Abstract
Microorganisms have become more resistant to pesticides, which increases their ability to invade and infect crops resulting in decreased crop productivity. The rhizosphere plays a crucial role in protecting plants from harmful invaders. The purpose of the study was to investigate the antagonistic efficiency of indigenous rhizospheric fungal isolates against phytopathogens of M. uniflorum plants so that they could be further used as potent Biocontrol agents. Thirty rhizospheric fungal isolates were collected from the roots of the Macrotyloma uniflorum plant and initially described morphologically for the present study. Further, in vitro tests were conducted to evaluate the antifungal activity of these strains against four myco-phytopathogens namely Macrophamina phaseolina, Phomopsis sp. PhSFX-1, Nigrospora oryzae, and Boeremia exigua. These pathogens are known to infect the same crop plant, M. uniflorum, and cause declines in crop productivity. Fifteen fungal strains out of the thirty fungal isolates showed some partial antagonistic activity against the myco-phytopathogens. The potent fungal isolates were further identified using molecular techniques, specifically based on the internal transcribed spacer (ITS) region sequencing. Penicillium mallochii, Cladosporium pseudocladosporioides, Aspergillus chevalieri, Epicoccum nigrum, Metarhizium anisopliae, and Mucor irregularis were among the strains that were identified. These potent fungal strains showed effective antagonistic activity against harmful phytopathogens. Current findings suggest that these strains may be taken into consideration as synthetic fungicides which are frequently employed to manage plant diseases alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Horse gram (Macrotyloma uniflorum) is an underutilized and unexplored crop plant belonging to Leguminaceae family. This crop is reported to be resistant to many abiotic stresses like heavy metal stress and drought stress. This crop is used to treat kidney stones, edema, menstrual pains, piles, renal stones, healing wounds, and many more medicinal purposes (Rawat et al. 2023a, 2023b). Microorganisms are present in almost every habitat of our planet including extreme hot and cold environments. They are responsible for various functions inside the living body and also help in recycling minerals as decomposers. They live together with mixed communities and form a complex microenvironment termed a microbiome (Li et al. 2021). The microbiome includes different microbial communities coexisting in a particular environment. The rhizosphere microbiome is responsible for plant growth and health and somehow the microbes residing around the roots of plants help them to grow properly by avoiding the invasion of harmful pathogens inside plant roots either by secreting some chemicals, siderophores, etc. (Rawat et al. 2020). A plant’s rhizosphere, or root system, affects its resistivity because it draws in beneficial bacteria and drives off undesirable ones. The myco-phytopathogens, like Macrophamina phaseolina, Nigrospora oryzae, Boeremia exigua, Phomopsis sp. PhSFX-1, are the most common phytopathogens infecting not only this legume plant but also commercially important crop plants like tomato, pepper, chickpea, mungbean, maize, and rice (Wang et al. 2017; Udayanga et al. 2011; Lan and Duan 2022). These pathogens affect the quality and quantity of crops resulting in a decline in production (Banaras et al. 2020; Khan and Javaid 2020). Synthetic pesticides are used for attaining a high yield of production but they come with greater risk to human health and the environment.
Fungi are the second largest group after insects and the key component of tropical ecosystems throughout the world and intimately associated with crucial processes like the decomposition, recycling, and transportation of nutrients in different environments (Chander 2016; Hawksworth and Lücking 2017; Wu et al. 2019). Fungi are one of the most diverse groups of Eukarya and represent an important functional component of the soil microbial communities (Tan et al. 2017), which constitute more of the soil biomass than bacteria, depending on soil depth and nutritional conditions (Paulina et al. 2016). Soil fungi are known to play an important role in decomposition via soil nutrient recycling and accumulation of soil organic matter and in plant health and development (Bridge and Spooner 2001; Martin et al. 2000). Various studies reported the following plant growth-promoting fungi genera Gliocladium, Penicillium, Aspergillus, Phoma, Phytophthora, Rhizoctonia, Talaromyces, Trichoderma are used to improve tomato, orange, apple, pear, cucumber, carrot, and other plants’ growth and further promote the plants’ innate immunity and the production of various necessary secondary metabolites by the plants (Khan et al. 2021, Khan and Javaid 2022a, 2022b; Rawat et al. 2023a, 2023b; Attia et al. 2022; Kuzin et al. 2020; Cantabella et al. 2020). Plant growth-promoting fungi (PGPF) perform the following functions in plants: antagonistic or biocontrol potential by competing for space and nutrients, growth hormone production (Akinola and Babalola 2021), mineral solubilization, mycoparasitic and saprophytic resistance, root colonization, and induced systemic resistance (ISR) in plants (Shasmita et al. 2022). Aside from the above roles mentioned, PGPF suppress the invasion of phytopathogens on tomato plants as well as other crop plants, they contribute to the improvement of nutrients in the soil, and they produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase and other phytohormones to reduce the production of ethylene in the plants (Adedayo et al. 2022). The most common fungi used as potent antagonist are genus Trichoderma but it also has some limitations. The present study is designed to isolate, characterize and identify the useful rhizosphere fungi and to test their antagonist potential against harmful pathogens and their effects on plant growth promotion. This will help in exploring more fungi against soil-borne pathogens and biopesticide development. Further, the active metabolites of potent fungi could be identified through metabolomic studies for biological activities like anti-fungal and anti-cancer.
