveals the predominance of species distinct from Mesorhizobium ciceri and Mesorhizobium mediterraneum. Microb Ecol 58:930–941. doi:10.1007/s00248-009-9536-6
Brígido C, Alexandre A, Oliveira S (2012) Transcriptional analysis of major chaperone genes in salt-tolerant and salt-sensitive mesorhizobia. Microbiol Res 167:623–629. doi:10.1016/j.micres.2012.01.006
Jarvis BDW, van Berkum P, Chen WX et al (1997) Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int J Syst Bacteriol 47:895–898. doi:10.1099/00207713-47-3-895
Alexandre A, Oliveira S (2011) Most heat-tolerant rhizobia show high induction of major chaperone genes upon stress. FEMS Microbiol Ecol 75:28–36. doi:10.1111/j.1574-6941.2010.00993.x
Brígido C, Alexandre A, Laranjo M, Oliveira S (2007) Moderately acidophilic mesorhizobia isolated from chickpea. Lett Appl Microbiol 44:168–174. doi:10.1111/j.1472-765X.2006.02061.x
Brígido C, Oliveira S (2013) Most acid-tolerant chickpea mesorhizobia show induction of major chaperone genes upon acid shock. Microb Ecol 65:145–153. doi:10.1007/s00248-012-0098-7
Schwyn R, Neilands J (1987) Universal chemical assay for detection and estimation of siderophores. Anal Biochem 160:47–56. doi:10.1016/0003-2697(87)90612-9
Alexander D, Zuberer D (1991) Use of chrome azurol-S-reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soil 12:39–45. doi:10.1007/BF00369386
Gupta R, Singal R, Skankar A et al (1994) A modified plate assay for screening phosphate solubilizing microorganisms. J Gen Appl Microbiol 40:255–260. doi:10.2323/jgam.40.255
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Patten CL, Glick BR (2002) The role of Pseudomonas putida indoleacetic acid in the development of the host plant system. Appl Environ Microbiol 68:3795–3801. doi:10.1128/AEM.68.8.3795-3801.2002
O’Gara F, Shanmugam KT (1976) Control of symbiotic nitrogen fixation in rhizobia regulation of NH4+ assimilation. Biochim Biophys Acta 451:342–352. doi:10.1016/0304-4165(76)90129-X
Gordon S, Weber R (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. doi:10.1104/pp.26.1.192
De Freitas J, Banerjee M, Germida J (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol Fertil Soil 24:358–364
Oliveira C, Alves V, Marriel IE et al (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 41:1782–1787. doi:10.1016/j.soilbio.2008.01.012
Charles TC, Newcomb W, Finan TM (1991) NDVF, a novel locus located on megaplasmid pRMESU47B (PEXO) of Rhizobium meliloti is required for normal nodule development. J Bacteriol 173:3981–3992
Hussain A, Hasnain S (2011) Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J Microbiol Biotechnol 27:2645–2654. doi:10.1007/s11274-011-0738-y
Carson K, Meyer J, Dilworth M (2000) Hydroxamate siderophores of root nodule bacteria. Soil Biol Biochem 32:11–21. doi:10.1016/S0038-0717(99)00107-8
Arora N, Kang S, Maheshwari D (2001) Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81:673–677
Vargas LK, Lisboa BB, Schlindwein G et al (2009) Occurrence of plant growth-promoting traits in clover-nodulating rhizobia strains isolated from different soils in Rio Grande do Sul state. Rev Bras Cienc Solo 33:1227–1235
Alikhani HA, Saleh-Rastin N, Antoun H (2006) Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 287:35–41. doi:10.1007/s11104-006-9059-6
Peix A, Rivas-Boyero AA, Mateos PF et al (2001) Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol Biochem 33:103–110. doi:10.1016/S0038-0717(00)00120-6
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181. doi:10.1016/j.micres.2006.04.001
Jida M, Assefa F (2011) Phenotypic and plant growth promoting characteristics of Rhizobium leguminosarum bv. viciae from lentil growing areas of Ethiopia. Afr J Microbiol Res 5:4133–4142
Oburger E, Jones D, Wenzel W (2011) Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 341:363–382. doi:10.1007/s11104-010-0650-5
