Abstract
Glioblastoma is the most common malignant brain cancer in adults, with poor prognosis. The blood–brain barrier limits the arrival of several promising anti-glioblastoma drugs, and restricts the design of efficient therapies. Recently, by using state-of-the-art technologies, including thymidine kinase targeting system in combination with glioblastoma xenograft mouse models, it was revealed that targeting glioblastoma-derived pericytes improves chemotherapy efficiency. Strikingly, ibrutinib treatment enhances chemotherapeutic effectiveness, by targeting pericytes, improving blood–brain barrier permeability, and prolonging survival. This study identifies glioblastoma-derived pericyte as a novel target in the brain tumor microenvironment during carcinogenesis. Here, we summarize and evaluate recent advances in the understanding of pericyte’s role in the glioblastoma microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are tumors that arise from glial cells [1]. Glioblastoma multiforme is the most aggressive type of these tumors [2], and the major brain primary tumor in adults worldwide [3]. Glioblastoma is a highly vascularized, invasive, diffuse, infiltrating, and a drug-resistant malignant cancer with the grimmest prognosis comparing to most tumors [4]. The median survival is approximately 1 year after diagnosis, despite current therapies [5]. Only less than 5% of patients with glioblastoma survive 5 years after diagnosis [6, 7]. The few accepted risk factors for glioblastoma comprise male gender, white ethnicity, increased age, high dose of ionizing radiation, and rare genetic syndromes [8]. Presently, the initial conventional therapy of patients diagnosed with glioblastoma consists of maximal surgical resection [9]. Nevertheless, the probability of recurrence is high due to the aggressiveness, and spread of these cancer cells in the brain [10]. Therefore, resection of glioblastoma primary tumors is followed by radiotherapy, and chemotherapy [1]. Regrettably, the main chemotherapeutic agent used temozolomide, alkylating drug which sensitizes glioblastoma cells to radiation, induces a small survival benefit [11]. Limited drug delivery through the blood–brain barrier is the main reason for failure of otherwise promising compounds for glioblastoma effective treatment.
The blood–brain barrier plays key roles in brain homeostasis, and consists of highly specialized endothelial cells surrounded by pericytes and glia [12,13,14,15]. It comprises a biochemical and a physical barrier for drug delivery in the adult brain [16]. Continuous tight junctions adjoin brain endothelial cells preventing diffusion in between them [17]. The presence of this physical interface between brain parenchyma and peripheral circulation was demonstrated more than a century ago, by dye injection into the blood stream that stained peripheral organs, without achieving the brain tissue [18]. Thus, in the presence of an intact blood–brain barrier, only lipophilic molecules from the bloodstream, smaller than 400 Da, can enter the brain parenchyma by transiting across endothelial cell luminal and abluminal plasma membranes [17]. Therefore, blood–brain barrier is neuroprotective in normal conditions, blocking the entrance of noxious agents to the brain, being advantageous [19]. In contrast, in glioblastoma patients, this barrier limits the arrival to the brain parenchyma of several oncologic drugs including hydrophilic molecules, monoclonal antibodies, and antibody-drug conjugates [20]. As a result, although the blood–brain barrier may be partially disrupted in brain tumor patients, multiple anti-glioblastoma agents have defective delivery and distribution in the brain parenchyma contributing to cancer recurrence [21]. To reach the invasive cancer cells in the brain, circumventing and counteracting the blocking effects of the blood–brain barrier, new strategies enhancing the efficacy of glioblastoma therapy are needed.
