Abstract
Bacillus subtilis SPB1 lipopeptides were evaluated as a natural antifungal agent against Fusarium solani infestation. In vitro antifungal assay showed a minimal inhibitory concentration of about 3 mg/ml with a fungicidal mode of action. In fact, treatment of F. solani by SPB1 lipopeptides generated excessive lyses of the mycelium and caused polynucleation and destruction of the related spores together with a total inhibition of spore production. Furthermore, an inhibition of germination potency accompanied with a high spore blowing was observed. Moreover, in order to be applied in agricultural field, in vivo antifungal activity was proved against the dry rot potato tubers caused by F. solani. Preventive treatment appeared as the most promising as after 20 days of fungi inoculation, rot invasion was reduced by almost 78 %, in comparison to that of non-treated one. When treating infected tomato plants, disease symptoms were reduced by almost 100 % when applying the curative method. Results of this study are very promising as it enables the use of the crude lipopeptide preparation of B. subtilis SPB1 as a potent natural fungicide that could effectively control the infection of F. solani in tomato and potato tubers at a concentration similar to the commercial fungicide hymexazol and therefore prevent the damage of olive tree.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fusariosis caused by the phytopathogenic fungus Fusarium spp., a vascular disease of diverse plants, causes great damages and leads to high mortalities (El-Kassas and Khairy 2009). The soil-borne fungus Fusarium solani is involved in the severe root-rot damping-off and wilt disease of several plants and is responsible for severe damages on many economically important plant species (El-Kassas and Khairy 2009). Such fungi are ubiquitous in soil and decaying plant material, where they act as decomposers. They are also host-specific pathogens of a great number of agriculturally important plants, including pea, cucurbits, sweet potato (root and tubers), and olive tree (El-Kassas and Khairy 2009). F. solani penetrates into the roots inducing root rots, and the infected tissues become usually dark red or brown and can form streaks which rise up to the ground level. Generally, affected plants lag behind growth. As the decay of roots progresses, the oldest leaves begin going yellow and the young leaves become flasks (El-Kassas and Khairy 2009).
So, an urgent need for the control of F. solani soft rot was developed. Chemical control methods have been widely used and discussed. But the abusive use of pesticides and fungicides to cure or prevent plant diseases has often been reported to bring about a wide array of pernicious effects, particularly on plants, soil, environment, and, ultimately, humans. Fungicide and pesticide treatments have often been used in conventional agriculture to protect against soil-borne diseases, to prevent or reduce plant mortality and losses, to enhance plant emergence, and, thus, to improve their overall production (Hammami et al. 2011). Nevertheless, disinfectants, such as sodium hypochlorite, or fumigants, such as methyl bromide, could be toxic to young plants and cause serious occupational and environmental risks to handlers and the environment (Yangui et al. 2013). They can also pose irreparable damage to the metallic structure of greenhouses (Hammami et al. 2011). Physical methods, such as heat treatment, are not always adequately appropriate for application and often produce large amounts of unviable seeds (Yangui et al. 2013). Therefore, due to the limitations associated with conventional chemical and physical control systems, biological treatment method appears as the most-promising techniques. The effectiveness of a potential alternative control agent should be conditioned by at least three main criteria. In fact, it must be highly specific against the target pathogens, easily degradable after use, and sufficiently cost-effective for wide-scale application (Hammami et al. 2011). Current research provides strong evidence that biological control offers one of the most promising, environmentally safe, and cost-effective tactics (Ongena and Jacques 2008). Actually, the use of bacteria as biocontrol agents has been extensively investigated, and a wide array of bioactive metabolites, such as bacteriocins and antibiotics, and others with antifungal, antiviral, insecticide, and herbicide properties have been described in the literature (Al-Reza et al. 2010; Hammami et al. 2009, 2011). It is well documented that many Bacillus species are well recognized as biocontrol agents against fungal diseases (Ongena and Jacques 2008) and their derived biomolecules were reported to inhibit fungal spore germination (Leelasuphakul et al. 2008; Matar et al. 2009; des Grades et al. 2012). In fact, Bacillus species are well known to produce many antimicrobial peptide substances or bacteriocins, such as BLIS produced by Bacillus cereus (Risoen et al. 2004) and Bac 14B produced by Bacillus subtilis (Hammami et al. 2009). It is well documented also that lipopeptides, the prevailing group of biosurfactant compounds, are among the most popular and powerful metabolites in combating and treating fungal disease infection in vitro and in vivo (Cao et al. 2012; Li et al. 2012).
Therefore, in view of the persistent search for natural alternatives to chemical control practices, the following work highlights the use of B. subtilis SPB1 lipopeptide as natural surfactant for plant pathogen elimination. In fact, in vitro and in vivo potential antifungal activities against F. solani infestation were done. Potato tubers and tomato root were defined as plant invasion materials. The evolution of the disease incidence was discussed when using SPB1 lipopeptide as preventive and curative treatments in comparison to a chemical fungicide.
Materials and methods
Bacterial strain and phytopathogen fungus
B. subtilis SPB1 (HQ392822), a lipopeptide biosurfactant-producing bacterium, was isolated in our laboratory from a Tunisian soil contaminated by hydrocarbons (Ghribi et al. 2012).
