Abstract
In addition to ammonia-oxidizing bacteria (AOB) the more recently discovered ammonia-oxidizing archaea (AOA) can also oxidize ammonia, but little is known about AOA community structure and abundance in subtropical forest soils. In this study, both AOA and AOB were investigated with molecular techniques in eight types of forests at surface soils (0–2 cm) and deep layers (18–20 cm) in Nanling National Nature Reserve in subtropical China. The results showed that the forest soils, all acidic (pH 4.24–5.10), harbored a wide range of AOA phylotypes, including the genera Nitrosotalea, Nitrososphaera, and another 6 clusters, one of which was reported for the first time. For AOB, only members of Nitrosospira were retrieved. Moreover, the abundance of the ammonia monooxygenase gene (amoA) from AOA dominated over AOB in most soil samples (13/16). Soil depth, rather than forest type, was an important factor shaping the community structure of AOA and AOB. The distribution patterns of AOA and AOB in soil layers were reversed: AOA diversity and abundances in the deep layers were higher than those in the surface layers; on the contrary, AOB diversity and abundances in the deep layers were lower than those in the surface layers. Interestingly, the diversity of AOA was positively correlated with pH, but negatively correlated with organic carbon, total nitrogen and total phosphorus, and the abundance of AOA was negatively correlated with available phosphorus. Our results demonstrated that AOA and AOB were differentially distributed in acidic soils in subtropical forests and affected differently by soil characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the first and rate-limiting step of the two-step nitrification process, ammonia oxidation is a pivotal process in the global nitrogen biogeochemical cycle (Kowalchuk and Stephen 2001). Ammonia-oxidizing bacteria (AOB) were long thought to be the only microbial players that perform this biochemical function (Kowalchuk and Stephen 2001; Winogradsky 1890) until the recent discovery of ammonia-oxidizing archaea (AOA) in the phylum Thaumarchaeota (Brochier-Armanet et al. 2008; Francis et al. 2005; Könneke et al. 2005). Both groups of ammonia-oxidizing microorganisms (AOM) can oxidize ammonia to nitrite aerobically, making them share similar ecological function in the environment (Schleper and Nicol 2010). AOB have been well studied for more than 100 years, but the newly discovered AOA and their relatively poorly understood characteristics and functioning in ecosystems has revived research on nitrification in the last decade.
Since the discovery of AOA, many studies have focused on the relative importance of AOA and AOB (Stahl and de la Torre 2012). A widely used approach is to compare their abundances in the environment through quantification of the amoA gene, which encodes the α-subunit of the ammonia monooxygenase, the catalytic subunit in both AOA and AOB. Through this approach, AOA have been demonstrated to be widely distributed and outnumber AOB in various environments, including marine sediments and water column, estuary sediments, hot springs, terrestrial soils and freshwater, and even arctic soils (Agogue et al. 2008; Alonso-Sáez et al. 2012; Alves et al. 2013; Beam et al. 2014; Ke et al. 2013; Lee et al. 2014a, b; Leininger et al. 2006; Wang et al. 2013; Wang and Gu 2013). Numerical dominance suggests that AOA play a more important role than AOB in nitrogen biogeochemistry, though this is still in debate because numerical dominance does not necessarily translate to higher activities in nature (Schleper 2010). Eco-physiological studies showed that although both AOA and AOB share the same broad function, they seem to have different niche requirements (Wessén et al. 2011). One of the differences is that AOA favor environments with low ammonium while AOB dominate habitats with high ammonium, which is due to the higher affinity of AOA for ammonia (Auguet et al. 2011, 2012). For example, studies have shown that “Cadidatus Nitrosopumilus maritimus” SCM1 has high affinity for ammonia, whereas AOB have lower affinities for ammonia. (Koper et al. 2010; Martens-Habbena et al. 2009).
Recent studies have shown that diverse and abundant AOA can exist in acidic habitats that are adverse to the survival of AOB (Hernández et al. 2014; Lehtovirta-Morley et al. 2011; Qin et al. 2013; Stopnisek et al. 2010; Zhang et al. 2012; Zhou et al. 2014). The concentration and availability of ammonia (NH3) are both very low in acidic soils because the concentration of NH3 decreases exponentially with decreasing pH (NH3 + H+ → NH4 +; pKa = 9.25). Because ammonia rather than ammonium (NH4 +) is the substrate for AOA and AOB, the high affinity of AOA for ammonia is recognized as the main mechanism permitting AOA survival in acidic environments (Martens-Habbena et al. 2009; Tourna et al. 2011; Verhamme et al. 2011). The investigated acidic environments included agriculture soils (Gubry-Rangin et al. 2010; Jiang et al. 2014; Lehtovirta-Morley et al. 2011; Qin et al. 2013), tea orchards and forests (Isobe et al. 2012; Lu et al. 2012; Yao et al. 2011; Zhang et al. 2012), forest peat soils (Stopnisek et al. 2010), acidic fens (Herrmann et al. 2012), and acidic geothermal springs (Beam et al. 2014). Investigation on AOA in subtropical forests in China is relatively poorly studied (Isobe et al. 2012).