Pericytes are defined based on their anatomical localization surrounding blood vessel walls, and communicating with endothelial cells [22,23,24]. Pericytes contribute to vascular stabilization [25], and blood flow regulation [26, 27]. Their role in the maintenance of functional integrity of the blood–brain barrier is well established [28]. Now, in a recent article in Cell Stem Cell, Zhou et al. investigated whether targeting glioblastoma-derived pericytes improves chemotherapy efficiency [29]. The authors revealed that high pericyte coverage of glioblastoma blood vessels is associated with poor response to chemotherapy in human patients, indicating that reducing pericyte coverage may improve chemotherapy efficacy. As part of pericytes in the glioblastoma microenvironment are derived from cancer stem cells [30], Zhou et al. targeted those cells by using state-of-the-art techniques, including thymidine kinase targeting system in combination with glioblastoma xenograft mouse model. These experiments revealed that elimination of glioblastoma-derived pericytes alters vascular permeability in the brain tumor, facilitating the efficient delivery of small molecules [29]. Furthermore, disruption of tumor-derived pericytes improved the anti-glioblastoma activity of etoposide, an anti-cancer drug that penetrates poorly in the blood–brain barrier, retarding tumor growth, and extending animal survival [29]. Interestingly, trying to identify specific glioblastoma-derived pericytes’ molecules for pharmacological targeting, the authors discovered that bone marrow tyrosine kinase on chromosome X (BMX) is highly expressed in neoplasia-derived pericytes in comparison to normal brain pericytes. Therefore, Zhou et al. treated glioblastoma-bearing mice with ibrutinib, a reported potent inhibitor of BMX. This therapy increased vascular permeability, improving the diffusion of small molecules into the tumor. Strikingly, ibrutinib treatment enhanced the effectiveness for a poor blood–brain barrier penetrating drug in a mouse model with glioblastoma xenograft, prolonging the survival of those mice [29]. Here, we discuss the findings from this study, and evaluate recent advances in our understanding of the pericyte’ biology in the glioblastoma microenvironment.
Perspectives/future directions
Glioblastoma immune microenvironment
Cancer mouse models try to mimic human disease; they expand greatly our capacity to decipher mechanistic details in vivo, and play a critical role in the development of novel therapies for glioblastoma. Immunocompromised mouse models preventing host immune rejection are widely used in glioblastoma research, as in the study by Zhou et al. [29], due to their certainty of glioblastoma initiation, speed of glioblastoma development, and simple establishment. Nevertheless, multiple compounds with significant anti-cancer effects in immuno-deficient mouse models do not work in human patients [31]. This may be due to the crucial roles that the immune system plays during tumor development [32, 33]. Because of the distinct tumor microenvironment in immunocompromised mice compared with humans, it is not possible to evaluate the effect of a specific therapy on the tumoral immune system by the use of this kind of model [34]. Thus, immuno-deficient mouse models should be used in combination with syngeneicly transplanted and genetically engineered immune-competent mouse models in order to minimize the disadvantages of each model. Future studies should reveal whether targeting glioblastoma pericytes in mice with active immune system also improves effectiveness of chemotherapy.
Pericytes, in addition to their role in the maintenance of functional integrity of the blood–brain barrier [28], have several immune functions [35]. They express adhesion molecules associated with the control of immune cells trafficking, such as VCAM-1 and ICAM-1 [36], and produce multiple chemokines important for immune cells functions [37,38,39]. Pericytes regulate lymphocytes activation [40,41,42,43], and attract innate leukocytes to exit through the sprouting blood vessels [44]. Pericytes also can affect blood coagulation, contribute to the clearance of toxic cellular byproducts, and have direct phagocytic activity as macrophages [37,46,47,48,49,50,51,52]. Importantly, recently it has been shown that in the brain pericytes are important for immunomodulation in the glioblastoma microenvironment [53, 54]. Therefore, it should be explored what is the effect of pericyte’ blockade on the immune cells that reside in the glioblastoma microenvironment, and how this affects brain tumors’ progression. Pericyte roles are complex, and our understanding of the cross-talk between pericytes and immune cells still remains restricted. Therefore, elucidating the details of the cross-talk between pericytes and different immune cell subsets in the brain tumor microenvironment is key to the development of anti-glioblastoma therapies.
Targeting pericytes in the glioblastoma microenvironment
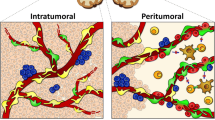
Pericytes are essential for the formation of new blood vessels, angiogenesis, during tumor growth [55]. For this reason, strategies targeting pericytes have been considered as anti-angiogenic treatments for different types of tumors [56]. Nonetheless, till now, clinical cancer studies with pericyte’ blockade have failed to ameliorate patients’ outcome [57, 58]. Higher pericytes’ coverage was related to better prognosis in some patients [59]. Importantly, in certain conditions, pericyte targeting even enhanced tumor metastatic progression [60,61,62,63]. Thus, the strategy to block pericytes requires a careful examination of glioblastoma and its microenvironment morphology and functional properties to determine whether a particular agent is having an effect. Zhou et al., using the thymidine kinase targeting system in glioblastoma xenograft mouse model, blocked exclusively glioblastoma-derived pericytes, which correspond to only part of pericytes present in the glioblastoma microenvironment [30]. Future studies should examine the effect of blocking also the other subset of pericytes non-glioblastoma-derived present in the tumor microenvironment. A better understanding of the molecular differences between glioblastoma-derived and non-glioblastoma-derived tumoral pericytes may reveal specific targets for anti-glioblastoma therapies (Fig. 1).