F. solani was kindly provided by Dr. Mohamed Ali Triki (Olive Tree Institute of Tunisia). It was maintained at 4 °C in Potato Dextrose Agar (PDA) plates and at −20 °C in tryptone salt medium (tryptone, 1 g; NaCl, 8.5 g; Tween 20, 1 %; glycerol, 15 %; and distilled water, 1 l). A conidial suspension of F. solani strain was prepared by culturing the fungus on PDA medium until sporulation for 1 week at 25 °C. The agar surface was then rinsed with 10 ml distilled water containing 8.5 g/l NaCl and 1 ml/l Tween 80. The concentration of spores was determined using a Malassez cytometer (Dutscher 140501), adjusted to 3.106 spores/ml and used to infect tomato roots and potato tubers.
Culture conditions and SPB1 biosurfactant extraction
Culture conditions were carried out as described by Mnif et al. (2013). At the end of the cultivation, the culture was centrifuged at 10,000 rpm and 4 °C for 20 min to remove bacterial cells. The supernatant-free cells served for lipopeptide extraction during three consecutive cycles of acid precipitation-dissolution (Mnif et al. 2013). In fact, each time, the pellet formed by acid precipitation was suspended in alkaline water, the pH was readjusted to 8 with NaOH 1 N, and the supernatant was collected by centrifugation at 10,000 rpm and 4 °C for 20 min followed by second acid precipitation. The final pellet formed was washed three times with acid water (pH = 2), dissolved in distilled water at a concentration of 10 mg/ml, the pH adjusted to 8 with NaOH 1 N, and lyophilized. This serves as crude lipopeptide preparation to study the antifungal activity.
Plant and fruit material
In vivo antifungal activity against infection by F. solani was studied on potato tubers and tomato plants. Potato tubers were selected free of wounds and rots and as much as possible homogeneous in maturity and size, and were stored at 4 °C for 2–4 days until use. Tomato seedlings were of the cultivar Riogrande (Societa Agricola Italiana Sementi, Centro Ricerca E Miglioramen to Sementi Horticole E Foraggere, Cesena, Italy; 2006/2007).
In vitro antifungal activity of SPB1 biosurfactant
The antifungal activity of SPB1 lipopeptide was checked initially by the disc diffusion method as described by Hammami et al. (2011). To try the antagonistic activity against hyphal growth, an agar with mycelia of F. solani was placed in the center of a Petri dish (diameter 6 cm) containing 10 ml of PDA with different concentrations of lipopeptide biosurfactant preparation dissolved in sterile distilled water (0.1, 0.2, 0.4, 1.0, 2.0, and 3.0 mg/ml, respectively) and incubated at 25 °C for 5 days. Control plates were mixed with distilled water alone. Radial growth was measured daily, and the inhibitory activity of mycelial growth of SPB1 lipopeptide was expressed as the percentage of the growth on the untreated medium according to the formula presented below. All assays consisted of three replicates and the averages of the repeated experimental results were determined. Then the mycelial growth inhibition was calculated according to the present formula:
MGI (%) = ((d c − d t)/d c) × 100; d c and d t represent mycelial growth diameter in control and treated Petri plates, respectively.
The minimal inhibitory concentration (MIC) is defined as the smallest concentration that inhibits the visible fungal growth totally (Zu et al. 2010). It was determined from growth observation of mycelial growth experiments done according to the disc diffusion method. The half maximal inhibitory concentration (IC50) is a measure of the effectiveness of a substance in inhibiting a specific biological or biochemical function (Soothill et al. 1992). It can be determined by constructing a dose–response curve and examining the effect of different concentrations of biosurfactant on the mycelial growth. It can be calculated for a given antagonist by determining the concentration needed to inhibit half of the maximum biological response (Soothill et al. 1992).
Mode of action of the lipopeptide biosurfactant
The fungistatic–fungicidal nature of the SPB1 lipopeptide biosurfactant was tested by controlling revival of growth of the inhibited mycelia disc following its transfer to non-treated PDA. A fungicidal effect was where there was no growth, whereas a fungistatic effect was where temporary inhibition of fungal growth occurred. The agar discs of F. solani, which failed to grow, were transferred onto agar media without SPB1 lipopeptide. Petri plates were incubated for 5 days. The experiments were conducted in triplicates.
Effect of SPB1 biosurfactant treatment on mycelium morphology
In order to assess the effect of SPB1 lipopeptides on the mycelium morphology, mycelium near the zone of inhibition was taken and observed by microscope (×40 magnification) after exposure to desired biosurfactant concentrations. An intact mycelium showing normal growth with no lipopeptide addition serves as negative control.
