Abstract
Plant rhizobacteria have been successfully used as biocontrol agents against fungal phytopathogens. However, their potential to control two important avocado diseases, namely Fusarium dieback (FD) and Phytophthora root rot (PRR), has been poorly studied. FD is an emerging disease triggered by fungi associated with two ambrosia beetle species (Euwallacea fornicatus species complex), while PRR is caused by Phytophthora cinnamomi, a soil-borne oomycete. In the present work, the antifungal activity of bacteria isolated from avocado rhizosphere was tested in dual culture assays against Fusarium euwallaceae, Graphium euwallaceae and Graphium sp., causal agents of FD, and against P. cinnamomi. In 2015, rhizosphere soil samples of FD infested and non-infested avocado trees were collected from a commercial avocado orchard in Escondido, California. In an initial screening, 72 of the 168 assessed bacterial isolates reduced mycelial growth of F. euwallaceae by up to 46%. Eight bacterial isolates showing inhibition percentages larger than 40% were then selected for further antagonism assays against the other fungal pathogens. Five bacterial isolates, determined by 16S rDNA sequencing to belong to the Bacillus subtilis/Bacillus amyloliquefaciens species complex, successfully inhibited the mycelial growth of both Graphium species by up to 30%. The same isolates and an additional isolate identified as Bacillus mycoides, inhibited the growth of P. cinnamomi by up to 25%. This is the first report of avocado rhizobacteria with antifungal activity against pathogens responsible for FD and PRR in avocado.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mexico is the world largest producer of avocados (Persea americana Mill.) with approximately 65% of the global production, followed by the United States of America (USA) with 23%, of which 85% is from California (AGMRC 2014; FAOSTAT 2015; Dunlap et al. 2017). Despite the economic importance of avocado production for these two neighboring countries, the productivity of avocado orchards has been hampered by fast-spreading diseases that are threatening avocado production in both countries. Those diseases include Fusarium dieback (FD), a new disease of avocado vectored by invasive shot hole borers (Euwallacea spp. nr. fornicatus) in California, and avocado root rot caused by Phytophthora cinnamomi Rands (PRR).
FD is caused by a complex of fungi including Fusarium euwallaceae Freeman, Mendel, Aoki & O’Donnell and Graphium euwallaceae Twizeyimana, Lynch & Eskalen, which form a symbiotic relationship with the invasive ambrosia beetle Euwallacea sp. nr. fornicatus, also known as Polyphagous Shot Hole Borer (PSHB) (Lynch et al. 2016). This new pest disease complex was first discovered in Los Angeles in 2012 (Eskalen et al. 2012). Another closely related Euwallacea species, the Kuroshio Shot Hole Borer (KSHB), was found in 2013 throughout Orange and San Diego Counties, very close to the Mexican border. More recently, KSHB was detected in Tijuana, Mexico (Garcia-Ávila et al. 2016). FD has a very wide host range and has been reported to pose a globally significant threat to natural forests, urban landscapes and fruit crops, particularly avocado (O’Donnell et al. 2016). The colonization by the fungi of the galleries burrowed by the beetle precedes a fungal invasion of vascular tissues, which blocks nutrient transport to higher parts of the tree leading to wilting, branch dieback, and in severe cases, tree mortality (Eskalen et al. 2013).
Phytophthora cinnamomi, on the other hand, is one of the most devastating plant pathogens worldwide. This soil-borne pathogen affects more than 3000 plant species (Pagliaccia et al. 2013) and is especially devastating in crop monocultures. It also causes root rot disease on avocado, which is estimated to affect about 70% of avocado orchards and to cause annual losses of 40 million US dollars in California alone (Toerien 2007). Following the root rot, defoliation and branch dieback occur, usually leading to tree mortality within 1–2 years (Coffey 1987).