Glioblastoma-derived pericytes as a novel therapeutic target. Pericytes associated to cerebral blood vessels residing within the brain tumor microenvironment can be subdivided into two big subpopulations: glioblastoma-derived and non-glioblastoma-derived. The study of Zhou et al. now reveals that targeting glioblastoma-derived pericytes improves chemotherapy efficacy [29]. Ibrutinib, a reported potent inhibitor of BMX, enhances the effectiveness of chemotherapy via reducing pericytes coverage. With the appearance of state-of-art modern technologies, future studies will reveal in detail all cellular components and their interaction with glioblastoma cells in the brain tumor microenvironment
Glioblastoma pericyte’ role as a stem cell, and as a niche cell for cancer stem cells
Pericytes are highly plastic cells [64], having the capacity to differentiate into distinct cellular populations, including osteoblasts [65], myoblasts [66], adipocytes [67], fibroblasts [68], smooth muscle cells [24], and chondrocytes [38]. Due to their multipotency, pericytes are promising targets for tissue regeneration and repair [25]. Recently, it has been shown that pericytes also have neurogenic potential, being able to generate neural and glial cells [69,70,71,72,73]. It remains completely unexplored pericyte’ plasticity in the glioblastoma microenvironment. Future studies should reveal whether pericytes have the ability also to form other stromal cells in the brain tumor microenvironment which may influence glioblastoma progression. Also, it remains undefined whether pericytes can become malignant cells, or whether glioblastoma-derived pericytes may de-differentiate into glioblastoma cancer cells in the glioblastoma microenvironment. These hypotheses can be evaluated by genetic fate-tracing pericyte-specific mouse models to access pericyte plasticity in vivo in the glioblastoma microenvironment.
In addition to its capacity to function as stem cells [74,75,76], pericytes can regulate the functioning of other stem cells, being important components of stem cell niches [38, 77,78,79,80,81]. The characteristic that several stem cells share is that they are concentrated in the proximity of blood vessels, which shelter them from noxious stimuli, and control the equilibrium between self-renewal and differentiation [38, 82]. Similarly, it has been suggested that glioblastoma stem cells as well reside in a perivascular niche that stimulate their self-renewal and long-term growth [83]. The role of pericyte as a niche cell for glioblastoma stem cells has not been explored yet. Identification of signals produced by pericytes important for glioblastoma stem cells maintenance may reveal whether, how, and when pericytes regulate glioblastoma stem cells behavior. If the pericyte functions as a cancer stem cell niche component as well, it raises interesting questions: Does targeting glioblastoma pericytes affect this role as well? Are glioblastoma stem cells attracted to the pre-existing pericytes, or do they previously generate glioblastoma-derived pericytes to support themselves? Also, it remains to be evaluated whether targeting glioblastoma-derived pericytes assists chemotherapy to eliminate glioblastoma stem cells.
Perivascular cells heterogeneity in the glioblastoma microenvironment
Even though pericytes are characterized by their anatomical perivascular localization, not all perivascular cells are pericytes [84, 85]. Several cells that may share molecular markers, including the ones used by Zhou et al. [29], with pericytes have been described as perivascular: e.g., macrophages [86,87,88], adventitial cells [89], smooth muscle cells [38], and fibroblasts [90]. Zhou et al. ablated genetically glioblastoma-derived pericyte-based desmin, a type-III intermediate-filament protein, expression. However, this marker could refer to other cell populations. For instance, desmin is known to be expressed in astrocytes, and other glial cells in the central nervous system [91,92,93]. Although none of brain pericyte markers are specific, when used in combination they clearly distinguish pericytes from other cell types [94]. Importantly, neoplastic astrocytes also may express desmin [95], therefore, it is possible that Zhou et al. eliminated malignant astrocytes, when ablation was done using the desmin-driven HSV-TK system [29]. Future studies will need to clarify whether the genetic ablation of pericytes was essential for the chemotherapeutic improvement, as probably other cell populations were affected as well.