Effect of SPB1 biosurfactant on spore germination
The evaluation of the effect of SPB1 lipopeptides on the germination of F. solani spores was performed as described by Rebib et al. (2012). Fresh spores were harvested from cultures of plugs containing mycelia of F. solani taken from a PDA Petri plate culture (3 days old) and suspended in sterile distilled water. Spores were separated from the mycelium fragment by filtration, and they were counted and used for germination test. The effect of SPB1 lipopeptides on spore germination was performed by mixing on microslides containing a thin layer of methylene blue colored PDA, 5 and 10 μl, respectively, of spore suspension adjusted at 106 spores/ml and different concentrations of SPB1 lipopeptide preparation. Controls consisted of 5 μl of spore suspension and 10 μl of sterile distilled water. Then microslides were incubated at 25 °C in a sterile Petri dish at constant humidity, and spore germination was microscopically evaluated. Conidia were considered germinated if the germ tube was longer than one half of the diameter of the spores (Rebib et al. 2012). Spore enumeration was performed on a total of 100 spores. All assays were conducted in triplicate. The percentage of inhibition of spores’ germination was calculated according to the present formula (Rebib et al. 2012):
where
- G 0 :
-
Number of spores germinated in the medium without SPB1 lipopeptide addition
- G :
-
Number of spores germinated in the medium containing SPB1 lipopeptides
Study of the in vivo antifungal activity of SPB1 lipopeptides towards F. solani infection
Preventive and curative treatments of infested potato tubers
Control of F. solani soft rot was evaluated in potato tubers cv. Spunta according to the protocol described by Yangui et al. (2013). Experiments were done in triplicates. Mature potato tubers used in all experiments were carefully selected on the basis of their size and on the absence of any disease or wounding symptoms. Tubers were surface disinfected by dipping in sodium hypochlorite at 2 % for 10 min and rinsed three times with sterile distilled water to eliminate saprophyte pathogen present at their surface and residual sodium hypochlorite. Then, they were dried under filter-sterilized air flow. Five-millimeter-wide and -deep wells were then artificially created with a sterile cork borer and 0.5 ml of spore suspension containing the pathogen agent at 3.106 spores/ml was poured on the well. Treatment of potato tubers by the minimal inhibitory concentration of SPB1 lipopeptides was realized according to two different ways. In the first one, preventive method, the required dose of lipopeptide preparation was added in the wells 24 h before infection by F. solani. In the second one, curative method, the required dose was administrated 24 h after infection by the pathogen. Negative controls were realized at the same way using sterile distilled water in place of lipopeptide preparation. Non-inoculated and non-treated tubers corresponding to negative controls were also made. Positive controls treated with hymexazol were also realized. Treated tubers were incubated 20 days at 30 °C in disinfected plastic bags at high relative humidity, and disease incidence was evaluated 20 days after pathogen challenge based on the diameter of spreading of F. solani lesions that developed around infected sites. After the incubation period, tubers were cut longitudinally via sites of inoculation. Parameters of dry rot induced (maximal width (w) and depth (d)) are noted. The penetration of the pathogen into tubers is calculated following the formula of Lapwood et al. as presented by Yangui et al. (2013):
where
- w :
-
width of soft rot (mm)
- d :
-
depth of the soft rot (mm)
- p :
-
depth of the inoculation well (mm)
Therefore, the inhibition of the rot extension was calculated according to the presented formula:
With
- Ti:
-
positive control: inoculated and not treated tuber
- Tr:
-
inoculated and treated tuber
Preventive and curative treatments of F. solani-infested tomato root
In vivo biocontrol of tomato root infestation by F. solani was conducted according to the method described by Cazorla et al. (2007) with little modifications. Roots of young tomato plants were soaked in 20 ml F. solani spore suspension (containing 3.106 spores/ml). A safe control was realized using sterile distilled water in place of F. solani culture. For preventive treatment, plants were submerged in biosurfactant solution at a concentration of 3 mg/ml, 1 day before infection by the pathogenic fungi. For curative treatment, plants were irrigated by SPB1 lipopeptide solution at a concentration of 3 mg/ml 1 day after infection. Inoculated and non-inoculated negative controls were treated the same way by sterile distilled water. Safe plants non-inoculated and untreated were also made as healthy control. Positive control treated with hymexazol (2 ml/l) was also realized in both protective and curative assays. Plants were transplanted in pots and placed in a hermetic cultivar room at a constant temperature of 22 ± 1 °C with a photoperiod of 16 h. They were irrigated regularly with water to maintain their survival. All experiments were realized in triplicate.
Pathogenicity of F. solani was estimated according to an annotation scale established by Muyolo et al. (1993) and based on the percentage of burnt leaf. During the period of observation, a grade was attributed to the plant every week following the annotation scale presented below (Muyolo et al. 1993):
-
0
leaf with healthy aspect
-
1
1 to 25 % of folia area was affected
-
3
26 to 50 % of folia surface was affected
-
4
51 to 75 % of folia surface was affected
-
5
76 to 100 % of folia surface was affected
On the basis of this annotation, disease severity in percentage was calculated according to this formula:
Results and discussion
In recent years, many studies have demonstrated problems arising from the presence of pesticide residues in the environment, foods, and feeds. This allowed us to avoid the use of some chemical fungicides to control the disease of plants and spoilage of their products used for food. Alternative methods to control these pathogens and spoilage organisms are now being investigated. Several microorganisms such as bacteria, yeasts, fungi, and their related metabolites could be exploited as biological control agents to replace hazardous chemical fungicides (Al-Reza et al. 2010; Hammami et al. 2009, 2011). In this article, we have described the use of a B. subtilis SPB1-derived lipopeptide as a potent antifungal agent against F. solani.
It was known that B. subtilis SPB1 was able to co-produce lipopeptide isoforms (unpublished results). After extraction, separation, and purification, different lipopeptide compounds were identified in the culture filtrate of SPB1. Mass spectroscopic analysis confirmed the presence of different lipopeptide compounds consisting of surfactin isoforms with molecular weights of 1007, 1021, and 1035 Da; iturin isoforms with molecular weights of 1028, 1042, and 1056 Da; and fengycin isoforms with molecular weights of 1432 and 1446 Da. Two new clusters of lipopeptide isoforms with molecular weights of 1410 and 1424 Da and 973 and 987 Da, respectively, were also detected.