Materials and methods
Isolation of myco-phytopathogens
The M. uniflorum plants were planted and collected in Bhimtal town of Nainital, Uttarakhand, India, during the 2019–2020 growing season, from September to November. The stem, pod, and leaf samples of M. uniflorum plants expressing symptoms of rot disease were collected in sterile bags. The samples were surface sterilized using 0.1% sodium hypochlorite for 3 min followed by three consecutive washings with sterilized distilled water (SDW). The samples were chopped into small pieces and inoculated in Potato Dextrose Agar media (PDA) for fungus isolation. All the PDA Plates were incubated at 28 ± 2 °C for 7 days.
Pathogenicity assay
Koch’s Postulates method was used for pathogenicity assay (Ross and Woodward 2016). The test was conducted on healthy plants at their early stage by injecting fungal spore suspension. The soil used for seed sowing was autoclaved thrice to kill native microorganisms. The pots were placed inside the greenhouse under controlled environment at 25 ± 2 °C up to 20 days.
Isolation of rhizosphere fungal strains
The M. uniflorum seeds of resistant varieties VG-8 and VG-19 were obtained from ICAR-Vivekananda Parvatiya Krishi Anusandhan Sansthan, Almora, Uttarakhand (33.5651° N, 73.0169° E) known to be resistant for many fungal rot diseases including Anthracnose. Rhizospheric soil samples along with healthy roots were collected from the M. uniflorum plants in mid-period and 10 days before harvesting plants.
Antagonistic assay against myco-phytopathogens
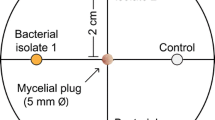
Antagonism assay was performed to check the potential of rhizospheric fungal strains in mycelial growth inhibition against Macrophamina phaseolina, Phomopsis sp. PhSFX-1, Nigrospora oryzae, and Boeremia exigua in vitro. A seven-day-old culture on potato dextrose agar (PDA) was used in this experiment. Briefly, a 5 mm Rhizospheric fungal mycelial disc was kept on one side of PDA plates and pathogenic cultures were kept on the other side of the fungal plugs at a 2 cm distance. Controls consisted of single cultures of the tested pathogen strains. Fungal antagonism was tested in triplicate and plates were incubated at 25 ± 2 °C for about 7 days. The antagonistic potential was evaluated as inhibition of the mycelial radial growth of pathogens against each Rhizospheric fungal strain, where R and r are the radii of fungal mycelial growth in control and treatment, respectively.
Calculation
The antagonistic index was accessed according to the following formula:
Antagonistic Index:
RM: radius of the pathogen in the control plate.
rm: radius of the pathogen in the dual culture plate.
Characterization of myco-phytopathogens and rhizosphere fungal strains
Microscopic analysis
Initially, the pathogens and Rhizosphere fungal strains were identified based on morphological characteristics. The shape, color, and texture of fungal isolate were observed visually on PDA plates. The sporangial shape and size of each isolate were observed at 200 × magnification under a compound microscope. Further, the pathogenic strains confirmed in pathogenicity assays and potent antagonist Rhizosphere fungal strains were then subjected to molecular-based identification.
Molecular characterization
The identification of isolates was carried out at the sequencing facility of the National Centre for Microbial Resource (NCMR), National Centre for Cell Science, Pune. Genomic DNA was isolated by the standard phenol/chloroform extraction method, followed by PCR amplification of the ITS regions using universal primers ITS1 [5′-TCC GTA GGT GAA CCT GCG G -3′] and ITS4 [5′-TCC TCC GCT TAT TGA TAT GC-3′]. The amplified ITS PCR product was purified by PEG-NaCl precipitation and directly sequenced on an ABI® 3730XL automated DNA sequencer (Applied Biosystems, Inc., Foster City, CA) as per the manufacturer’s instructions. Essentially, sequencing was carried out from both ends so that each position was read at least twice. Assembly was carried out using Lasergene package followed by NCBI BLAST against sequences from type material for tentative identification (Boratyn et al. 2013). All the retrieved and tested sequences were aligned using the ClustalW program and subjected to phylogenetic analysis. The phylogenetic tree was constructed using the neighbor-joining (NJ) method in MEGA-X version 10.1.7 with 1000 bootstrap replications and the evolutionary distances were calculated by using the Jukes–Cantor model.