Different management strategies have been implemented to control or mitigate the negative effects of FD and PRR in avocado orchards. Since the use of agrochemicals in commercial orchards is often restricted due to the inherent risks posed by their residues, more efforts should be placed in finding some other effective and environmental friendly management strategies such as biological control (Umeda et al. 2016). Biocontrol strategies using naturally occurring beneficial bacteria have been recently explored to control laurel wilt and FD (Dunlap et al. 2017). These authors used an in-house collection of Bacillaceae strains to test for antifungal activity, and reported that three Paenibacillus species and one Bacillus species caused antagonism in vitro against F. euwallaceae. In other studies, different strains of Bacillus, Pseudomonas and Streptomyces have shown some level of antagonistic activity against P. cinnamomi (You et al. 1996; Cazorla et al. 2007; Vida et al. 2017). Bacterial isolates belonging to the genus Pseudomonas have also been reported to produce antifungal substances and to be able to inhibit the mycelial growth of P. cinnamomi in dual cultures (Stirling et al. 1992; Vida et al. 2017), while Actinobacteria or Bacillus species have been associated with P. cinnamomi suppressiveness (You et al. 1996; Yin et al. 2004).
Microorganisms associated with the rhizosphere are of particular importance in the search for successful biological agents since they secrete a wide range of substances that could act in the suppression of pathogens (Yang et al. 2001; Bais et al. 2006; Compant et al. 2010). Recently, rhizobacteria such as Bacillus subtilis (Ehrenberg) Cohn and Serratia plymuthica (Lehmann & Neumann) Breed et al. were proved effective to inhibit the growth of the pathogenic fungi Moniliophtora perniciosa (Stahel) Aime & Phillips-Mora and Rhizoctonia solani J.G. Kuhn respectively, through the emission of diffusible and volatile compounds (Chaves-López et al. 2015; Neupane et al. 2015). The objective of this study was therefore to identify bacterial strains in avocado rhizospheres with antagonistic activity against F. euwallaceae, G. euwallaceae, and Graphium sp., three fungal pathogens responsible for FD that are affecting avocado orchards in California and threatening avocado production in Mexico. Furthermore, since these beneficial bacteria were recovered from the rhizosphere of avocado trees, their antifungal effects were also evaluated against the avocado root rot agent P. cinnamomi.
Materials and methods
Isolation of bacteria associated with avocado rhizospheres
Rhizosphere soil samples were collected in December 2015 from an avocado orchard located in Escondido, San Diego County, California, where the majority of the trees were infested with both PRR and FD. Five non symptomatic avocado trees and five avocado trees presenting symptoms of FD were selected. Four soil and root samples were taken per tree, approximately 50 cm away from the trunk and at a depth of 5–10 cm, where most of the feeder roots of avocado grow, and subsequently mixed to obtain one bulk sample of rhizosphere soil per tree. The hand shovel used for sample collection was disinfected between each tree with 70% ethanol. Samples were transported in a cooler and immediately processed upon arrival at the laboratory at UC Riverside. Loose soil was removed from the roots, and the remaining soil, which was strongly adhered to the roots, was recovered as rhizosphere soil. Solutions were subsequently prepared from 1 g rhizosphere soil and 99 ml distilled water, and homogenized by shaking vigorously. Dilutions of 1:10 and 1:100 were then streaked onto Petri dishes with Luria–Bertani agar (Difco), in triplicate. Plates were incubated at room temperature and isolates were taken from the plates as they grew and subcultured in nutrient agar until pure cultures were obtained.
In vitro antagonism assays against F. euwallaceae, Graphium spp. and P. cinnamomi
The bacterial isolates that were obtained from the rhizosphere of healthy and infected avocado trees were first screened for in vitro antagonism against F. euwallaceae. To prepare the dual cultures for the antagonism assays, bacterial isolates were re-streaked onto nutrient agar plates (nutrient broth (Difco) and granulated agar (Fisher)) and incubated at 25 °C for 48 h. An isolate of F. euwallaceae (strain UCR4511 provided by the Eskalen Lab.) was incubated on Potato Dextrose Agar (PDA; Difco Laboratories) medium at 25 °C for 5 days prior dual plating.