In vitro antifungal activity of SPB1 biosurfactant
The antagonistic activity of SPB1 lipopeptides against F. solani was, firstly, demonstrated by the disc diffusion method. The SPB1 lipopeptides exhibited a moderate to high antifungal activity against the plant pathogenic fungi, F. solani. The anti-fungal activity was dose dependent and is confirmed by the radial growth method. According to literature reviews and preliminary studies, different lipopeptide concentrations ranging from 0.1 to 3 mg/ml were tried. In fact, findings demonstrate that, comparatively to the negative control, the radial growth of F. solani was affected in the presence of SPB1 lipopeptides. As presented in Fig. 1, a gradual decrease of fungi diameter was observed with increasing SPB1 lipopeptide concentration. The growth inhibition percentages in the presence of different lipopeptide doses were counted. Mean values were statistically significant according to Duncan test at p values <0.05. SPB1 lipopeptides biosurfactant was shown to be effective in inhibiting the in vitro growth of the phytopathogenic fungus F. solani, and a MIC and an IC 50 % value of about 3 and 2 mg/ml were determined, respectively. According to literature studies, the related MIC value was much higher than that recorded for the FK463 lipopeptide antifungal agent as presented by Tawara et al. (2000) and the minimum inhibitory concentration of the cyclic lipopeptide antibiotics from B. subtilis C2 recorded against F. solani (Li et al. 2012). Moreover, the IC 50 % value was, mainly, much higher than that described for iturin A (0.51 mg/ml) of B. subtilis strain BS-99-H against Pestalotiopsis eugeniae (Lin et al. 2010) and for the antifungal active compounds of B-FS01 strain (20 μg/ml) against Fusarium moniliforme (Hu et al. 2007).
To assert the antifungal potency of SPB1 biosurfactant, mycelium of F. solani was microscopically observed near the zone of inhibition showing excessive lyses of the mycelium with polynucleated and excessive destructed spores (Fig. 2). However, untreated negative control test demonstrated normal mycelium aspect and smooth surfaces. This observation suggested that SPB1 biosurfactant acted on the cell surface by the potent permeabilizing activity leading to cellular death. These results confirm the fungicidal action of SPB1 lipopeptide biosurfactant by F. solani. In fact, we observe the inadequacy of F. solani to regain growth. This could be also proved by the excessive lysis of the mycelium with polynucleated and destructed spores as described previously. Similar results were demonstrated by Kim et al. (2004) revealing that Colletotrichum gloeosporioides treated for 12 h with Bacillus thuringiensis CMB26-derived lipopeptides showed cell surface shrinking leading to a fungicidal effect towards the phytopathogenic fungi. Comparable findings were described by Senthilkumar et al. (2009) and Lin et al. (2010) reporting the cell lysis of the pathogenic fungi Rhizoctonia bataticola when treated by antifungal metabolite produced by Paenibacillus sp. and the swelling and the deformation of fungus hyphae of P. eugeniae when treated by the iturin A of B. subtilis BS-99-H.
Effect of SPB1 lipopeptide on F. solani mycelial growth: representative microscopic pictures (10 × 40 magnifications) of mycelium and spores of F. solani grown in medium with a negative control without SPB1 lipopeptide treatment showing normal mycelia aspect and spore germination, b, c F. solani mycelia treated with 0.4 and 2 mg/ml SPB1 lipopeptide respectively showing an excessive lysis of the mycelia with polynucleated and destructed spores. d F. solani mycelia treated with 3 mg/ml SPB1 lipopeptide with polynucleated and non-germinated, deformed, and blooming spores
Effect of SPB1 lipopeptides on F. solani spore production and germination
Spore production and germination of F. solani were determined in the presence of B. subtilis SPB1 lipopeptides. The results obtained showed a significant inhibition of fungal spore production at varied concentrations of the antifungal compound. SPB1 lipopeptide preparation exhibited a potent inhibitory effect on the spore production of F. solani within the range of 78 to 100 % at a concentration ranging from 1.5 to 3 mg/ml. Therefore, the inhibition effectiveness was dose dependent and an increase of inhibition potency was observed when increasing lipopeptide concentration. In fact, the number of spores was about 1.1 × 106 spores/ml in the presence of 1.5 mg/ml SPB1 lipopeptides consisting towards 5 × 106 spores/ml in its absence. Distilled water, used as negative control, did not inhibit the spore production.
Spores of F. solani were treated with different lipopeptide concentrations and subjected to germination. Microscopic observations were made in course of time (after 6, 24, and 48 h of incubation). The results presented in Fig. 3 showed an inhibition of germination potency accompanied with a high spore blowing in comparison with the negative control that showed normal spore germination potency. In fact, in the control incubated with distilled water, conidia germinated by forming a germ tube and produced appressoria. It appears that the crude lipopeptide preparation also affected the permeability of the membrane of conidiospores, preventing, thus, their germination.
According to the present study, the antifungal compound may be effective in the control of fungal growth. SPB1 lipopeptides were effective also in inhibiting spore formation and germination. Regarding literature reviews and studies, biomolecules derived from B. subtilis strains were reported to inhibit fungal spores’ germination (Leelasuphakul et al. 2008; Matar et al. 2009). Also, results are in accordance to those published by Chitarra et al. (2003) suggesting that B. subtilis YM 10–20 iturin causes Penicillium roqueforti conidiospore permeabilization inhibiting therefore their germination. Under these conditions, the spores will lose the ability to initiate biochemical activities and to start morphological changes. In fact, a novel antifungal protein derived from B. subtilis B29 was reported to inhibit highly conidial spore germination of Fusarium oxysporum and suppress germ-tube elongation, and induced distortion, tumescence, and rupture of a portion of the germinated spores (Jing et al. 2009). Lipopeptides are also well known to inhibit spore germination of many fungi. A cluster of fengycin homologues produced by B. subtilis B-FS01 was demonstrated to strongly inhibit growth and germination of spores of F. moniliforme (Hu et al. 2007). The culture filtrate of B. subtilis strain C2 containing lipopeptide compounds belonging to fengycin family was shown to induce chlamydospore in F. solani (Li et al. 2012). Iturin A produced by B. subtilis BS-99-H (Lin et al. 2010) and fengycin produced by B. subtilis LB5 (Ruangwong et al. 2012) inhibit Pestalotiopsis eugeniae spore and Colletotrichum gloeosporioides conidia germination, respectively.