Forests are an important ecosystem on Earth, accounting for more than 90 % biomass of terrestrial ecosystems (FAOSTAT 2011). Subtropical forests, however, are continuously fragmented because of economic development and have been extensively transformed into commercial plantations (FAOSTAT 2011). Guangdong Province is mainly located in the subtropics, with a forest area of 9.849 × 104 km2 in 2013 (FDGP 2014). Nanling National Nature Reserve, the largest reserve in Guangdong Province, protects various typical subtropical forests (Chen 2012). In this study, we initiated a molecular study on AOA and AOB in acidic soils of different types of forests in Nanling National Nature Reserve, aiming to uncover the distribution patterns of AOA and AOB in the soils and the effects of soil characteristics and forest type on their distribution.
Materials and methods
Description of sites and sample collection
Soil samples were collected on October 15, 2013 from Guangdong Nanling National Nature Reserve, which is located in South China (24°37′–24°57′N, 112°30′–113°04′E) with an area of 58,368.4 hm2. This nature reserve has a subtropical monsoon climate with an annual precipitation of 2108.4 mm. The average temperature in the hottest month (July) is 26.2 °C, while in the coldest month (January) 7.1 °C. The soils in this nature reserve are basically classified into subtropical yellow soil (above 700 m) and red soil (below 700 m), with the inorganic fraction dominated by granite, metamorphic rock or sandstone (NNNR 2015). Vegetation consists of evergreen broadleaf forest, bamboo forest, needle and broadleaf mixing forest, mountain dwarf forest, and mountain grassland. In this study, eight types of forests were investigated, including one type of mountainous dwarf forest, two types of bamboo forests at different elevations, two types of evergreen broadleaf forests at different elevations, and three types of coniferous and broadleaf mixing forests consisting of different tree species (Table 1).
For each of the eight forests, one composite sample of surface soil (0–2 cm deep) and one composite sample of deep layer soil (18–20 cm deep) were collected as follows. At each forest, three soil profiles (at least 10 m apart) were made in a 30 × 30 m plot. Before soil collection, the organic matter horizon was removed. Then one kg of surface and deep layer soils were collected from each of the three profiles, and the three soils of the same layer (surface or deep layer) were pooled and mixed thoroughly to form a composite sample. For each composite sample, 100 g of soil was kept in a small plastic bag for molecular analysis and 900 g of soil was kept in a larger plastic bag for physicochemical analysis; the rest was then discarded. The soil samples were immediately put onto ice bags in a heat-insulated cooler after collection and carried back to the laboratory on the same day of sampling. In the laboratory, samples for subsequent molecular studies were kept at −80 °C and those for physicochemical analysis were processed immediately.
Physicochemical analysis
Physicochemical analysis was carried out in Guangdong Institute of Eco-environment and Soil Sciences according to Methods of Agriculture Chemical Analysis (Lu 2000). Briefly, pH was measured with a pH meter (Starter 3C, OHAUS). Organic carbon was measured using the sulfuric acid dichromate digestion method. Total nitrogen was measured using the Kjeldahl method. Available nitrogen was measured using the KOH diffusion method. Ammonium-N and nitrate–N were extracted with KCl first and then analyzed with Nessler’s reagent colorimetry and ultraviolet spectrophotometry, respectively. Total phosphorus was digested with HClO4 + H2SO4 first and then measured with the colorimetry method. Available phosphorus was extracted with NaHCO3 first and then measured with the colorimetry method. All the spectrophotometry was done with UV–Vis spectrophotometer (752 N type, Shanghai Jingke Co.).
Soil DNA extraction
Total DNA from each composite soil sample was extracted in duplicate using the SoilMaster DNA Extraction kit (Epicentre Biotechnologies, Madison, WI) according to the manual of the manufacturer. The duplicate DNA extracts from the same composite soil sample were then pooled and stored at −20 °C for subsequent molecular analysis.
Polymerase chain reaction amplification
The archaeal amoA genes were amplified using the primers Arch-amoAF (5′—STAATGGTCTGGCTTAGACG—3′) and Arch-amoAR (5′—GCGGCCATCCATCTGTATGT—3′) (Francis et al. 2005). Based on the standard procedures in the manufacturer’s instructions (Promega) and results of previous studies (Francis et al. 2005), the optimized polymerase chain reaction (PCR) mixture contained in a final volume of 50 µl and consisted of the followings: GoTaq Flexi buffer (1× , Promega), MgCl2 (1.5 mM, Promega), dNTPs (0.2 mM of each, Promega), GoTaq Flexi polymerase (0.025 U µl−1, Promega), DNA template (~0.5 ng µl−1), forward and reverse primers (each 0.4 µM), and BSA (0.01 %). PCR conditions were set as follows: 95 °C for 5 min; 30 cycles of 94 °C for 45 s, 53 °C for 1 min, and 72 °C for 1 min; and finally 72 °C for 15 min. PCR products were checked by electrophoresis in a 1 % agarose gel stained with GelRed (1:10000, v/v).