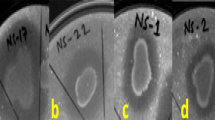
One agar plug of 5 mM of diameter was taken from the border of the fungus mycelial growth with a sterile cork borer and placed on the center of a PDA plate. Bacterial isolates were taken from a single colony with a toothpick and inoculated at a 2-cm distance from the mycelial plug (Fig. 1). Three different bacterial isolates were tested per plate. Additionally, mock inoculation with a sterile toothpick was used as a control on each experimental plate. The antagonism assays were carried out in triplicate. Dual culture plates were incubated at 25 °C and after 5 days, the mycelium radial growth was measured towards the bacterial and control treatments. The percentage of inhibition of mycelial growth was calculated using the formula reported by Idris et al. (2007): % inhibition = [(R-r)/R] × 100, where R is the radius of fungal growth from the center of the plate towards the control treatment, and r is the radius of fungal growth towards the bacterial treatment.
Schematic design of the dual culture antagonism assays. Bacterial isolates were inoculated with a toothpick at a 2-cm distance of the central mycelial plug. Three different bacterial isolates were tested per plate. Additionally, a sterilized toothpick mark was used as a control. The antagonism assays were carried out in triplicate
Eight bacterial isolates were then selected from those isolates showing high inhibition of F. euwallaceae mycelial growth (inhibition percentage higher than 40%), to be further evaluated for antagonism against other fungal pathogens of avocado. The selected bacterial isolates were tested against G. euwallaceae (fungal symbiont of PSHB), Graphium sp. (fungal symbiont of KSHB) and P. cinnamomi (strain UCR3458, provided by the Eskalen Lab.), following the same procedure as the in vitro antagonism assays against F. euwallaceae. The incubation time used to grow the fungal culture prior to set up the antagonism assays varied depending on the fungal species (11 days for Graphium species, 3 days for P. cinnamomi). Antagonism assays against Graphium spp. were carried out using five replicates whilst 10 replicates were used for P. cinnamomi.
Molecular identification of antagonistic bacterial isolates
DNA was extracted from each bacterial isolate showing in vitro antagonism against F. euwallaceae following the method proposed by Bollet et al. (1991). Briefly, the bacterial pellet was washed with 1 ml TE buffer and resuspended in 100 µl TE. The lysis step was carried out by adding 50 µl of 10% SDS and incubating the sample at 65 °C for 30 min. After centrifuging and removing the supernatants, the remaining pellets were heated for 2 × 1 min in a microwave and resuspended in 200 µl TE. An equal volume of chloroform—isoamyl alcohol—phenol (24:1:25) was then added and samples were shaken for 15 min, after which the aqueous phase was recovered by a 20 min centrifugation step and precipitated in ethanol. DNA integrity was verified by electrophoresis. Unsuccessful DNA extractions were repeated using DNeasy® Blood and Tissue kit (Qiagen, Germany) following the manufacturer’s instructions.
The 16S rRNA region was amplified by PCR using universal primers 27F (5′- AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′- TACGGYTACCTTGTTACGACTT-3′), in 50 μl reactions containing 25–150 ng of template DNA, 1X of Taq buffer, 200 µM of each dNTP, 1.25 mM of MgCl2, 0.4 µM of forward and reverse primers, and 0.5U of Taq DNA polymerase (Qiagen, Germany). Reactions were performed in a SureCycler 8800 thermal cycler (Agilent, California, USA) under the following conditions: initial denaturation at 95 °C for 4 min; 30 cycles of denaturation at 95 °C for 45 s, annealing at 53 °C for 45 s and extension at 72 °C for 2 min; and a final extension step at 72 °C for 5 min. Amplified DNA products were visually checked on an electrophoresis gel and purified using QiaQuick® Purification kit (Qiagen, Germany), according to the manufacturer’s instructions. Purified DNA amplicons were then sent to Macrogen Inc. for sequencing. Sequences were deposited in GenBank (accession numbers MF377554 to MF377573).