Study of in vivo antifungal activity of SPB1 lipopeptides
Effect of biosurfactant treatment on potato tuber rot development
In order to evaluate the efficacy of the antifungal activity of B. subtilis SPB1 against F. solani in vivo, experiments were done on infected potato tubers. According to the data from tuber experiments, the results obtained showed that all the treatments with 3 mg/ml SPB1 lipopeptides were significantly effective (P = 0.05) in controlling F. solani potato tuber rot infection when they were treated 24 h before and after their inoculation for preventive and curative treatments, respectively (Fig. 4). A total rot was observed on the untreated and inoculated tubers and those treated with distilled water, whereas important reductions of decay severity were obtained with all biological treatments tested. Penetration values for negative and the positive control and the treated tubers are presented in Fig. 5. For those treated with the antifungal agent produced by B. subtilis SPB1, we observed an important decrease of the tuber rot when treated before infection by F. solani, evaluated to 53.56 % towards the untreated positive control. When treated after F. solani infestation (curative method), only a slight decrease of tuber rot of about 6.6 % towards positive control was observed. Accordingly, the antagonist SPB1 lipopeptides were shown to be the most effective treatment when applied as preventive treatment. In order to compare the efficacy of the antifungal potential of SPB1 lipopeptides towards chemical antifungal agent, hymexazol was used as positive control in parallel. It was demonstrated as an excellent antifungal in vivo and in vitro against Fusarium infestation. It could powerfully reduce tuber rot incidence occasioned by F. solani species. However, SPB1 lipopeptide preparation was more effective than hymexazol in suppressing tuber rot penetration. The efficiency of preventive treatment towards curative method was similar when using SPB1 lipopeptides and hymexazol. This drastic decrease of the antifungal activity when treating tubers after spore inoculation can be due to the rapidity of spore invasion leading to tuber rots. Accordingly, lipopeptide preparation could be used as an excellent preventive treatment of tubers before their storage in order to inhibit phytopathogenic fungi penetration by injuries carried during crop collection.
Effect of B. subtilis SPB1 biosurfactant and hymexazol treatment on soft rot development occasioned by F. solani after 20 days of incubation at 30 °C (inoculated tubers, cv. Spunta). a Healthy control (HC); b inoculated control (IC); c preventive treatment by biosurfactant (Trb+I); d preventive treatment by hymexazol (TrH+I); e curative treatment by biosurfactant (I+TrB); f curative treatment by hymexazol (I+TrH)
Effect of biosurfactant and hymexazol treatment on the penetration of F. solani into potato tubers in both preventive ( ) and curative (
) and curative ( ) treatment (mean values are statistically significant at p <0.05) with the negative control = healthy control and the positive control = inoculated with F. solani and not treated
) treatment (mean values are statistically significant at p <0.05) with the negative control = healthy control and the positive control = inoculated with F. solani and not treated
Effect of SPB1 lipopeptide treatment on tomato root infestation
In order to investigate the protective effect of SPB1 lipopeptides against F. solani, in vivo biocontrol of tomato root infestation was conducted (Fig. 6). Therefore, after 1 week of inoculation, untreated tomato plants manifested burning symptoms on their leaf. These symptoms developed necrotic appearance on the collar and roots of inoculated plants 2 weeks after. Control plants were not inoculated and did not present any necrosis or symptoms at the time of assessment. Thus, we can assume that there has been no cross-contamination of the fungus between pots. However, inoculated and treated plants are similar to the healthy control and did not develop any symptoms. SPB1 lipopeptides positively affected plant development as well as increased biomass and root shoot length by inhibiting F. solani growth when tested in pot experiments (Fig. 6). The temporal evolution of foliar alteration occasioned by F. solani demonstrates the same fact. Results presented in Fig. 6 showed a total alteration of inoculated tomato leaf after 20 days of incubation. Also, curative treatment using SPB1 lipopeptides or hymexazol appeared as more interesting than preventive treatment. In fact, no foliar alterations were observed when curative treatments were followed. Actually, results demonstrated that SPB1 lipopeptide treatment was significantly effective in avoiding and suppressing symptom apparition. As presented in Fig. 7, the degree of disease reduction varied according to the treatment method. In contrast to the results observed for potato tuber rot, curative treatment method appeared as more interesting to inhibit F. solani infection symptom development. In fact, when treating tomato plants after F. solani infection, by SPB1 antifungal agent, disease development was inhibited to about 100 % in contrast to 75 % when applying the preventive method. Studies showed similar results when using hymexazol. Furthermore, SPB1 lipopeptides were more efficient than hymexazol; a reduction of disease incidence of about 55 % was obtained by preventive treatment by hymexazol in contrast to 75 % recorded when using SPB1 lipopeptides.