Pericytes are heterogeneous in their morphology, distribution, molecular markers, origin, and function [96]. Pericytes associated with distinct blood vessel types differ in their morphology, markers, and function [38, 97,98,99]. At least two pericyte subsets have been described in the brain: type-1 and type-2 pericytes distinguished based on their Nestin-GFP expression [68, 100]. Interestingly, not all central nervous system pericytes express desmin [101]. Thus, in addition to non-glioblastoma-derived pericytes, glioblastoma-derived pericytes not-expressing desmin were not targeted by the desmin-driven HSV-TK system [29]. Whether only a fraction of pericytes is important for the maintenance of blood–brain barrier integrity remains to be studied.
Molecular targeting of glioblastoma pericytes
Recent advances in the understanding of the molecular and cellular mechanisms involved in glioblastoma progression pave the way for the development of targeted therapies that would decrease chemotherapeutic toxicity, while increasing therapeutic efficacy. Targeting pericytes have been proposed as a therapy in several cancers, due especially to their angiogenic potential. Unfortunately, experimental data do not invariably anticipate success at the clinic. Zhou et al. revealed that BMX is upregulated in glioblastoma-derived pericytes [29]. However, several cell types may express BMX in addition to pericytes [102, 103]. BMX was not conditionally deleted from glioblastoma pericytes or from other cell populations that express it in the glioblastoma microenvironment, so there is no direct evidence that pericytes will be the only/main functionally important target when blocking BMX. Transgenic mouse models have been applied to study specific cell populations within distinct tissue-microenvironments [104, 105]. The ability, not only to eliminate cells, but to delete single genes in specific cellular populations in adult mice has allowed us to answer specific questions regarding the roles of molecules derived from different cell subsets in the regulation of physiologic and pathologic processes. The exact molecular mechanisms in which pericytes are involved during glioblastoma progression in vivo are yet not completely clear, and will need to be revealed in future studies. The generation of BMX-floxed mice to be crossed with pericyte-specific inducible CreER driver will allow us to specifically delete this molecule in pericytes in vivo. In addition to studies in genetic mouse models, transcriptomic and single pericyte analysis represents fundamental tools that will help us develop targeted therapies for pericytes in the glioblastoma microenvironment during different stages of cancer progression.
Efforts are underway in the field to identify tumoral pericytes inhibitors that will influence uniquely the tumor niche. Zhou et al. proposed to use the FDA-approved drug ibrutinib to disrupt selectively glioblastoma-derived pericytes [29]. Ibrutinib, previously known as PCI-32765, is a potent inhibitor of BMX. Yet, ibrutinib also have significant activity against 19 other kinases, including BLK, BTK, ITK, TEC, EGFR, ERBB2, and JAK3 [106]. Possibly, for this reason, ibrutinib use have reported side effects which may limit its use, such as hypertension, atrial fibrillation, bleeding, diarrhea, infection, arthralgia, and skin toxicity [107,108,109,110,111]. Therefore, the discovery of new molecular targets within pericytes, not expressed by other cells, will lead to the development of more effective, less toxic drugs. A deeper characterization of ibrutinib effects on the glioblastoma microenvironment should be performed. Are cancer stem cells, angiogenesis, other stromal and inflammatory cells also affected? Future studies should explore the most effective use of ibrutinib in glioblastoma patients. Need to be defined: chemotherapeutic drugs that are effective in combination with ibrutinib; the timing of its use; and the optimal dosage.
Conclusion
The study by Zhou et al. reveals glioblastoma-derived pericytes as a novel important target in the glioblastoma microenvironment. However, our understanding of pericytes biology in the brain tumor microenvironment still remains limited, and future studies should shed light on the complexity and interactions of different cellular components of the glioblastoma microenvironment during carcinogenesis. A great challenge for the future will be to translate experimental data into humans. Improving the availability of human glioblastoma samples will be essential to reach this goal.