The bacterial amoA genes were amplified using the primers amoA-1F (5′—GGGGGTTTCTACTGGTGGT—3′) and amoA-2R (5′—CCCCTCKGSAAAGCCTTCTTC—3′) (Rotthauwe et al. 1997). The optimized PCR reaction mixture contained in a final volume of 50 µl and consisted of the followings: GoTaq Flexi buffer (1 × , Promega), MgCl2 (1.25 mM, Promega), dNTPs (0.2 mM of each, Promega), GoTaq Flexi polymerase (0.025 U µl−1, Promega), DNA template (~0.5 ng µl−1), forward and reverse primers (each 0.4 µM), and BSA (0.01 %). PCR conditions were set as follows: 94 °C for 3 min; 30 cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 50 s; and finally 72 °C for 10 min. PCR products were checked by electrophoresis in a 1 % agarose gel stained with GelRed (1:10000, v/v).
Cloning and sequencing
Clone libraries were established from the PCR products as described by Friedrich et al. (2001). Briefly, the PCR-amplified products were purified using the Gel Advanced™ Gel Extraction System (Viogene-Bio Tek Co., Taiwan, ROC) according to the manufacturer’s instructions, and cloned into the PMD18 T-vector (Takara, Japan). The insertion of an appropriate-sized DNA fragment was determined by PCR amplification with the primer set M13F and M13R. About 30 clones in each library were randomly selected for sequencing. Sequencing was performed with ABI 3730xl DNA analyzer (Applied Biosystems).
Phylogenetic analysis and biodiversity calculation
The retrieved sequences were blasted on the web (http://blast.ncbi.nlm.nih.gov) (Altschul et al. 1990) and the most similar sequences were downloaded to rebuild the phylogenetic trees. For each sample, clones with more than 97 % putative protein sequence identity were grouped into the same OTU using the furthest neighbor algorithm in DOTUR (Distance-Based OTU and Richness) program (Schloss and Handelsman 2005), and their representative putative protein sequences (198 and 150 amino acids for AOA and AOB, respectively) were used for phylogenetic analysis using the software MEGA 6 (Tamura et al. 2013). Phylogenetic trees were constructed with the neighbor-joining method with 1000 bootstraps to estimate the confidence of the tree topologies.
Besides OTU assignments, the DOTUR program was also used to generate biodiversity indices such as Chao1, Shannon index (H), and numbers of OTU for each sample.
Real-time quantitative PCR analysis
The abundances of archaeal and bacterial amoA genes were determined in triplicate with real-time quantitative PCR amplification using a FastStart Universal SYBR Green Master (Rox) Kit (Roche, Germany). Real-time qPCR was performed in 96-well optical plates placed in the ABI PRISM® 7000 Sequence Detection System (applied biosystems). The primer sets composed of Arch-amoAF and Arch-amoAR, and amoA-1F and amoA-2R were used for the amplification of the amoA genes of AOA and AOB, respectively. The final reaction volume was 20 µl and the reaction composition and cycling conditions were in accordance with the manual.
The specificity of the PCR amplification was determined by the melting curve and gel electrophoresis. Cycle thresholds were determined by comparing with the standard curves constructed using a 10 fold serial dilution (102 × 107 gene copies µl−1) of newly extracted plasmids containing corresponding gene fragments. Relative copy numbers among target groups were evaluated and some replicates of large deviation (|xi−u| >2.41σ) were excluded in order to decrease standard error. The correlation coefficient R 2 values were higher than 0.97 for all of the standard curves.
Principal coordinates analysis of AOM community structure
Fast UniFrac provides a suite of tools for the comparison of microbial communities using phylogenetic information (Goecks et al. 2010). To compare microbial communities in different environments, the phylogenetic trees of AOA and AOB were analyzed online using principal coordinates analysis (PCoA) on the website of Fast UniFrac (http://unifrac.colorado.edu).
Nucleic acid sequence accession numbers
The AOA and AOB amoA gene sequences determined in this study are available in GenBank under accession numbers KP736255 to KP736365 and KP736366 to KP736411, respectively.
Results
Physicochemical characteristics of forest soils
A total of eight parameters, including pH, organic carbon, ammonium-N, nitrate–N, available nitrogen, total nitrogen, available phosphorus and total phosphorus were investigated for the 16 forest soil samples (Table 2). All the soils were acidic with pH between 4.24 and 5.10. Soil nutrient concentrations varied considerably among different forest types. For example, organic carbon in the surface of one evergreen broadleaf forest (middle-elevation) was very high (151.8 g kg−1), but that in the surface of another evergreen broadleaf forest (low-elevation) was relatively low (69.2 g kg−1). Soil depth was a significant factor affecting nutrient levels. For each nutrient investigated, the average concentration of the surface soils was significantly higher than that of the deep layer soils (Table 2). For example, NH4 + concentration of the surface soils was 74.7 ± 25.0 mg kg−1 dry soil (n = 8), whereas that of the deep layers was only 35.6 ± 7.0 mg kg−1 dry soil (n = 8).