Effect of B. subtilis SPB1 biosurfactant and hymexazol treatment on soft rot tomato root development occasioned by F. solani after 20 days of incubation at 30 °C (inoculated tomato, cv. Riogrande); healthy control (HC); inoculated control (IC); preventive treatment by biosurfactant (TrB+I); preventive treatment by hymexazol (TrH+I); curative treatment by biosurfactant (I+TrB); curative treatment by hymexazol (I+TrH)
The results of this study suggested that the crude lipopeptide preparation of B. subtilis SPB1 would be a potential natural fungicide that could effectively control tomato infestation by F. solani with efficiency significantly higher than the commercial fungicide hymexazol.
Showing efficient in vitro antifungal activity, SPB1 lipopeptides were evaluated as an in vivo biocontrol agent against potato tuber and tomato root rot occasioned by F. solani. According to the data from the experiments done on infested potato tubers and tomato root, all the treatments with 3 mg/ml SPB1 lipopeptides including both the curative and preventive method were significantly effective in controlling F. solani infection. Regarding many literature reviews and studies, many microbial derived compounds were described as inhibitors of in vivo fungi rot development (Leelasuphakul et al. 2008). In fact, fengycin was described by Hu et al. (2007), Cao et al. (2012), and Rebib et al. (2012) as inhibitor of in vivo F. moniliforme spread causing maize infection, suppressor of Fusarium wilt of cucumber, and suppressor of Fusarium foot rot of wheat. Also, Kita et al. (2005) evaluated the suppressive ability of iturin A isolated from B. subtilis RB14 against damping-off of tomato seedlings and Phomopsis root rot of cucumber. Biosurfactant-type lipopeptides are involved in the multifaceted biocontrol potential of B. subtilis species against fungi spread and infection. In similar studies realized by Triki et al. (2012), the authors reported that preventive treatment by B. subtilis filtrate permits a decrease of rot extension of about 62 %, whereas curative treatment permits a decrease of about 37 % only. These findings are also similar to our results indicating the effectiveness of the preventive method in avoiding F. solani potato tuber rot spreading towards the curative method. Also, De Corato et al. (2014), Zhang et al. (2013), and Yangui et al. (2013) reported the effectiveness of the preventive treatment towards the curative treatment in avoiding phytopathogenic fungi invasion. Indeed, the effectiveness of the preventive versus curative treatment suggests the in vivo stability of the crude lipopeptide preparation before 24 h of treatment.
Showing a high efficiency in treating infected potato tubers, SPB1 lipopeptide preparation was tested as potent treatment against tomato root rot. In fact, regarding literature studies, diverse biological control systems were applied to treat tomato infestation by phytopathogenic fungus such us Trichoderma viride (Ebtsam et al. 2009) and Trichoderma harzianum and Paenibacillus lentimorbus (Montealegre et al. 2005). B. subtilis species and their metabolites were largely used as in vivo biocontrol agent against fungal infestation (Nihorimbere et al. 2010; des Grades et al. 2012). Our study depicted the potential role of B. subtilis-derived lipopeptide as in vivo biocontrol agent against F. solani tomato root rot.
SPB1 lipopeptide preparation was effective in inhibiting F. solani growth when tested in pot experiments. Also, it affected positively plant development as well as increased biomass and root shoot length especially when applied as curative treatment. In fact, des Grades et al. (2012) reported the effectiveness of the application of B. subtilis (strain FZB24) cells and their excreted metabolites in the biological control of leaf pathogens of tomato. But, in contrast to our findings, they demonstrated that there were no significant differences in the reduction of disease severity when applying the biological treatment before and after inoculation of Phytophthora infestans (des Grades et al. 2012). Nihorimbere et al. (2010) investigated the beneficial effects of B. subtilis on field-grown tomato in Burundi by a growth promotion and a disease reduction of about 65–70 % towards local Fusarium infestation. Accordingly, lipopeptides produced mainly by Bacillus species are very popular as antifungal agent to suppress plant tomato disease development (Ongena and Jacques 2008). Kita et al. (2005) discussed the suppressive ability of iturin A produced by B. subtilis RB14-C against the damping-off of tomato seedlings caused by R. solani and Phomopsis root rot of cucumber. A mycosubtilin from a B. subtilis overproducing strain was effective for reducing a Pythium infection of tomato seedlings marked by a significant increase in the germination rate of seeds with a greater fresh weight of emerging seedlings (Leclère et al. 2005).
The effectiveness of the curative treatment towards the preventive treatment was depicted also by Oxenham et al. (2005), Soylu et al. (2010), and Wang et al. (2013) reporting the in vivo antifungal activities of essential oil of basil (Ocimum basilicum) against Botrytis fabae, of the essential oils of various plants against tomato grey mold disease agent Botrytis cinerea, and of lactoferrin against gray mold on tomato plants caused by B. cinerea, respectively. Moreover, the higher curative effect of SPB1 lipopeptide against F. solani mold decay of tomato plant than the preventive effect may be directly related to the inhibition of spore germination, germ-tube growth, and/or mycelial growth of F. solani in vivo with a direct fungitoxic property as indicated in in vitro studies. Also, it could be hypothesized that SPB1 lipopeptide presented a better effect as curative rather than protective treatment because they might poorly be absorbed by fungi and plant cells if they are applied before pathogen inoculation (Soylu et al. 2010).