Data analysis
Statistical analyses were carried out with R version 3.4.1. Means, standard deviation, and standard error values were calculated using the PLYR package (Wickham 2011). Fungal growth data was analyzed using a multiple linear regression model, with fungal species and bacterial isolates as independent factors. A contrast matrix was generated in order to compare all treatments to the control and a Post-hoc analysis was subsequently implemented by using the “multcomp” package (Holthorn et al. 2008) in R with link function glht (general linear hypothesis testing).
Sequences were manually checked in BioEdit 7.2.5. (Hall 1999). An alignment was constructed in MEGA 7 (Kumar et al. 2016), using the multiple alignment program MUSCLE with the edited sequences and their best matches in GenBank nucleotide database (www.ncbi.nlm.nih.gov). The resulting alignment was manually edited. Sequences with 99% of identity were grouped in operational taxonomic units (OTUs) using the rdp pipeline (Cole et al. 2009). A Maximum-Likehood tree was constructed, using a Kimura two parameter model with uniform rates, and a Bootstrap method with 1000 replicates.
Results
Antifungal activity against Fusarium euwallaceae
In total, 168 bacterial isolates from rhizospheres of avocado trees were tested in dual cultures against F. euwallaceae. The mycelial radial growth of F. euwallaceae was reduced by 72 bacterial isolates, with inhibition percentages ranging from 15 to 46% (Online Resource 1). These 72 antagonistic bacterial isolates were grouped into 9 morphotypes based on Gram-staining results and microscopic characteristics such as cellular shape and size and presence of endospores. Up to 3 bacterial isolates per morphotype (20 isolates in total) were then selected for sequencing.
The inhibition percentages of mycelial growth of F. euwallaceae caused by the 20 sequenced bacterial isolates are shown in Fig. 2. All isolates belonged to the bacterial genus Bacillus and were clustered into two OTUs: OTU 1 was represented by sequences phylogenetically similar to Bacillus amyloliquefaciens Priest et al. and B. subtilis, whilst OTU 2 was represented by close relatives of Bacillus mycoides Flugge and B. thuringiensis Berliner (Table 1). A phylogeny of the 20 sequenced bacterial isolates with antagonism against F. euwallaceae is included in Online Resource 2.
Inhibition percentage of mycelial radial growth of Fusarium euwallaceae grown in dual cultures with antagonistic bacterial isolates. Values represent the average of 3 replicates. Bars represent standard errors (s.e.). All isolates significantly inhibited mycelial radial growth in comparison with a control (Post-hoc analysis implemented using the “multcomp” package (Holthorn et al. 2008) in R, with link function glht (general linear hypothesis testing), P ≤ 0.05)
Antifungal activity against Graphium spp. and P. cinnamomi
Eight bacterial isolates with inhibition percentage higher than 40% were randomly selected from the isolates that were showing high antagonistic activity against F. euwallaceae, to be tested for antagonism against G. euwallaceae, Graphium sp. and P. cinnamomi. The selected isolates were isolates: INECOL-4720, INECOL-4740, INECOL-4742, INECOL-4743, INECOL-5920, INECOL-5922, INECOL-5924, and INECOL-5927. Isolates INECOL-4742, INECOL-4743, INECOL-5922, INECOL-5924, and INECOL-5927, phylogenetically related with B. amyloliquefaciens, significantly reduced the mycelial radial growth of both Graphium species. In particular, bacterial isolate INECOL-5922 exhibited the greatest inhibition (30.2%) against G. euwallaceae (Fig. 3), whilst isolate INECOL-4742 inhibited the growth of Graphium sp. by 27% (Fig. 4). The mycelial radial growth of P. cinnamomi was significantly reduced by bacterial isolates INECOL-4740, INECOL-4742, INECOL-4743, INECOL-5922, INECOL-5924 and INECOL-5927, with isolate INECOL-5924 showing the greatest inhibition (25.5%, Fig. 5). Interestingly, the growth of P. cinnamomi seems to be promoted by bacterial isolates INECOL-4720 and INECOL-5920, although not significantly. Five isolates were able to inhibit the mycelial growth of all four avocado fungal pathogens: isolates INECOL-4742, INECOL-4743, INECOL-5922, INECOL-5924, and INECOL-5927 (Table 2; Fig. 6).