Phylogeny of ammonia-oxidizing microorganisms
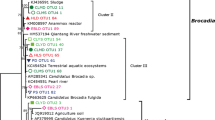
The phylogenetic tree of AOA in the eight types of forests analyzed using putative protein sequences of amplified amoA genes is shown in Fig. 1. A total of 488 archaeal amoA clones were acquired from the soil samples, representing the eight types of forests. The phylogenetic tree showed that Nanling National Nature Reserve harbored a wide range of AOA phylotypes, which fell into Nitrosotalea-like, Nitrososphaera-like, and six unknown clusters that have not been retrieved elsewhere. No sequences fell into the Nitrosopumilus-like cluster, which is mainly found in oceans (Bertagnolli et al. 2015). Nitrosotalea-like AOA detected in this study were also frequently detected in other acid soils (Lehtovirta-Morley et al. 2011). Nitrososphaera-like AOA were demonstrated to be widely distributed in various soils (Tourna et al. 2011). Cluster 1 and Cluster 4 included sequences from acidic red soils. Cluster 2 and Cluster 6 included sequences from tributary sediments. Cluster 3 included sequences from forest soils, tributary sediments and sea sediments. Cluster 5 appears to be a new cluster of AOA.
Phylogenetic tree based on deduced protein sequences of the amoA gene sequences of ammonia-oxidizing archaea (198 amino acids). The phylogenetic tree was constructed with the neighbor-joining method with 1000 bootstrapping to estimate the confidence of the tree topologies. Bootstrap values (>50 %) are indicated at the branch points. The scale bar represents 0.02 sequence divergence. The solid squares in blue represent the clone sequences from surface soils (0–2 cm deep). The solid triangles in green represent the clone sequences from deep soils (18–20 cm deep)
The phylogenetic tree of AOB in the eight types of forests analyzed using putative protein sequences of amplified amoA genes is shown in Fig. 2. A total of 348 bacterial amoA gene clone sequences were acquired from the soils of most forests, except the surface soils of the evergreen broadleaf forest at middle elevation, the deep layers of the coniferous and broad-leaved mixed forest, the mountainous bamboo forest and the evergreen broad-leaved forest. Previous studies have shown that AOB in terrestrial environments are mainly classified into two genera: Nitrosomonas-like and Nitrosospira-like (Kowalchuk and Stephen 2001). In this study, only Nitrosospira-like AOB were retrieved from the forests. These sequences can be further classified into three clusters. The three clusters were closely related, and members of the three clusters were also reported elsewhere (Fig. 2). Most of them were found in acidic environments. Cluster 1 included phylotypes from acidic red soil and lake sediment. Cluster 2 included Nitrosospira multiformis and Nitrosospira briensis. Cluster 3 included sequences from acidic red soils and agriculture soils.
Phylogenetic tree based on deduced protein sequences of the amoA gene sequences of ammonia-oxidizing bacteria (150 amino acids). The phylogenetic tree was constructed with the neighbor-joining method with 1000 bootstraps to estimate the confidence of the tree topologies. Bootstrap values (>50 %) are indicated at the branch points. The scale bar represents 0.03 sequence divergence. The solid squares in blue represent the clone sequences from surface soils (0–2 cm deep). The solid triangles in green represent the clone sequences from deep soils (18–20 cm deep)
Comparison of AOM community structures in different soils
For each forest, AOA communities of the surface soil and the deep layer were not clustered together (Fig. 3a), indicating AOA community structures in surface and deep layers in the same forest were different. However, the surface soils and deep soils each clustered together, regardless of forest type. (Figure 3b). This phenomenon indicated that soil depth or a factor associated with soil depth was pivotal in shaping AOA community structure, while forest type did not affect AOA community structure very much.
Principal coordinates analysis (PCoA) based on the Unifrac distance metric of deduced archaeal AmoA protein (a, b) and deduced bacterial AmoA protein (c, d), refer to Table 1 for detailed information of soil sample IDs
Similar to AOA, AOB communities of the surface and deep layer in the same forest were not clustered together (Fig. 3c). However, AOB communities in the surface soils of all the investigated forests were clustered together; and those in the deep layers of all investigated forests were clustered together (Fig. 3d). Because of that, soil depth or a factor associated with soil depth is important in shaping AOB community structure, while forest type had less effect on shaping AOB community structure compared to soil depth.
Diversity of ammonia-oxidizing microorganisms
Shannon indices (H′), indicating the diversity of AOA and bacteria, are shown in Table 3. The diversity of AOA (H′ = 1.43 ± 0.55) in most forest soils was higher than that of AOB (H′ = 0.73 ± 0.47). When observed by depth, the AOA diversity of the deep layer was higher than that of the surface layer in most individual forests. For AOB, on the contrary, the surface soil generally had higher diversity than that of the deep layer in most individual forests, indicating that the diversity pattern of AOA in the layers was opposite to that of AOB in this study.
Abundances of ammonia-oxidizing archaea and bacteria
In these subtropical forests, the abundance of AOA in 13 out of 16 soil samples was higher than AOB (except the surface soils of a coniferous and broad-leaved mixed forest, mountainous bamboo forest at high elevation, and evergreen broad-leaved forest at low elevation) (Fig. 4), suggesting AOA numerically dominated over AOB in those forests. When observed by depth, in 6 out of 8 forests, AOA abundances in the deep layers were higher than those in the surface layers (Fig. 4). On the contrary, for most forests (6 out of 7), AOB abundances in the deep layers were lower than those in the surface layers.