Regarding the two in vivo studies, SPB1 lipopeptide preparation was shown to be more effective than hymexazol in suppressing F. solani rot. Our results were similar to those presented by Daami-Remadi et al. (2006) and Yun-feng et al. (2012) indicating the efficiency of biological treatment in comparison to chemical method against in vivo fungi spore spreading.
Conclusion
Antifungal activity of SPB1 lipopeptides against F. solani was assessed. In vitro antifungal assay determined a minimal inhibitory concentration of 3 mg/ml with a fungicidal mode of action towards F. solani. Microscopic observations showed excessive lysis of the mycelium with polynucleated and destructed spores of the treated fungi. Experimental study showed a total inhibition of spore production when treating F. solani with 3 mg/ml of lipopeptides. Furthermore, results showed an inhibition of the germination potency accompanied with a high spore blowing compared to the negative control with normal spore germination potency. In order to investigate the applicability of SPB1 lipopeptides as antifungal agent in agricultural field, in vivo potential activity was tried against potato tubers and tomato root infested by F. solani. For potato tuber treatment, results showed that SPB1 lipopeptide biosurfactant preparation could be used as preventive treatment of tubers before their storage in order to inhibit phytopathogenic fungi penetration by injuries carried during crop collection. For tomato plant treatment, disease development was inhibited to about 100 % in contrast to 80 % when applying the preventive method. To conclude, the crude lipopeptide preparation of B. subtilis SPB1 would be a potential natural fungicide that could effectively control F. solani infestation in tomato and potato tubers.
References
Al-Reza SM, Rahman A, Ahmed Y, Kang SC (2010) Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic Biochem Physiol 96:86–92
Cao Y, Xu Z, Ling N, Yuan Y, Yang X, Chen L, Shen B, Shen Q (2012) Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Sci Hortic 135:32–39
Cazorla FM, Romero D, Pérez-Garcia A, Lugtenberg BJJ, de Vicente A, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103:1950–1959
Chitarra GS, Breeuwer P, Nout MJR, van Aelst AC, Rombouts FM, Abee T (2003) An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. J Appl Microbiol 94:159–166
Daami-Remadi M, Jabnoun-Khiareddine H, Ayed F, Hibar K, Znaidi IEA, El Mahjoub M (2006) In vitro and in vivo evaluation of individually compost fungi for potato Fusarium dry rot biocontrol. J Biol Sci 6(3):572–580
De Corato U, Viola E, Arcieri G, Valerio V, Cancellara FA, Zimbardi F (2014) Antifungal activity of liquid waste obtained from the detoxification of steam-exploded plant biomass against plant pathogenic fungi. Crop Prot 55:109–118
des Grades ZE, der Agrarwissenschaften D, Fakultät HL, Wilhelms RF (2012) Biological control of leaf pathogens of tomato plants by Bacillus subtilis (strain FZB24): antagonistic effects and induced plant resistance. Inaugural-Dissertation, Institute of Crop Science and Resource Conservation—Phytomedicine, vorgelegt am 06.06.2012
Ebtsam MM, Abdel-Kawi KA, Khalil MNA (2009) Efficiency of Trichodermaviride and Bacillus subtilis as biocontrol agents against Fusarium solani on tomato plants. Egypt J Phytopathol 37:47–57
El-Kassas HY, Khairy HM (2009) A trial for biological control of a pathogenic fungus (Fusarium solani) by some marine microorganisms. Am Eurasian J Agric Environ Sci 5:434–440
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I, Maktouf S, Chaabouni-Ellouze S (2012) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol. doi:10.1155/2012/373682
Hammami I, Rhouma A, Jaouadi B, Rebai A, Nesme X (2009) Optimization and biochemical characterization of a bacteriocin from a newly isolated Bacillus subtilis strain 14B for biocontrol of Agrobacterium spp. strains. Lett Appl Microbiol 48:253–260
Hammami I, Triki MA, Rebai A (2011) Purification and characterization of the novel bacteriocin Back IH7 with antifungal and antibacterial properties. J Plant Pathol 93:443–454
Hu LB, Shi ZQ, Zhang T, Yang ZM (2007) Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC38932. FEMS Microbiol Lett 272:91–98
Jing L, Yang Q, Zhao L-H, Zhang S-M, Wang Y-X, Zhao X-Y (2009) Purification and characterization of a novel antifungal protein from Bacillus subtilis strain B29. J Zhejiang Univ Sci B 10:264–272
Kim PI, Bai H, Chae H, Chung S, Kim Y, Park Y, Chi Y-T (2004) Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J Appl Microbiol 97:942–949
Kita N, Ohya T, Uekusa H, Nomura K, Manago M, Shoda M (2005) Biological control of damping-off of tomato seedlings and cucumber Phomopsis root rot by Bacillus subtilis RB14-C. Jpn Agric Res Q 39:109–114
Leclère V, Béchet M, Adam A, Guez J-S, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584
Leelasuphakul W, Hemmanee P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Technol 48:113–121
Li L, Ma MC, Huang R, Qu Q, Li GH, Zhou JW, Zhang KQ, Lu KP, Niu XM, Luo J (2012) Induction of chlamydospore formation in Fusarium by cyclic lipopeptide antibiotics from Bacillus subtilis C2. J Chem Ecol 38:966–974