Results and discussion
The present work was carried out on isolated rhizospheric fungal strains and their antagonistic effects on phytopathogens. The rhizosphere fungal isolates were Penicillium mallochii, Penicillium sp. FKI-4429, Cladosporium pseudocladosporioides, Metarhizium anisopliae, Fusarium tricinctum, Cladosporium sp. MBC003, Penicillium citrinum, Aspergillus chevalieri, Mucor irregularis, Aspergillus versicolor, Epicoccum nigrum, Aspergillus udagawae, and Schizophyllum commune. They were tested against the phytopathogens Macrophomina phaseolina, Nigrospora oryzae, Boeremia exigua, and Phomopsis sp. PhSFX-1. These were identified according to their cultural, morphological, microscopical, and genetic characteristics. This study has provided useful information about the pathogenic fungi associated with Macrotyloma uniflorum plant parts which may affect the plant health, agricultural production, and also economic loss. Also, the antagonistic activity of potent fungi isolated from the rhizosphere region of Macrotyloma uniflorum plant against the phytopathogens.
Isolation and pathogenicity assay of myco-phytopathogens
After 15 days of incubation, out of 10 strains, 4 fungal strains were observed repeatedly which were named as PP 101, PP 102, PP 103, and PP 104 and further preceded.
Symptoms of rot diseases in the field were black, brown, and grey-colored rings. The infected leaves were initially light spotted. Later, they were brownish and slightly wrinkled to wither and eventually die. The dark patches were present on the infected stems and they dried eventually. The seed pods were also infected and seeds were less in numbers in the infected pods as compared to the healthy ones. On Potato Dextrose Agar media, the morphology of all the isolates was different. Purely isolated cultures of IsolatePP101 have macroscopic features of grey colonies, filamentous resembling cotton, and spreading growths and forming sclerotia. The results of microscopic observations showed that isolatePP101 had elliptical-shaped spores, and branched and aseptate hyphae. IsolatePP102 has black colonies, filamentous like cotton, elliptical spores, and branched and septate hyphae. IsolatePP103 has black colonies with powdery and thread-like structures forming sclerotia and septate hyphae, and isolatePP104 has white-colored colonies with cottony wavy texture, spherical-shaped spores, filamentous, and septate hyphae.
All the plants that were injected with isolates for pathogenic test were infected with rot diseases (Fig. 1). The pathogens recovered from the experimented plants were morphologically similar to the isolates. This experiment confirmed the pathogenicity of the isolated strains. The symptoms of the rot disease with black and brown spots were observed on leaves, pods, and stem part of Macrotyloma uniflorum plant.
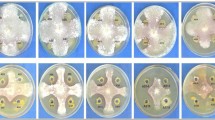
Isolation and antagonistic assay of rhizosphere fungal strains against myco-phytopathogens
A total of thirty rhizosphere fungal isolates were recovered among which fifteen strains showed potent antagonistic activity against the myco-phytopathogens (Fig. 2). The results of the antagonist test obtained fifteen isolates that were able to inhibit the isolated pathogens PP101, PP102, PP103, and PP104 (Table 1).
Calculation
Antagonistic Index %:
RM: radius of the pathogen in the control plate.
rm: radius of the pathogen in the dual culture plate.