Quantification of archaeal and bacterial amoA genes in the surfaces and deep layers of subtropical forests by qPCR. See Table 1 for the explanation of the sample IDs
Correlation of characteristics of soils and biodiversity and abundance of AOM
The correlation between the eight physicochemical parameters of soils and biodiversity and abundance of AOA and AOB is shown in Table 4. The results showed that the biodiversity of AOA was significantly correlated with pH, organic carbon, total nitrogen and total phosphorus (p < 0.05, n = 16). The abundance of AOA was significantly correlated with available phosphorus (p < 0.05, n = 16). However, the biodiversity and abundance of AOB were not significantly correlated with any of the physicochemical parameters, although some correlations were high (Table 4).
Discussion
The abundant occurrence of AOA and AOB in the acidic forest soils
AOB were long thought to be the only ammonia oxidizers in the microbial N cycle (Kowalchuk and Stephen 2001), but recent studies showed that the newly discovered AOA are ubiquitous and numerically dominant over AOB in various habitats (reviewed by Stahl and de la Torre 2012). Moreover, some studies demonstrated that AOA were responsible for the observed nitrification in those environments (Dodsworth et al. 2011; Gubry-Rangin et al. 2010; Levičnik-Höfferle et al. 2012; Nicol et al. 2008; Stopnisek et al. 2010; Verhamme et al. 2011; Yao et al. 2011). These findings indicated that AOA might play a more important role than AOB in the global N cycle. More interestingly, a number of acidic soils, which were believed to be an adverse habitat to AOB, harbored diverse and abundant AOA (Lu et al. 2012; Tripathi et al. 2013; Zhang et al. 2012). The successful survival of AOA in acidic soils was attributed to the high affinity of AOA for ammonia, because ammonia concentrations are usually very low in acidic soils and the high affinity of AOA for ammonia helps them acquire ammonia in environments with low ammonia concentration (Prosser and Nicol 2012; Stahl and de la Torre 2012; Verhamme et al. 2011).
Subtropical forest soils are usually acidic (Zhang et al. 2013). The pH values of Nanling forest soils in this study ranged between 4.24 and 5.10 (Table 2). In these acidic forest soils, we observed diverse and abundant AOA. Moreover, the diversity of AOA observed in this study was apparently higher than that observed in Dinghushan Nature Reserve (Isobe et al. 2012), another reserve in Guangdong Province more than two hundred km south from Nanling Reserve, but receiving high N deposition (32–34 kg N ha−1 year−1 NH4 + and NO3 − in 2004 and 2005) as it was located in Pearl River Delta which is an important industrial manufacturing region in China (Fang et al. 2008).
A total of eight clusters were found in the present study, including Nitrosotalea-like, Nitrososphaera-like and six unclassified clusters. Most sequences fell into the Nitrosotalea-like cluster, in which the cultivated Nitrosotalea devanaterra is considered to be a model acidic AOA (Lehtovirta-Morley et al. 2011). Of all the retrieved sequences, none belonged to the Group I.1a Nitrosopumilus cluster that are thought to exist mainly in oceans despite one strain, Nitrosopumilus sp., which has been enriched from agriculture soil (Jung et al. 2011). The high diversity of AOA observed in Nanling Nature Reserve indicated that a wide range of AOA phylotypes can adapt to acidic conditions, rather than the former speculation that only a small subset of AOA were specialized to adapt to acidic environments (Gubry-Rangin et al. 2011). The ability of AOA to adapt to acidic environments probably is a shared characteristic for most AOA.
Consistent with the findings in many other acidic environments (Tripathi et al. 2013; Zhang et al. 2012), AOA in Nanling forest soils numerically dominated over AOB, suggesting AOA might play a more important role than AOB in nitrification in acidic soils. Isobe et al. (2012) found that AOA were responsible for the observed ammonia oxidation in Dinghushan Nature Reserve (more than 200 km south Nanling), where no AOB were detected. However, in Nanling Reserve AOB were also abundant in these acidic forest soils, though not as high abundance as AOA. All detected AOB in Nanling fell into the genus Nitrosospira, which was consistent with the fact that cultivated AOB from acidic environments mostly belonged to Nitrosospira (De Boer and Kowalchuk 2001). Since both AOA and AOB exist in these acidic soils, future work may focus on determining which group is responsible for nitrification activity in these soils.