Neila A, Adnane B, Mustapha F et al (2014) Phaseolus vulgaris-rhizobia symbiosis increases the phosphorus uptake and symbiotic N2 fixation under insoluble phosphorus. J Plant Nutr 37:643–657. doi:10.1080/01904167.2013.872275
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. App Microbiol Biotechnol 71:137–144. doi:10.1007/s00253-006-0380-z
Caballero-Mellado J, Onofre-Lemus J, Estrada-de los Santos P, Martinez-Aguilar L (2007) The tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Appl Environ Microbiol 73:5308–5319. doi:10.1128/aem.00324-07
Hamdali H, Bouizgarne B, Hafidi M et al (2008) Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19. doi:10.1016/j.apsoil.2007.08.007
Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y (2008) Rock phosphate-solubilizing Actinomycetes: screening for plant growth-promoting activities. World J Microbiol Biotechnol 24:2565–2575. doi:10.1007/s11274-008-9817-0
Viruel E, Lucca ME, Sineriz F (2011) Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Arch Microbiol 193:489–496. doi:10.1007/s00203-011-0692-y
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2. doi:10.1186/2193-1801-2-587
Renella G, Landi L, Ascher J et al (2006) Phosphomonoesterase production and persistence and composition of bacterial communities during plant material decomposition in soils with different pH values. Soil Biol Biochem 38:795–802. doi:10.1016/j.soilbio.2005.07.005
Tao G, Tian S, Cai M et al (2008) Phosphate solubilizing and mineralizing abilities of bacteria isolated from soils. Pedosphere 18:515–523. doi:10.1016/S1002-0160(08)60042-9
Arkhipova T, Prinsen E, Veselov S et al (2003) Cytokinin producing bacteria enhances plant growth in drying soil. Plant Soil 292:305–315. doi:10.1007/s11104-007-9233-5
Arkhipova T, Veselov S, Melentiev A et al (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272:201–209. doi:10.1007/s11104-004-5047-x
Mehnaz S, Lazarovits G (2006) Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb Ecol 51:326–335. doi:10.1007/s00248-006-9039-7
Caba J, Centeno M, Fernandez B et al (2000) Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a super nodulating mutant and the wild type. Planta 211:98–104. doi:10.1007/s004250000265
Pii Y, Crimi M, Cremonese G et al (2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7:21. doi:10.1186/1471-2229-7-21
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. doi:10.1111/j.1574-6976.2007.00072.x
Pavlova ZB, Lutova LA (2000) Nodulation as a model for studying differentiation in higher plants. Russ J Genet 36:975–988
Akimova GP, Sokolova MG (2012) Cytokinin content during early stages of legume-rhizobial symbiosis and effect of hypothermia. Russ J Plant Physiol 59:656–661. doi:10.1134/S1021443712030028
Ona O, Smets I, Gysegom P et al (2003) The effect of pH on indole-3-acetic acid (IAA) biosynthesis of Azospirillum brasilense Sp7. Symbiosis 35:199–208
Ona O, Van Impe J, Prinsen E, Vanderleyden J (2005) Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol Lett 246:125–132. doi:10.1016/j.femsle.2005.03.048
Brandl MT, Quinones B, Lindow SE (2001) Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc Natl Acad Sci U S A 98:3454–3459. doi:10.1073/pnas.061014498
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol 48:635–642. doi:10.1139/W02-053
Nukui N, Minamisawa K, Ayabe S-I et al (2006) Expression of the 1-aminocyclopropane-1-carboxylic acid deaminase gene requires symbiotic nitrogen-fixing regulator gene nifA2 in Mesorhizobium loti MAFF303099. Appl Environ Microbiol 72:4964–4969. doi:10.1080/AEM.02745-05
Pereira S, Lima A, Figueira E (2006) Screening possible mechanisms mediating cadmium resistance in Rhizobium leguminosarum bv. viciae isolated from contaminated Portuguese soils. Microb Ecol 52:176–186. doi:10.1007/s00248-006-9057-5
Nonnoi F, Chinnaswamy A, de la Torre VSG et al (2012) Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. and Trifolium spp.) growing in mercury-contaminated soils. Appl Soil Ecol 61:49–59. doi:10.1016/j.apsoil.2012.06.004