Inhibition percentage of mycelial radial growth of Graphium euwallaceae grown in dual cultures with antagonistic bacterial isolates. Values represent the average of 5 replicates. Bars represent standard errors (s.e.). * indicates significant inhibition of mycelial growth in comparison with a control (Post-hoc analysis implemented using the “multcomp” package (Holthorn et al. 2008) in R, with link function glht (general linear hypothesis testing), P ≤ 0.05)
Inhibition percentage of mycelial radial growth of Graphium sp. grown in dual cultures with antagonistic bacterial isolates. Values represent the average of 5 replicates. Bars represent standard errors (s.e.). * indicates significant inhibition of mycelial growth in comparison with a control (Post-hoc analysis implemented using the “multcomp” package (Holthorn et al. 2008) in R, with link function glht (general linear hypothesis testing), P ≤ 0.05)
Inhibition percentage of mycelial radial growth of Phytophthora cinnamomi grown in dual cultures with antagonistic bacterial isolates. Values represent the average of 10 replicates. Bars represent standard errors (s.e.). * indicates significant inhibition of mycelial growth in comparison with a control (Post-hoc analysis implemented using the “multcomp” package (Holthorn et al. 2008) in R, with link function glht (general linear hypothesis testing), P ≤ 0.05). Bacterial isolates INECOL-4720 and INECOL-5920 stimulated the mycelial growth of P. cinnamomi, although not significantly
Discussion
The use of chemical pesticides in agriculture has allowed the reduction of crop losses due to microbial phytopathogens, but is associated with environmental pollution, emergence of resistant pathogens and human health hazards (Prabhukarthikeyan et al. 2017). In order to counteract the negative effects of agrochemicals and provide an alternative solution to problems caused by pathogenic microorganisms, several reports have recommended the exploitation of beneficial rhizobacteria as biocontrol agents (Abdallah et al. 2016; Tokpah et al. 2016; Egamberdieva et al. 2017). Identifying rhizobacteria with antifungal properties constitutes the first step for the development of formulations that could biologically control FD or PRR. Such biological fungicides may include beneficial bacterial consortia in the form of concentrated powder, or oil-based or polymer-based products (Shaikh and Sayyed 2015), which could be sprayed directly onto the soil or directly injected into the stem of avocado trees, as shown by Na (2016).
In this study, all sequenced avocado rhizobacteria showing significant inhibition of the mycelial growth of F. euwallaceae belonged to the genus Bacillus. Recently, Dunlap et al. (2017) reported that Bacillus velezensis Ruiz-García et al. and several Paenibacillus species, isolated from human feces and honey bee larvae, presented antagonistic activity against F. euwallaceae. In another study, strains of B. subtilis, isolated from California sycamore (Platanus racemosa Nutt.) and avocado wood samples, significantly inhibited the growth of F. euwallaceae (Na 2016). Bacillus subtilis endophytic strains, isolated from avocado roots, were also found to reduce P. cinnamomi mycelial growth by up to 28% (Hakizimana et al. 2011). Interestingly, in our study, the bacterial isolates which presented antagonistic activity against all fungal pathogens also belonged to the B. subtilis species complex. Within the B. subtilis species complex, representatives of the subgroup B. amyloliquefaciens subsp. plantarum are known to be plant-associated strains with plant-growth promoting and antifungal activities and are therefore widely used as biofertilizer and biocontrol agents in agriculture (Dunlap et al. 2017; Fan et al. 2017). Our results corroborate these findings, and constitute the first report of avocado rhizobacteria with antifungal properties against FD and PRR causal agents.