The potent antagonistic fungal strains were PA, PB, PC, PD, PF, PG, PH, PL, PM, PN, PR, PT, PV, and PX which later on were identified as Penicillium mallochii, Penicillium sp. FKI-4429, Cladosporium pseudocladosporioides, Metarhizium anisopliae, Fusarium tricinctum, Cladosporium sp. MBC003, Penicillium citrinum, Aspergillus chevalieri, Mucor irregularis, Aspergillus versicolor, Epicoccum nigrum, Aspergillus udagawae, and Schizophyllum commune. The rest of the fungus did not show antagonistic activity against the phytopathogens. Among these fungal isolates for Macrophomina phaseolina pathogen, isolatePM (55%) showed maximum fungal mycelial growth inhibition while isolatePO (20%) showed the least inhibition similarly for Nigrospora oryzae IsolatePQ (66%) showed maximum growth inhibition while IsolatePC (23%) the least inhibition; for Boeremia exigua IsolatePF (60%) showed maximum growth inhibition while IsolatePD (20%) showed the least inhibition, and for Phomopsis sp. PhSFX-1 IsolatePH (60%) showed maximum growth inhibition while IsolatePG (23%) showed the least inhibition as shown in Table 1. Isolates that showed fungal mycelial growth inhibition of more than 40% against isolated phytopathogens were further selected for molecular identification. The natural capacity to suppress pathogens has been studied in many disease-suppressive soils against the oomycetes and fungi Pythium ultimum, Pythium irregulare, Pythium aphanidermatum, Phytophthora nicotianae, Phytophthora capsici, Phytophthora cinnamomi, Rhizoctonia solani, and Fusarium oxysporum. The understanding of disease suppressive mechanisms is a crucial step to enhance the suppressive effect by manipulation of the soil microbiota. More specifically, the suppressive properties can be explained through combined antimicrobial actions exerted by molecules and microbes or mechanisms of antagonism among microbes and pathogens. The biological factors based on disease suppression generally include a combination of different actions. The mechanisms underlying the suppressive effect are primarily associated with the biological activity of soil microbiota which interacts with the soil organic matter (SOM) as well as the host plant. The most important factors are represented by the increased microbial activity (Melero et al. 2006) and fungistasis (Bonanomi et al. 2017), enhanced soil structure (Bronick and Lal 2005), release of mineral nutrients during SOM decomposition (Berry et al. 2002), activation of competition for space and nutrients (Noble and Coventry 2005), elicitation of microbiostasis and hyperparasitism, release of diffusible antibiotic-like compounds (Weller et al. 2002), and activation of systemic disease-resistance in the host plant (Bulluck Iii et al. 2002). A direct inhibition on conidial germination and mycelium growth of plant pathogens induced by Bacillus, Pseudomonas, Streptomyces, Trichoderma, and Penicillium has been documented against phytopathogenic fungi using compost water extract (El-Masry et al. 2002; McQuilken et al. 1994). Suppression of Fusarium melonis in wilt-suppressive soils and composted green wastes-amended soils has been associated with populations of Aspergillus, Streptomyces, and fluorescent Pseudomonas (Cha et al. 2016; Suàrez-Estrella et al. 2007). Sewage sludge compost suppresses F. oxysporum sp. melonis wilt on tomato if combined with selected Trichoderma asperellum isolates (Cotxarrera et al. 2002). Other authors instead concluded that species of Penicillium can act as top BCAs against Fusarium oxysporum sp. lycopersici of tomato (Hussain et al. 2016).
Based on the analysis of the variety of testing of potential antagonist isolates against the fungus Macrophomina phaseolina, Nigrospora oryzae, Phomopsis sp. PhSFX-1, and Boeremia exigua on 7 days after inoculation showed that the inoculation of the potential antagonist isolates had a significant effect on the percentage of incidence of phytopathogens fungal disease in vitro. The following is presented about the average percentage of incidence of pathogenic fungal disease in vitro in Table 1.
Microscopic and molecular characterization of myco-phytopathogens
The fungi isolated were examined morphologically, and their spores and thallus structure were analyzed under the compound microscope (Figs. 3, 4, 5, 6). The detailed characteristic features of myco-phytopathogens are shown in Table 2.
The selected phytopathogens were sent for molecular identification based on ITS sequencing. Pathogenic fungi PP101, PP102, PP103, and PP104 were closely related to Nigrospora oryzae, Boeremia exigua, Macrophamina phaseolina, and Phomopsis sp. PhSFX-1 and showed 100% identity similarity with accessions KX219801.1, MH550515.1, HQ392782.1, and MH371253.1. These sequences are submitted to the Genbank nucleotide database with accession numbers obtained as OK244648.1, ON791481, OK244650.1, and OK244651.1, respectively, as shown in Table 2.
The evolutionary relationship of all the sequences was determined by constructing a phylogenetic tree (Fig. 7).