Gray CW, McLaren RG, Roberts AHC, Condron LM (1998) Sorption and desorption of cadmium from some New Zealand soils: effect of pH and contact time. Aust J Soil Res 36:199–216. doi:10.1071/S97085
Abou-Shanab RA, Ghozlan H, Ghanem K, Moawad H (2005) Behaviour of bacterial populations isolated from rhizosphere of Diplachne fusca dominant in industrial sites. World J Microbiol Biotechnol 21:1095–1101. doi:10.1007/s11274-004-0005-6
Wani PA, Khan MS, Zaidi A (2008) Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol Lett 30:159–163. doi:10.1007/s10529-007-9515-2
Wani PA, Khan MS, Zaidi A (2008) Effect of metal-tolerant plant growth-promoting Rhizobium on the performance of pea grown in metal-amended soil. Arch Environ Contam Toxicol 55:33–42. doi:10.1007/s00244-007-9097-y
Lakzian A, Murphy P, Turner A et al (2002) Rhizobium leguminosarum bv. viciae populations in soils with increasing heavy metal contamination: abundance, plasmid profiles, diversity and metal tolerance. Soil Biol Biochem 34:519–529. doi:10.1016/S0038-0717(01)00210-3
Wani PA, Khan MS (2013) Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9. Bull Environ Contam Toxicol 91:117–124. doi:10.1007/s00128-013-1002-y
Joseph B, Patra R, Lawrence R (2007) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Prod 1:141–151
Rajkumar MA, Ae N, Prasad MN, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149. doi:10.1016/j.tibtech.2009.12.002
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374. doi:10.1016/j.biotechadv.2010.02.001
Glick BR, Stearns JC (2011) Making phytoremediation work better: maximizing a plant’s growth potential in the midst of adversity. Int J Phytoremediation 13:4–16. doi:10.1080/15226514.2011.568533
Hao X, Xie P, Johnstone L et al (2012) Genome sequence and mutational analysis of plant-growth-promoting bacterium Agrobacterium tumefaciens CCNWGS0286 isolated from a zinc-lead mine tailing. Appl Environ Microbiol 78:5384–5394. doi:10.1128/AEM.01200-12
Acknowledgements
This work was supported by FEDER Funds through the Operational Programme for Competitiveness Factors—COMPETE and National Funds through FCT-Foundation for Science and Technology under the Project UID/AGR/00115/2013 and Project no. FCOMP-01-0124-FEDER-028316 (PTDC/BIA-EVF/4158/2012), the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 247669, and InAlentejo Project ALENT-07-0262-FEDER-001871. C. Brígido acknowledges a FCT fellowship (SFRH/BPD/94751/2013). B.R. Glick was supported by the Natural Science and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Maximum likelihood phylogeny of chickpea mesorhizobia isolates and type strains, based on partial 16S rRNA gene analysis (alignment length 579 bp). Kimura’s two-parameter model with a discrete gamma distribution and invariant sites was used. The three main clusters generated are marked with letters A to C. (JPG 226 kb)
Supplementary Table 1
Results obtained from the Portuguese chickpea mesorhizobia characterization in terms of specific plant growth-promoting traits and tolerance to heavy metals. nd- not determined; Classes of P solubilization: 0- no solubilization; 1-low solubilization; 2- high solubilization. Classes of siderophore production: 0 no production, 1- low production, 2- medium production, 3- high production. (JPG 31 kb)
Supplementary Table 2
Some characteristics of the soils used to obtain chickpea rhizobia isolates [data from 30 and 32]. Classes of soil pH: 1) soils with pH values below 6.5 (acid soils), 2) soils with pH values ranging from 6.5 to 7.4 (neutral soils), 3) soils with pH values above 7.4 (alkaline soils). nd- not determined (JPG 17 kb)
Rights and permissions
About this article
Cite this article
Brígido, C., Glick, B.R. & Oliveira, S. Survey of Plant Growth-Promoting Mechanisms in Native Portuguese Chickpea Mesorhizobium Isolates. Microb Ecol 73, 900–915 (2017). https://doi.org/10.1007/s00248-016-0891-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0891-9