References
DeAngelis LM (2001) Brain tumors. N Engl J Med 344(2):114–123. https://doi.org/10.1056/NEJM200101113440207
Fisher PG, Buffler PA (2005) Malignant gliomas in 2005: where to GO from here? JAMA 293(5):615–617. https://doi.org/10.1001/jama.293.5.615
Reardon DA, Rich JN, Friedman HS, Bigner DD (2006) Recent advances in the treatment of malignant astrocytoma. J Clin Oncol 24(8):1253–1265. https://doi.org/10.1200/JCO.2005.04.5302
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Kortmann RD, Jeremic B, Weller M, Plasswilm L, Bamberg M (2003) Radiochemotherapy of malignant glioma in adults. Strahlentherapie und Onkologie 179(4):219–232. https://doi.org/10.1007/s00066-003-1027-y
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncology. https://doi.org/10.1093/neuonc/not151
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain Radiation Oncology T G, National Cancer Institute of Canada Clinical Trials G (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Inskip PD, Linet MS, Heineman EF (1995) Etiology of brain tumors in adults. Epidemiol Rev 17(2):382–414
Woernle CM, Peus D, Hofer S, Rushing EJ, Held U, Bozinov O, Krayenbuhl N, Weller M, Regli L (2015) Efficacy of surgery and further treatment of progressive glioblastoma. World Neurosurg 84(2):301–307. https://doi.org/10.1016/j.wneu.2015.03.018
Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, Mintz A (2017) Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Transl Med 6(2):471–481. https://doi.org/10.5966/sctm.2016-0007
Darefsky AS, King JT Jr, Dubrow R (2012) Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 118(8):2163–2172. https://doi.org/10.1002/cncr.26494
Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, Prazeres PHDM, Filev R, Mintz A, Birbrair A (2017) Endothelial cells maintain neural stem cells quiescent in their niche. Neurosci 363:62–65
Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavaçana N, Assoni AF, Pelatti MV, Birbrair A, de Lima ACP, Singer JM, Rocha RMM, Da Silva GL, Mantovani MS, Macedo-Souza LI, Ferrari MFR, Zatz M (2017) Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient. Stem Cell Rev Rep 13(5):686–698
Prazeres PHDM, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, Mintz A, Gonçalves R, Birbrair A (2018) Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol 38(4):777–782
Santos GSP, Prazeres P, Mintz A, Birbrair A (2017) Role of pericytes in the retina. Eye. https://doi.org/10.1038/eye.2017.220
Theodorakis PE, Muller EA, Craster RV, Matar OK (2017) Physical insights into the blood-brain barrier translocation mechanisms. Phys Biol 14(4):041001. https://doi.org/10.1088/1478-3975/aa708a
Parrish KE, Sarkaria JN, Elmquist WF (2015) Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood-brain barrier. Clin Pharmacol Ther 97(4):336–346. https://doi.org/10.1002/cpt.71
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34(1):207–217
Karim R, Palazzo C, Evrard B, Piel G (2016) Nanocarriers for the treatment of glioblastoma multiforme: current state-of-the-art. J Control Release 227:23–37. https://doi.org/10.1016/j.jconrel.2016.02.026
Oberoi RK, Parrish KE, Sio TT, Mittapalli RK, Elmquist WF, Sarkaria JN (2016) Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro-Oncology 18(1):27–36. https://doi.org/10.1093/neuonc/nov164
Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32(11):1959–1972. https://doi.org/10.1038/jcbfm.2012.126
Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, Sena IFG, Prazeres PHDM, Borges IT, Azevedo V, Mintz A, Birbrair A (2017) Endothelial Cells as Precursors for Osteoblasts in the Metastatic Prostate Cancer Bone. Neoplasia 19(11):928–931
Prazeres PHDM, Turquetti Anaelise OM, Azevedo PO, Barreto RSN, Miglino MA, Mintz A, Delbono O, Birbrair A (2018) Perivascular cell αv integrins as a target to treat skeletal muscle fibrosis. Int J Biochem Cell Biol 99:109–113
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10(1):67–84. https://doi.org/10.1016/j.scr.2012.09.003
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2015) Pericytes at the intersection between tissue regeneration and pathology. Clin Sci 128(2):81–93. https://doi.org/10.1042/CS20140278
Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, Birbrair A (2018) Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol. https://doi.org/10.1016/j.yjmcc.2018.01.014
Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A (2017) Pericytes make spinal cord breathless after injury. Neuroscientist. https://doi.org/10.1177/1073858417731522