Phylogenetic tree based on neighbor-joining analysis of the rDNA ITS sequences of the pathogenic fungal isolates obtained from various tissues of the M. uniflorum plant. The pathogenic fungal isolates along with their obtained accession numbers are highlighted. For the closely related species, the taxonomic names are written with their respective accession number. Significant bootstrap values (> 50%) are indicated at the branching points
These fungi are reported as highly pathogenic strains causing huge harm to the crops. There are many reports on the pathogenicity of Nigrospora sp. (Jia et al. 2024; Raza et al. 2010; Wright et al. 2008; Zhao et al. 2014; Dutta et al. 2015), Boeremia exigua (Kadir and Umaerus 1987; Gorny et al. 2015; Michel et al. 2018; Grinbergs and France 2014; Michel et al. 2018; Gao et al. 2019; Banerjee and Panja 2020), Macrophomina phaseolina (Basandrai et al. 2021; de Sousa Linhares et al. 2020; Marquez et al. 2021; Teja et al. 2020; Lodha and Mawar 2020), and Phomopsis sp. PhSFX-1 (Chaisiri et al. 2020; Anwar et al. 2017; Asad et al. 2015; Correia et al. 2017; Brumer et al. 2018) on the stem, fruit, leaf, and root parts of wheat, potato, lima bean, sugarcane, rice, legumes, sesame, tea, etc. These are very aggressive types of fungal phytopathogens infecting plant health, yield, and growth promotion. The genus of Nigrospora is a widely distributed fungus, which can exist as an endophyte and plays a role as a pathogen to affect plant health (Wang et al. 2017; Ebada et al. 2016). The pathogenic fungus, Boeremia exigua, infects many other plants as their hosts. For example, 11 varieties of B. exigua reportedly infect 45 plant species belonging to 31 genera and 19 families (Berner et al. 2015). Macrophomina phaseolina is a soil-borne fungal pathogen that incites charcoal rot in more than 500 plant species (Marquez et al. (2021). Diaporthe/Phomopsis species are widely distributed around the world; they are pathogens of many important crops and can grow as parasites on humans and animals also (Van Warmelo et al. 1970; Udayanga et al. 2011; Gomes et al. 2013). Pathogenic Diaporthe species can grow in plant tissue without causing clearly visible symptoms for a long time. But later, they do kill the host tissue so they should be categorized as hemibiotrophs (Udayanga et al. 2011).
Microscopic and morphological characterization of rhizospheric fungi
The rhizospheric isolates are given in Fig. 8.
Molecular characterization of selected potent antagonist rhizospheric fungi
Among all the tested fungal strains, 15 strains showed some sort of antagonism against the phytopathogens in dual culture assay on PDA media. The fungal isolates that showed partial antagonistic activity against the phytopathogens were sent for molecular identification based on ITS sequencing. The sequences are submitted to the Genbank Nucleotide database with accession numbers given in Table 3. Phylogenetic trees constructed from 16S rRNA sequences (Fig. 9). The phylogenetic tree indicated that isolatePA, PB, PC, PD, PF, PG, PH, PL, PM, PN, PR, PT, PV, and PX were closely related to Penicillium mallochii, Penicillium sp. FKI-4429, Cladosporium pseudocladosporioides, Metarhizium anisopliae, Fusarium tricinctum, Cladosporium sp. MBC003, Penicillium citrinum, Aspergillus chevalieri, Mucor irregularis, Aspergillus versicolor, Penicillium citrinum, Epicoccum nigrum, Aspergillus udagawae, and Schizophyllum commune and showed 99–100% identity with accessions MN944416.1, AB548364.1, MT582794.1, MN710409.1, MN594466.1, JQ885448.1, MG748682.1, MT316337.1, MZ423089.1, MN547369.1, MT558921.1, MT166336.1, MN882827.1, and MK647986.1, respectively (Table 3).
Phylogenetic tree based on neighbor-joining analysis of the rDNA ITS sequences of the rhizospheric fungal isolates obtained from rhizosphere soil of M. uniflorum plant. The rhizospheric fungal codes along with their accession numbers obtained are highlighted. For the closely related species, the taxonomic names are written with their respective accession number. Significant bootstrap values (> 50%) are indicated at the branching points
Conclusion
The present investigation concludes that out of thirty fungal strains, fifteen strains efficiently suppressed the mycelial growth of pathogenic Macrophomina phaseolina, Nigrospora oryzae, Boeremia exigua, and Phomopsis sp. PhSFX-1 fungi in direct interactions-assays in vitro. The ITS sequence analysis of rhizospheric fungal strains showed 98 to 100% identity with close relatives belonging to Penicillium mallochii, Penicillium sp. FKI-4429, Cladosporium pseudocladosporioides, Metarhizium anisopliae, Fusarium tricinctum, Cladosporium sp. MBC003, Penicillium citrinum, Aspergillus chevalieri, Mucor irregularis, Aspergillus versicolor, Epicoccum nigrum, Aspergillus udagawae, and Schizophyllum commune. These fungi have antagonistic potential against pathogenic fungi. These fungal isolates should further be analyzed for metabolomics study to study their active constituents. These results confirmed the significant role of native rhizospheric fungi for the control of soil-borne fungal pathogens and the potential use of identified isolates in bio-fertilizers and bio-fungicides development.