Influence of factors on AOA abundance and diversity
Environmental factors are important drivers shaping the community structures and abundance of microorganisms in soils. Studies showed that the community structures and abundances of AOA and AOB were differently affected by some factors such as pH (Nicol et al. 2008), salinity (Wang and Gu 2014), organic carbon (Wang et al. 2014), and elevation (Zhang et al. 2009). Before this study, we had hypothesized that forest type was an important factor shaping AOM communities based on two reasons. Firstly, different forests have litters containing different natural inhibitors such as phenolic acids, flavonoids and terpenoids, which may influence AOM differently (De Boer and Kowalchuk 2001). Secondly, AOA and AOB respond to ammonia sources differently. In addition, in other studies AOA seemed to better utilize ammonia released from organic matter, while AOB tended to favor inorganic ammonia such as fertilizer (Yamamoto et al. 2011). In our study, soil depth, rather than forest type, had a significant influence on community structures of both AOA and AOB. This may be because soils of different layers had distinct characteristics (Table 2), and soil parameters can shape AOM community structures. Compared to soil parameters, forest type had relatively little effect on AOM communities. To our knowledge, this is the first report of AOM community composition being determined by soil depth rather than by forest type (Fig. 3).
Plenty of studies have shown that AOA can occur in habitats with a wide range of pH values (pH 3.5–8.7) and observed relationships between pH and AOA abundance include negative, positive or no correlation depending on the study system (Gubry-Rangin et al. 2011; Prosser and Nicol 2012). In this study, pH and seven nutrients (organic carbon, ammonium-N, nitrate–N, available nitrogen, total nitrogen, available phosphorus and total phosphorus) were investigated along with the molecular quantification of putative AOM. AOA abundance showed no significant correlation with pH (p > 0.05, n = 16; Table 2). However, the diversity of AOA was significantly correlated with pH (p < 0.05, n = 16; Table 2), indicating more phylotypes of AOA favored habitats of higher pH values. Our finding that AOA diversity decreases with decreasing pH was consistent with the result of Gubry-Rangin et al. (2011), but opposite to that of Tripathi et al. (2013). This result suggests that survival in acidic environments is only an adaptive strategy for AOA, and that acidic environments themselves are not a favorable condition for AOA.
Studies have demonstrated that AOA tend to exist in oligotrophic environments such as low ammonium habitats (Sauder et al. 2012; Verhamme et al. 2011). To our knowledge, the relationship between AOA abundance and phosphorus has not been studied in soils. In this study AOA abundance was negatively correlated with available phosphorus (p < 0.05, n = 16; Table 4), suggesting that the acidic forest soils with higher available phosphorus were adverse to AOA. Besides available phosphorus, AOA diversity was negatively correlated with organic carbon, total nitrogen and total phosphorus (p < 0.05, n = 16). These phenomena again suggested that AOA favored oligotrophic environments of low nitrogen and phosphorus. Former studies mainly focused on the effect of ammonia on AOA as ammonia is a substrate of AOA. Our study for the first time revealed that AOA favored oligotrophic habitats in terms of other nutrients.
The mechanism explaining why phosphorus inhibited AOA in these acidic forest soils might be as follows. Phosphorus is usually a limiting factor for tree growth in many ecosystems, especially in acidic subtropical forests (Porder et al. 2005; Wardle et al. 2004). In subtropical China, available P is generally low in the acidic forest soils (Hou et al. 2015; Zheng et al. 2015), thus it is a factor limiting the growth of plants and microorganisms (Elser et al. 2007). When more available P can be obtained, plants and heterotrophic microorganisms will utilize them quickly and grow much faster than the autotrophic AOA. The fast-growing plants and copiotrophic, heterotrophic microorganisms will then enhance the absorption of other elements, which would in turn inhibit AOA.
References
Agogue H, Brink M, Dinasquet J, Herndl GJ (2008) Major gradients in putatively nitrifying and non-nitrifying archaea in the deep North Atlantic. Nature 456:788–791
Alonso-Sáez L et al (2012) Role for urea in nitrification by polar marine archaea. Proc Natl Acad Sci USA 109:17989–17994. doi:10.1073/pnas.1201914109
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Alves RJE, Wanek W, Zappe A, Richter A, Svenning MM, Schleper C, Urich T (2013) Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J 7:1620–1631. doi:10.1038/ismej.2013.35
Auguet J-C, Nomokonova N, Camarero L, Casamayor EO (2011) Seasonal changes of freshwater ammonia-oxidizing archaeal assemblages and nitrogen species in oligotrophic Alpine lakes. Appl Environ Microbiol 77:1937–1945. doi:10.1128/aem.01213-10
Auguet J-C, Triado-Margarit X, Nomokonova N, Camarero L, Casamayor EO (2012) Vertical segregation and phylogenetic characterization of ammonia-oxidizing archaea in a deep oligotrophic lake. ISME J 6:1786–1797. doi:10.1038/ismej.2012.33