Lin HF, Chen TH, Liu SD (2010) Bioactivity of antifungal substance iturin A produced by Bacillus subtilis strain BS-99-H against Pestalotiopsis eugeniae, a causal pathogen of wax apple fruit rot. Plant Pathol Bull 19:225–233
Matar SM, El-Kazzaz SA, Wagih EE, Al-Diwany AI, Moustafa HE, Abo-Zaid GA, Abd-Elsalam HE, Hafez EE (2009) Antagonistic and inhibitory effect of Bacillus subtilis against certain plant pathogenic fungi, I. Biotechnology 8:53–61
Mnif I, Sahnoun R, Ellouze-Chaabouni S, Ghribi D (2013) Evaluation of B. subtilis SPB1 biosurfactants’ potency for diesel-contaminated soil washing: optimization of oil desorption using Taguchi design. Environ Sci Pollut Res. doi:10.1007/s11356-013-1894-4
Montealegre JR, Errera R, Velásquez JC, Silva P, Besoaín X, Pérez LM (2005) Biocontrol of root and crown rot in tomatoes under greenhouse conditions using Trichoderma harzianum and Paenibacillus lentimorbus. Additional effect of solarization. Electron J Biotechnol 8:250–257
Muyolo NG, Lipps PE, Schmitthenner AF (1993) Reactions of dry bean, lima bean, and soybean cultivars to Rhizoctonia root and hypocotyl rot and web blight. Plant Dis 77:234–238
Nihorimbere V, Ongena M, Cawoy H, Brostaux Y, Kakana P, Jourdan E, Thonart P (2010) Beneficial effects of Bacillus subtilis on field-grown tomato in Burundi: reduction of local Fusarium disease and growth promotion. Afr J Microbiol Res 4:1135–1142
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Oxenham SK, Svoboda KP, Walters DR (2005) Antifungal activity of the essential oil of basil (Ocimum basilicum). J Phytopathol 153:174–180
Rebib H, Hedi A, Rousset M, Boudabous A, Limam F, Sadfi-Zouaoui N (2012) Biological control of Fusarium foot rot of wheat using fengycin-producing Bacillus subtilis isolated from salty soil. Afr J Biotechnol 11:8464–8475
Risoen PA, Ronning P, Hegna IK, Kolsto AB (2004) Characterization of a broad range antimicrobial substance from Bacillus cereus. J Appl Microbiol 96:648–655
Ruangwong OU, Chang C-I, Lamine SA, Liang W-J (2012) Identification of antifungal compound produced by Bacillus subtilisLB5 with ability to control anthracnose disease caused by Colletotrichum gloeosporioides. Afr J Microbiol Res 6:3732–3738
Senthilkumar M, Swarnalakshmi K, Govindasamy V, Lee YK, Annapurna K (2009) Biocontrol potential of soybean bacterial endophytes against charcoal rot fungus, Rhizoctonia bataticola. Curr Microbiol 58:288–293
Soothill JS, Ward R, Girling AJ (1992) The IC50: an exactly defined measure of antibiotic sensitivity. J Antimicrob Chemother 29:137–139
Soylu EM, Kurt S, Soylu S (2010) In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int J Food Microbiol 143:183–189
Tawara S, Ikeda F, Maki K, Morishita Y, Otomo K, Teratani N, Goto T, Tomishima M, Ohki H, Yamada A, Kawabata K, Takasugi H, Sakane K, Tanaka H, Matsumoto F, Kuwahara S (2000) In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother 44:57–62
Triki MA, Hammami I, KridHadj-Taieb S, Daami-Remadi M, Mseddi A, El Mahjoub M, Gdoura R, Khammasy N (2012) Biological control of atypical pink rot disease of potato in Tunisia. Glob Sci Books Pest Tech 6:60–64
Wang J, Xia XM, Wang HY, Li PP, Wang KY (2013) Inhibitory effect of lactoferrin against gray mould on tomato plants caused by Botrytis cinerea and possible mechanisms of action. Int J Food Microbiol 161:151–157
Yangui T, Sayadi S, Dhouib A (2013) Sensitivity of Pectobacteriu mcarotovorum to hydroxytyrosol-rich extracts and their effect on the development of soft rot in potato tubers during storage. Crop Prot 53:52–57
Yun-feng Y, Qi-qin L, Gang F, Gao-qing Y, Jian-hua M, Wei L (2012) Identification of antifungal substance (Iturin A2) produced by Bacillus subtilis B47 and its effect on southern corn leaf blight. J Integ Agric 11:90–99
Zhang Y-L, Li S, Jiang D-H, Kong L-C, Zhang P-H, Xu J-D (2013) Antifungal activities of metabolites produced by a termite associated Streptomyces canus BYB02. J Agric Food Chem 61:1521–1524
Zu W, Yu H, Liang L, Fu Y, Efferth T, Liu X, Wu N (2010) Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 15:3200–3210
Acknowledgments
This work has been supported by grants from the Tunisian Ministry of Higher Education, Scientific Research and Technology and the Tunisian Ministry of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Mnif, I., Hammami, I., Triki, M.A. et al. Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani . Environ Sci Pollut Res 22, 18137–18147 (2015). https://doi.org/10.1007/s11356-015-5005-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5005-6


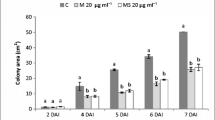








 ); inoculated control (
); inoculated control ( ); preventive treatment by SPB1 lipopeptide (
); preventive treatment by SPB1 lipopeptide ( ); preventive treatment by hymexazol (
); preventive treatment by hymexazol ( ); curative treatment by SPB1 lipopeptide (
); curative treatment by SPB1 lipopeptide ( ); curative treatment by hymexazol (
); curative treatment by hymexazol ( )
)