Data availability
No datasets were generated or analysed during the current study.
References
Adedayo AA, Babalola OO, Prigent-Combaret C, Cruz C, Stefan M, Kutu F, Glick BR (2022) The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: a review. PeerJ 10:e13405
Akinola SA, Babalola OO (2021) The fungal and archaeal community within plant rhizosphere: a review on their contribution to crop safety. J Plant Nutr 44(4):600–618
Anwar A, Bhat M, Ganaie N, Ambardar VK, Hassan MG (2017) Prevalence and management through relative performance of organic mulches and fungi toxicants of noxious Phomopsis fruit rot (Phomopsis vexans, Sacc. & Syd.) Harter, in brinjal ecology of Kashmir. Innov Pharm J 6:318–323
Asad HA, Meah MB, Begum SN, Khalil MI, Rafii MY, Latif MA (2015) Study of genetic variation of eggplant cultivars by using RAPD-PCR molecular markers and the relationship with Phomopsis blight disease reaction. Genet Mol Res 14(4):17007–17018
Attia MS, Abdelaziz AM, Al-Askar AA, Arishi AA, Abdelhakim AM, Hashem AH (2022) Plant growth-promoting fungi as biocontrol tool against fusarium wilt disease of tomato plant. J Fungi 8(8):775
Banaras S, Javaid A, Shoaib A (2020) Non-chemical control of charcoal rot of urdbean by Sonchus oleraceous application. Planta Daninha 38:e020216088
Banerjee A, Panja B (2020) First report of Boeremia exigua var. exigua as a pathogen of Cycas circinalis in India. J Plant Pathol 102:935–936
Basandrai AK, Pandey AK, Somta P, Basandrai D (2021) Macrophomina phaseolina–host interface: insights into an emerging dry root rot pathogen of mungbean and urdbean, and its mitigation strategies. Plant Pathol 70(6):1263–1275
Berner D, Cavin C, Woudenberg JH, Tunali B, Büyük O, Kansu B (2015) Assessment of Boeremia exigua var. rhapontica, as a biological control agent of Russian knapweed (Rhaponticum repens). Biol Control 81:65–75
Berry PM, Sylvester-Bradley R, Philipps L, Hatch DJ, Cuttle SP, Rayns FW, Gosling P (2002) Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag 18:248–255
Bonanomi G, Gaglione SA, Cesarano G, Sarker TC, Pascale M, Scala F, Zoina A (2017) Frequent applications of organic matter to agricultural soil increase fungistasis. Pedosphere 27(1):86–95
Boratyn, G. M., Camacho, C., Cooper, P. S., Coulouris, G., Fong, A., Ma, N., ... & Zaretskaya, I. (2013). BLAST: a more efficient report with usability improvements. Nucleic acids research, 41(W1), W29-W33
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232:147–154
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124(1–2):3–22
Brumer, B. B., Lopes-Caitar, V. S., Chicowski, A. S., Beloti, J. D., Castanho, F. M., Gregório da Silva, D. C., ... & Marcelino-Guimarães, F. C. (2018). Morphological and molecular characterization of Diaporthe (anamorph Phomopsis) complex and pathogenicity of Diaporthe aspalathi isolates causing stem canker in soybean. Eur J Plant Pathol, 151:1009–1025
Bulluck Iii LR, Brosius M, Evanylo GK, Ristaino JB (2002) Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl Soil Ecol 19(2):147–160
Cantabella D, Dolcet-Sanjuan R, Casanovas M, Solsona C, Torres R, Teixidó N (2020) Inoculation of in vitro cultures with rhizosphere microorganisms improve plant development and acclimatization during immature embryo rescue in nectarine and pear breeding programs. Sci Hortic 273:109643
Cha, J. Y., Han, S., Hong, H. J., Cho, H., Kim, D., Kwon, Y., ... & Kwak, Y. S. (2016). Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J, 10(1):119–129
Chaisiri C, Liu XY, Lin Y, Li JB, Xiong B, Luo CX (2020) Phylogenetic analysis and development of molecular tool for detection of Diaporthe citri causing melanose disease of citrus. Plants 9(3):329
Chander H (2016) Diversity and distribution of macrofungi and lichens in the Nanda Devi Biosphere Reserve. Biological Diversity and Ecology. Discovery Publishing House, New Delhi, pp 184–207
Correia KC, de Queiroz JVJ, Martins RB, Nicoli A, Del Ponte EM, Michereff SJ (2017) Development and evaluation of a standard area diagram set for the severity of phomopsis leaf blight on eggplant. Eur J Plant Pathol 149:269–276