Different mechanisms of fungal growth inhibition are reported for species of the genus Bacillus. Several studies indicate that, for members of the B. subtilis species complex, the antagonism is related to the secretion of antibiotic lipopeptides. Cawoy et al. (2015) showed that, in dual culture tests, B. subtilis/B. amyloliquefaciens secreted lipopeptides such as iturin and fengycin, which inhibited the growth of Fusarium oxysporum Schlecht. emend. Snyder & Hansen. Cazorla et al. (2007) also reported that B. subtilis strains, isolated from avocado rhizosphere, inhibited the avocado pathogens Rosellinia necatrix Berl. ex Prill. and the tomato pathogen F. oxysporum f.sp. radicis-lycopersici Jarvis & Shoemaker through iturin and fengycin secretion. Moreover, the authors concluded that other compounds, such as hydrolytic enzymes, were also likely to act as antifungal molecules. The antifungal activity of bacterial lipopeptides was confirmed by Mnif et al. (2015) in an in vitro assay, using an extract of lipopeptides produced by B. subtilis SPB1. The authors observed that the bacterial extract generated mycelial lysis, polynucleation, spore destruction and inhibition of mycelial growth in Fusarium solani, which is phylogenetically closely related to F. euwallaceae (O’Donnell et al. 2015). The inhibition zone observed in some of our dual culture assays may therefore be due to the secretion of diffusible lipopeptide compounds by the tested bacterial isolates. Further studies thus need to be performed to confirm the identity of the antifungal diffusible compounds that were involved in the inhibition. The variety of antifungal compounds secreted by B. subtilis/B. amyloliquefaciens may also explain the fact that different bacterial strains, although belonging to the same OTU, differed in their capacity to inhibit fungal pathogens. Moreover, B. subtilis and B. amyloliquefaciens are also known to emit volatiles with antifungal properties (Fiddaman and Rossall 1993; Yuan et al. 2012). The inhibitory effect of volatiles emitted by the avocado rhizobacteria that were isolated in this study also needs to be tested, in order to assess the full potential of these bacterial strains to control avocado fungal pathogens. Bacillus species are considered as good candidates to develop biopesticide formulations due to their ability to produce a wide range of antibiotics and antifungal volatile compounds and to form heat- and UV-resistant spores (Ji et al. 2013). The effectiveness of the five Bacillus isolates with strong antifungal activity in vitro against F. euwallaceae, G. euwallaceae, Graphium sp., responsible for FD, and P. cinnamomi responsible for PRR in avocado, should also be evaluated in vivo, to confirm their potential use as biocontrol agents of these important avocado diseases.