Beam JP, Jay ZJ, Kozubal MA, Inskeep WP (2014) Niche specialization of novel Thaumarchaeota to oxic and hypoxic acidic geothermal springs of Yellowstone National Park. ISME J 8:938–951. doi:10.1038/ismej.2013.193
Bertagnolli AD, Meinhardt KA, Pannu M, Brown S, Strand S, Fransen SC, Stahl DA (2015) Influence of edaphic and management factors on the diversity and abundance of ammonia-oxidizing Thaumarchaeota and bacteria in soils of bioenergy crop cultivars. Environ Microbiol Rep 7:312–320. doi:10.1111/1758-2229.12250
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Micro 6:245–252. doi:10.1038/nrmicro1852
Chen Z-M (2012) Ecosystem functions in Nanling National Nature Reserve and protective measures concerned. Shaanxi For Sci Technol 61–63:019
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866. doi:10.1016/s0038-0717(00)00247-9
Dodsworth JA, Hungate BA, Hedlund BP (2011) Ammonia oxidation, denitrification and dissimilatory nitrate reduction to ammonium in two US Great Basin hot springs with abundant ammonia-oxidizing archaea. Environ Microbiol 13:2371–2386. doi:10.1111/j.1462-2920.2011.02508.x
Elser JJ et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fang YT, Gundersen P, Mo JM, Zhu WX (2008) Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 5:339–352. doi:10.5194/bg-5-339-2008
FAOSTAT (2011) <http://faostat.fao.org/>
FDGP (2014) <http://www.gdf.gov.cn/>
Francis CA, Roberts KJ, Beman JM, Alyson ES, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Friedrich MW, Schmitt-Wagner D, Lueders T, Brune A (2001) Axial differences in community structure of Crenarchaeota and Euryarchaeota in the highly compartmentalized gut of the soil-feeding termite Cubitermes orthognathus. Appl Environ Microbiol 67:4880–4890. doi:10.1128/aem.67.10.4880-4890.2001
Goecks J, Nekrutenko A, Taylor J, Team TG (2010) Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 11:R86
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74:566–574. doi:10.1111/j.1574-6941.2010.00971.x
Gubry-Rangin C et al (2011) Niche specialization of terrestrial archaeal ammonia oxidizers. Proc Natl Acad Sci USA 108:21206–21211. doi:10.1073/pnas.1109000108
Hernández M, Dumont MG, Calabi M, Basualto D, Conrad R (2014) Ammonia oxidizers are pioneer microorganisms in the colonization of new acidic volcanic soils from South of Chile. Environ Microbiol Rep 6:70–79. doi:10.1111/1758-2229.12109
Herrmann M, Hädrich A, Küsel K (2012) Predominance of thaumarchaeal ammonia oxidizer abundance and transcriptional activity in an acidic fen. Environ Microbiol 14:3013–3025. doi:10.1111/j.1462-2920.2012.02882.x
Hou E, Chen C, Wen D, Liu X (2015) Phosphatase activity in relation to key litter and soil properties in mature subtropical forests in China. Sci Total Environ 515–516:83–91
Isobe K et al (2012) High abundance of ammonia-oxidizing archaea in acidified subtropical forest soils in southern China after long-term N deposition. FEMS Microbiol Ecol 80:193–203
Jiang Y, Jin C, Sun B (2014) Soil aggregate stratification of nematodes and ammonia oxidizers affects nitrification in an acid soil. Environ Microbiol 16:3083–3094. doi:10.1111/1462-2920.12339
Jung M-Y et al (2011) Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal Group I.1a from an agricultural soil. Appl Environ Microbiol 77:8635–8647. doi:10.1128/aem.05787-11
Ke X, Angel R, Lu Y, Conrad R (2013) Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil. Environ Microbiol 15:2275–2292. doi:10.1111/1462-2920.12098
Könneke M, Bernhard AE, Torre JRDL, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Koper TE, Stark JM, Habteselassie MY, Norton JM (2010) Nitrification exhibits Haldane kinetics in an agricultural soil treated with ammonium sulfate or dairy-waste compost. FEMS Microbiol Ecol 74:316–322. doi:10.1111/j.1574-6941.2010.00960.x
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529. doi:10.1146/annurev.micro.55.1.485
Lee K-H, Wang Y-F, Li H, Gu J-D (2014a) Niche specificity of ammonia oxidizing communities in a freshwater interior city wetland receiving municipal wastewater in Daqing, Northeast China. Ecotoxicology 23:2081–2091. doi:10.1007/s10646-014-1334-3
Lee K-H, Wang Y-F, Zhang G-X, Gu J-D (2014b) Distribution patterns of ammonia-oxidizing bacteria and anammox bacteria in the freshwater marsh of Honghe wetland in Northeast China. Ecotoxicology 23:1930–1942. doi:10.1007/s10646-014-1333-4
Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW (2011) Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA 108:15892–15897. doi:10.1073/pnas.1107196108
Leininger S et al (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Levičnik-Höfferle Š, Nicol GW, Ausec L, Mandić-Mulec I, Prosser JI (2012) Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol Ecol 80:114–123. doi:10.1111/j.1574-6941.2011.01275.x
Lu R (2000) Methods of agriculture chemical analysis. China Agriculture Scientech Press, Beijing
Lu L et al (2012) Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J 6:1978–1984
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature 461:976–979. doi:10.1038/nature08465
Nicol G, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
NNNR (2015) <http://www.gdnl.org/>