Cotxarrera L, Trillas-Gay MI, Steinberg C, Alabouvette C (2002) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biol Biochem 34(4):467–476
Dutta J, Gupta S, Thakur D, Handique PJ (2015) First report of Nigrospora leaf blight on tea caused by Nigrospora sphaerica in India. Plant Dis 99(3):417–417
Ebada SS, Eze P, Okoye FB, Esimone CO, Proksch P (2016) The fungal endophyte Nigrospora oryzae produces quercetin monoglycosides previously known only from plants. ChemistrySelect 1(11):2767–2771
El-Masry MH, Khalil AI, Hassouna MS, Ibrahim HAH (2002) In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World J Microbiol Biotechnol 18:551–558
Gao, P., Nan, Z. B., Christensen, M. J., Barbetti, M. J., Duan, T. Y., Liu, Q. T., ... & Huang, J. F. (2019). Factors influencing rust (Melampsora apocyni) intensity on cultivated and wild Apocynum venetum in Altay Prefecture, China. Phytopathology, 109(4):593–606
Gomes RR, Glienke C, Videira SIR, Lombard L, Groenewald JZ, Crous PW (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31(1):1–41
Gorny AM, Kikkert JR, Dunn AR, Dillard HR, Smart CD, Pethybridge SJ (2015) Tan spot of lima bean caused by Boeremia exigua var. exigua in New York State, USA. Can J Plant Pathol 37(4):523–528
Grinbergs, D. E., & France, R. A. (2014, November). Black root rot of industrial chicory (Cichorium intybus L. var. sativum) in Chile caused by Boeremia exigua var. exigua. In Phytopathology 104(11):47–47. 3340 PILOT KNOB ROAD, ST PAUL, MN 55121 USA: AMER PHYTOPATHOLOGICAL SOC
Hawksworth DL, Lücking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectr 5(4):10–1128
Hussain I, Alam SS, Khan I, Shah B, Naeem A, Khan N, ... & Iqbal, M (2016) Study on the biological control of fusarium wilt of tomato. J EntomolZool Studies 4(2):525-8
Jia W, Luo M, Wei T, Zhang H, Zeng Y, Jiang Y (2024) Nigrospora musae and N. oryzae as new causal agents of broad bean leaf spot disease in China. Crop Prot 177:106567
Kadir S, Umaerus V (1987) Varietal differences to infection of potato stems by the gangrene pathogen Phoma Exigua Var Foveata. Potato Res 30:1–8
Khan IH, Javaid A (2020) Comparative antifungal potential of stem extracts of four quinoa varieties against Macrophomina phaseolina. Int J Agric Biol 24(3):441–446
Khan IH, Javaid A (2022a) DNA cleavage of the fungal pathogen and production of antifungal compounds are the possible mechanisms of action of biocontrol agent Penicillium italicum against Macrophomina phaseolina. Mycologia 114(1):24–34
Khan IH, Javaid A (2022b) Antagonistic activity of Aspergillus versicolor against Macrophomina phaseolina. Braz J Microbiol 53(3):1613–1621
Khan IH, Javaid A, Ahmed D (2021) Trichoderma viride controls Macrophomina phaseolina through its DNA disintegration and production of antifungal compounds. Int J Agric Biol 25(4):888–894
Kuzin A, Solovchenko A, Stepantsova L (2020) Soil fertility management in apple orchard with microbial biofertilizers. E3S Web of Conferences (Vol. 222, p. 03020). EDP Sciences
Lan Y, Duan T (2022) Characterization of Boeremia exigua causing stem necrotic lesions on Luobuma in northwest China. Sci Rep 12(1):21609
Li J, Wang C, Liang W, Liu S (2021) Rhizosphere microbiome: the emerging barrier in plant-pathogen interactions. Front Microbiol 12:772420
Lodha S, Mawar R (2020) Population dynamics of Macrophomina phaseolina in relation to disease management: a review. J Phytopathol 168(1):1–17
Marquez N, Giachero ML, Declerck S, Ducasse DA (2021) Macrophomina phaseolina: general characteristics of pathogenicity and methods of control. Front Plant Sci 12:634397
Martin, F. M., Perotto, S., & Bonfante, P. (2000). Mycorrhizal fungi: a fungal community at the interface between soil and roots. In The rhizosphere (pp. 279–312). CRC Press
McQuilken MP, Whipps JM, Lynch JM (1994) Effects of water extracts of a composted manure-straw mixture on the plant pathogen Botrytis cinerea. World J Microbiol Biotechnol 10:20–26
Melero S, Porras JCR, Herencia JF, Madejon E (2006) Chemical and biochemical properties in a silty loam soil under conventional and organic management. Soil and Tillage Research 90(1–2):162–170