References
Abdallah RAB, Mokni-Tlili S, Nefzi A, Jabnoun-Khiareddine H, Daami-Remadi M (2016) Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol Control 97:80–88. https://doi.org/10.1016/j.biocontrol.2016.03.005
Ag Marketing Resource Center (AGMRC) (2014) http://www.agmrc.org/
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bollet C, Gevaudan MJ, De Lamballerie X, Zandotti C, De Micco P (1991) A simple method for the isolation of chromosomal DNA from gram positive or acid-fast bacteria. Nucleic Acids Res 19:1955
Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M (2015) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8:281–295. https://doi.org/10.1111/1751-7915.12238
Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, Vicente AD, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103:1950–1959. https://doi.org/10.1111/j.1365-2672.2007.03433.x
Chaves-López C, Serio A, Gianotti A, Sacchetti G, Ndagijimana M, Ciccarone C, Stellarini A, Corsetti A, Paparella A (2015) Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J Appl Microbiol 119:487–499. https://doi.org/10.1111/jam.12847
Coffey MD (1987) Phytophthora root rot of avocado: an integrated approach to control in California. Plant Dis 71:1046–1053
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:141–145. https://doi.org/10.1093/nar/gkn879
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Dunlap CA, Lueschow S, Carrillo D, Rooney AP (2017) Screening of bacteria for antagonistic activity against phytopathogens of avocados. Plant Gene 11:17–22. https://doi.org/10.1016/j.plgene.2016.11.004
Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199. https://doi.org/10.3389/fmicb.2017.00199
Eskalen A, Gonzalez A, Wang DH, Twizeyimana M, Mayorquin JS, Lynch SC (2012) First report of a Fusarium sp. and its vector Tea Shot Hole Borer (Euwallacea fornicatus) causing Fusarium dieback on avocado in California. Plant Dis 96:1070. https://doi.org/10.1094/PDIS-03-12-0276-PDN
Eskalen A, Stouthamer R, Lynch SC, Rugman-Jones PF, Twizeyimana M, Gonzalez A, Thibault T (2013) Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis 97:938–951. https://doi.org/10.1094/PDIS-11-12-1026-RE
Fan B, Blom J, Klenk HP, Borriss R (2017) Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an “Operational Group B. amyloliquefaciens” within the B. subtilis species complex. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00022
Fiddaman PJ, Rossall S (1993) The production of antifungal volatiles by Bacillus subtilis. J Appl Bacteriol 74:119–126
Food and Agriculture Organization of the United Nations (FAOSTAT) (2015) http://www.fao.org/
García-Avila CDJ, Trujillo-Arriaga FJ, López-Buenfil JA, González-Gómez R, Carrillo D, Cruz LF, Ruiz-Galván I, Quezada-Salinas A, Acevedo-Reyes N (2016) First report of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Mexico. Fla Entomol 99:555–556. https://doi.org/10.1653/024.099.0335
Hakizimana JD, Gryzenhout M, Coutinho TA, Van den Berg N (2011) Endophytic diversity in Persea americana (avocado) trees and their ability to display biocontrol activity against Phytophthora cinnamomi. In: Proceedings VII World Avocado Congress 2011, Cairns, Australia, 5–9 September 2011
Hall TA (1999) BioEdit: a friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Holthorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Idris HA, Labuschagne N, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106. https://doi.org/10.1016/j.biocontrol.2006.07.017
Ji SH, Paul NC, Deng JX, Kim YS, Yun BS, Yu SH (2013) Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 41:234–242. https://doi.org/10.5941/MYCO.2013.41.4.234
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol msw054. https://doi.org/10.1093/molbev/msw054
Lynch SC, Twizeyimana M, Mayorquin JS, Wang DH, Na F, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A (2016) Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.—two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108:313–329. https://doi.org/10.3852/15-063
Mnif I, Hammami I, Triki MA, Azabou MC, Ellouze-Chaabouni S, Ghribi D (2015) Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Environ Sci Pollut Res 22:18137–18147. https://doi.org/10.1007/s11356-015-5005-6
Na F (2016) Identification of two novel fungal species associated with Kuroshio shot hole borer (Euwallacea sp.) and evaluation of novel biological control method to inhibit the fungal associates of the invasive ambrosia beetle species in California (Order No. 10153676). Available from ProQuest Dissertations and Theses Global. (1836080563). Retrieved from https://search.proquest.com/docview/1836080563?accountid=27949