Porder S, Asner GP, Vitousek PM (2005) Ground-based and remotely sensed nutrient availability across a tropical landscape. Proc Natl Acad Sci USA 102:10909–10912. doi:10.1073/pnas.0504929102
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531. doi:10.1016/j.tim.2012.08.001
Qin H et al (2013) Ammonia-oxidizing archaea are more important than ammonia-oxidizing bacteria in nitrification and NO3 −-N loss in acidic soil of sloped land. Biol Fertility Soils 49:767–776. doi:10.1007/s00374-012-0767-1
Rotthauwe J, Witzel K, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Sauder LA, Peterse F, Schouten S, Neufeld JD (2012) Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ Microbiol 14:2589–2600. doi:10.1111/j.1462-2920.2012.02786.x
Schleper C (2010) Ammonia oxidation: different niches for bacteria and archaea? ISME J 4:1092–1094
Schleper C, Nicol GW (2010) Ammonia-oxidising archaea—physiology, ecology and evolution. In: Robert KP (ed) Advances in microbial physiology, vol 57. Academic Press, Oxford, pp 1–41. doi:10.1016/b978-0-12-381045-8.00001-1
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506. doi:10.1128/aem.71.3.1501-1506.2005
Stahl DA, de la Torre JR (2012) Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol 66:83–101. doi:10.1146/annurev-micro-092611-150128
Stopnisek N, Gubry-Rangin C, Hofferle S, Nicol GW, Mandic-Mulec I, Prosser JI (2010) Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl Environ Microbiol 76:7626–7634. doi:10.1128/aem.00595-10
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tourna M et al (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108:8420–8425. doi:10.1073/pnas.1013488108
Tripathi BM, Kim M, Lai-Hoe A, Shukor NAA, Rahim RA, Go R, Adams JM (2013) pH dominates variation in tropical soil archaeal diversity and community structure. FEMS Microbiol Ecol 86:303–311. doi:10.1111/1574-6941.12163
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5:1067–1071. doi:10.1038/ismej.2010.191
Wang Y-F, Gu J-D (2013) Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl Microbiol Biotechnol 97:7015–7033. doi:10.1007/s00253-012-4430-4
Wang Y-F, Gu J-D (2014) Effects of allylthiourea, salinity and pH on ammonia/ammonium-oxidizing prokaryotes in mangrove sediment incubated in laboratory microcosms. Appl Microbiol Biotechnol 98:3257–3274. doi:10.1007/s00253-013-5399-3
Wang Y-F, Feng Y-Y, Ma X, Gu J-D (2013) Seasonal dynamics of ammonia/ammonium-oxidizing prokaryotes in oxic and anoxic wetland sediments of subtropical coastal mangrove. Appl Microbiol Biotechnol 97:7919–7934. doi:10.1007/s00253-012-4510-5
Wang Y-F, Li X-Y, Gu J-D (2014) Differential responses of ammonia/ammonium-oxidizing prokaryotes in mangrove sediment to amendment of acetate and leaf litter. Appl Microbiol Biotechnol 98:3165–3180. doi:10.1007/s00253-013-5318-7
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–513. doi:10.1126/science.1098778
Wessén E et al (2011) Spatial distribution of ammonia-oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J 5:1213–1225. doi:10.1038/ismej.2010.206
Winogradsky S (1890) Investigations on nitrifying organisms. Ann Inst Pasteur 4:213–321
Yamamoto N, Asano R, Yoshii H, Otawa K, Nakai Y (2011) Archaeal community dynamics and detection of ammonia-oxidizing archaea during composting of cattle manure using culture-independent DNA analysis. Appl Microbiol Biotechnol 90:1501–1510
Yao H et al (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microbiol 77:4618–4625. doi:10.1128/aem.00136-11
Zhang L-M, Wang M, Prosser JI, Zheng Y-M, He J-Z (2009) Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:208–217. doi:10.1111/j.1574-6941.2009.00775.x
Zhang L-M, Hu H-W, Shen J-P, He J-Z (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Zhang J-B, Cai Z-C, Zhu T-B, Yang W-Y, Müller C (2013) Mechanisms for the retention of inorganic N in acidic forest soils of southern China. Sci Rep 3:2342. doi:10.1038/srep02342
Zheng M, Huang J, Chen H, Wang H, Mo J (2015) Responses of soil acid phosphatase and beta-glucosidase to nitrogen and phosphorus addition in two subtropical forests in southern China. Eur J Soil Biol 68:77–84. doi:10.1016/j.ejsobi.2015.03.010
Zhou Z, Shi X, Zheng Y, Qin Z, Xie D, Li Z, Guo T (2014) Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur J Soil Biol 60:24–33
Acknowledgments
This research was supported by a research grant to Yong-Feng Wang from National Natural Science Foundation of China [31470562] and fund to Guangdong Nanling Forest Ecology Station from The National Forestry Bureau of China. We would like to thank Mr. Sanwu Zhang for support in field sampling and Ms. Kelly Lau at The University of Hong Kong for general laboratory support in this research work. We are grateful to the editor and the reviewers for their careful revisions and constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Xian-Hua Gan and Fang-Qiu Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gan, XH., Zhang, FQ., Gu, JD. et al. Differential distribution patterns of ammonia-oxidizing archaea and bacteria in acidic soils of Nanling National Nature Reserve forests in subtropical China. Antonie van Leeuwenhoek 109, 237–251 (2016). https://doi.org/10.1007/s10482-015-0627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0627-8