Michel VV, Daepp M, Woudenberg JH, de Gruyter J, de Wit PJ (2018) First report of Boeremia exigua var. exigua causing stem and leaf spot on common speedwell in Switzerland. Plant Disease 102(2):440–440
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Tech 15(1):3–20
Paulina A, Fatima M, Sagaya GR (2016) Studies on soil mycoflora in different tomato fields of four districts in Tamil Nadu, India. Int J Curr Microbiol App Sci 5(7):92–99
Rawat J, Sanwal P, Saxena J, Prasad R (2023a) Exploring the biochar as a suitable carrier for a bioinoculant Aspergillus niger K7 and its consequence on Eleusine coracana in field studies. Journal of Agriculture and Food Research 14:100825
Rawat J, Saxena J, Sanwal P, Maddela NR, Nain L, Prasad R (2023b) Improving the growth and productivity of Macrotyloma uniflorum medicinal plant by the co-inoculation of P, Zn and K-solubilizing fungi under field conditions. Curr Microbiol 80(9):277
Rawat J, Yadav N, Pande V (2020) Role of rhizospheric microbial diversity in plant growth promotion in maintaining the sustainable agrosystem at high altitude regions. Recent Advancements in Microbial Diversity. Academic Press, pp 147–196
Raza G, Ali K, Mukhtar Z, Mansoor S, Arshad M, Asad S (2010) The response of sugarcane (Saccharum officinarum L) genotypes to callus induction, regeneration and different concentrations of the selective agent (geneticin-418). Afr J Biotech 9(51):8739–8747
Ross LN, Woodward JF (2016) Koch’s postulates: an interventionist perspective. Stud Hist Philos Sci A 59:35–46
Shasmita, Swain BB, Mohapatra PK, Naik SK, Mukherjee AK (2022) Biopriming for induction of disease resistance against pathogens in rice. Planta 255(6):113
de Sousa Linhares CM, Ambrósio MMQ, Castro G, Torres SB, Esteras C, de Sousa Nunes GH, Picó B (2020) Effect of temperature on disease severity of charcoal rot of melons caused by Macrophomina phaseolina: implications for selection of resistance sources. Eur J Plant Pathol 158:431–441
Suárez-Estrella F, Vargas-Garcia C, Lopez MJ, Capel C, Moreno J (2007) Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Protection 26(1):46–53
Tan Y, Cui Y, Li H, Kuang A, Li X, Wei Y, Ji X (2017) Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol Res 194:10–19
Teja TS, Kelayia DS, Asha R (2020) Impact of environmental factors on Macrophomina phaseolina causing charcoal rot of soybean. Int J Curr Microbiol App Sci 9:3784–3790
Udayanga D, Liu X, McKenzie EH, Chukeatirote E, Bahkali AH, Hyde KD (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 50:189–225
Van Warmelo KT, Marasas WFO, Adelaar TF, Kellerman TS, Van Rensburg IBJ, Minne J (1970) Experimental evidence that lupinosis of sheep is a mycotoxicosis caused by the fungus, Phomopsis leptostromiformis(Kuhn) Bubak. J S Afr Vet Assoc 41(3):235–247
Wang M, Liu F, Crous PW, Cai L (2017) Phylogenetic reassessment of Nigrospora: ubiquitous endophytes, plant and human pathogens. Persoonia 39(1):118–142
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40(1):309–348
Wright ER, Folgado M, Rivera MC, Crelier A, Vasquez P, Lopez SE (2008) Nigrospora sphaerica causing leaf spot and twig and shoot blight on blueberry: a new host of the pathogen. Plant Dis 92(1):171–171
Wu F, Zhou LW, Yang ZL, Bau T, Li TH, Dai YC (2019) Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Diversity 98:1–76
Zhao H, Liu HY, Yang XS, Liu YX, Ni YX, Wang F, Tang L (2014) First report of Nigrospora leaf blight on sesame caused by Nigrospora sphaerica in China. Plant Dis 98(6):842–842
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by P.P. and A.N. The first draft of the manuscript was written by P.P., J.R. and R.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pant, P., Negi, A., Rawat, J. et al. Characterization of rhizospheric fungi and their in vitro antagonistic potential against myco-phytopathogens invading Macrotyloma uniflorum plants. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00520-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00520-y