Neupane S, Finlay RD, Alström S, Elfstrand M, Högberg N (2015) Transcriptional responses of the bacterial antagonist Serratia plymuthica to the fungal phytopathogen Rhizoctonia solani. Environ Microbiol Rep 7:123–127. https://doi.org/10.1111/1758-2229.12203
O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Na F, Geiser DM, Bateman C, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP (2015) Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genet Biol 82:277–290. https://doi.org/10.1016/j.fgb.2014.10.014
O’Donnell K, Libeskind-Hadas R, Hulcr J, Bateman C, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Lynch SC, Geiser DM, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP (2016) Invasive Asian Fusarium—Euwallacea ambrosia beetle mutualists pose a serious threat to forests, urban landscapes and the avocado industry. Phytoparasitica 44:435–442. https://doi.org/10.1007/s12600-016-0543-0
Pagliaccia D, Pond E, McKee B, Douhan GW (2013) Population genetic structure of Phytophthora cinnamomi associated with avocado in California and the discovery of a potentially recent introduction of a new clonal lineage. Phytopathology 103:91–97. https://doi.org/10.1094/PHYTO-01-12-0016-R
Prabhukarthikeyan SR, Manikandan R, Durgadevi D, Keerthana U, Harish S, Karthikeyan G, Raguchander T (2017) Bio-suppression of turmeric rhizome rot disease and understanding the molecular basis of tripartite interaction among Curcuma longa, Pythium aphanidermatum and Pseudomonas fluorescens. Biol Control 111:23–31. https://doi.org/10.1016/j.biocontrol.2017.05.003
Shaikh SS, Sayyed RZ (2015) Role of plant growth promoting rhizobacteria and their formulation in biocontrol of plant diseases. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, New Delhi
Stirling AM, Hayward AC, Pegg KG (1992) Evaluation of the biological control potential of bacteria isolated from a soil suppressive to Phytophthora cinnamomi. Australas Plant Path 21:133–142
Toerien J (2007) The Phytophthora challenge. Calif Avocado Soc Yearb 90:89–101
Tokpah DP, Li H, Wang L, Liu X, Mulbah QS, Liu H (2016) An assessment system for screening effective bacteria as biological control agents against Magnaporthe grisea on rice. Biol Control 103:21–29. https://doi.org/10.1016/j.biocontrol.2016.07.009
Umeda C, Eskalen A, Paine TD (2016) Polyphagous shot hole borer and Fusarium dieback in California. In: Paine T, Lieutier F (eds) Insects and diseases of Mediterranean forest systems. Springer International Publishing, New York
Vida C, Cazorla FM, de Vicente A (2017) Characterization of biocontrol bacterial strains isolated from a suppressiveness-induced soil after amendment with composted almond shells. Res Microbiol. https://doi.org/10.1016/j.resmic.2017.03.007
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40:1–29. https://doi.org/10.18637/jss.v040.i01
Yang C, Crowley DE, Menge JA (2001) 16S rDNA fingerprinting of rhizosphere bacterial communities associated with healthy and Phytophtora infected avocado roots. FEMS Microbiol Ecol 35:129–136. https://doi.org/10.1111/j.1574-6941.2001.tb00796.x
Yin B, Scupham AJ, Menge JA, Borneman J (2004) Identifying microorganisms which fill a niche similar to that of the pathogen: a new investigative approach for discovering biological control organisms. Plant Soil 259:19–27
You MP, Sivasithamparam K, Kurtböke DI (1996) Actinomycetes in organic mulch used in avocado plantations and their ability to suppress Phytophthora cinnamomi. Biol Fertil Soils 22:237–242
Yuan J, Raza W, Shen Q, Huang Q (2012) Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl Environ Microbiol 78:5942–5944. https://doi.org/10.1128/AEM.01357-12
Acknowledgements
We thank Clemente García-Ávila and Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA) for facilitating the import of bacterial strains isolated from a California avocado orchard to Mexico. We also thank Eneas Aguirre, Diana Sánchez and Yonatan Escudero for their help with isolating and morphotyping bacterial isolates, and Ofelia Ferrera for her technical assistance. This study was supported by a 2015 The University of California Institute for Mexico and the United States and El Consejo Nacional de Ciencia y Tecnología (UC MEXUS – CONACYT) collaborative research grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guevara-Avendaño, E., Carrillo, J.D., Ndinga-Muniania, C. et al. Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi . Antonie van Leeuwenhoek 111, 563–572 (2018). https://doi.org/10.1007/s10482-017-0977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0977-5