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68(3):409–427. https://doi.org/10.1016/j.neuron.2010.09.043
Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, Huang Z, Zhai K, Ke SQ, Ping YF, Feng H, Rich JN, Yu JS, Bao S, Bian XW (2017) Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem C21(5):591–603. https://doi.org/10.1016/j.stem.2017.10.002
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153(1):139–152. https://doi.org/10.1016/j.cell.2013.02.021
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA (2001) Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 84(10):1424–1431. https://doi.org/10.1054/bjoc.2001.1796
Gotthardt D, Putz EM, Grundschober E, Prchal-Murphy M, Straka E, Kudweis P, Heller G, Bago-Horvath Z, Witalisz-Siepracka A, Cumaraswamy AA, Gunning PT, Strobl B, Muller M, Moriggl R, Stockmann C, Sexl V (2016) STAT5 is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov 6(4):414–429. https://doi.org/10.1158/2159-8290.CD-15-0732
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14(10):1014–1022. https://doi.org/10.1038/ni.2703
Azevedo PO, Paiva AE, Santos GSP, Lousado L, Andreotti JP, Sena IFG, Mintz A, Birbrair A (2018) Cross-talk between lung cancer and bones results in neutrophils that promote tumor progression. Cancer Metastasis Rev (in press)
Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, Cunha FQ, Mintz A, Birbrair A (2018) Pericytes modulate myelination in the central nervous system. J Cell Physiol 233(8):5523–5529
Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, Alvarez-Vallina L, Sanz L (2014) Lipopolysaccharide activates toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289(4):2457–2468. https://doi.org/10.1074/jbc.M113.521161
Andreotti JP, Paiva AE, Prazeres P, Guerra DAP, Silva WN, Vaz RS, Mintz A, Birbrair A (2018) The role of natural killer cells in the uterine microenvironment during pregnancy. Cell Mol Immunol. https://doi.org/10.1038/s41423-018-0023-1
Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19(3):214–223. https://doi.org/10.1038/ncb3475
Sena IFG, Paiva AE, Prazeres PHDM, Azevedo PO, Lousado L, Bhutia SK, Salmina AB, Mintz A, Birbrair A (2018) Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. https://doi.org/10.1002/cam4.1375
Balabanov R, Beaumont T, Dore-Duffy P (1999) Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J Neurosci Res 55(5):578–587
Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, Lin F (2011) Retinal pericytes inhibit activated T cell proliferation. Investig Ophthalmol Vis Sci 52(12):9005–9010. https://doi.org/10.1167/iovs.11-8008
Verbeek MM, Westphal JR, Ruiter DJ, de Waal RM (1995) T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J Immunol 154(11):5876–5884
Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN (1993) Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol 47(1):23–34
Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S (2013) Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 14(1):41–51. https://doi.org/10.1038/ni.2477
Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ (2006) Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab 26(2):209–217. https://doi.org/10.1038/sj.jcbfm.9600181
Fisher M (2009) Pericyte signaling in the neurovascular unit. Stroke 40(3 Suppl):S13–S15. https://doi.org/10.1161/STROKEAHA.108.533117
Bouchard BA, Shatos MA, Tracy PB (1997) Human brain pericytes differentially regulate expression of procoagulant enzyme complexes comprising the extrinsic pathway of blood coagulation. Arterioscler Thromb Vasc Biol 17(1):1–9
Jeynes B (1985) Reactions of granular pericytes in a rabbit cerebrovascular ischemia model. Stroke 16(1):121–125
Balabanov R, Washington R, Wagnerova J, Dore-Duffy P (1996) CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res 52(2):127–142. https://doi.org/10.1006/mvre.1996.0049
Thomas WE (1999) Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Rev 31(1):42–57
Hasan M, Glees P (1990) The fine structure of human cerebral perivascular pericytes and juxtavascular phagocytes: their possible role in hydrocephalic edema resolution. J Hirnforsch 31(2):237–249
Castejon OJ (2011) Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol 49(3):162–173
Valdor R, Garcia-Bernal D, Bueno C, Rodenas M, Moraleda JM, Macian F, Martinez S (2017) Glioblastoma progression is assisted by induction of immunosuppressive function of pericytes through interaction with tumor cells. Oncotarget 8(40):68614–68626. https://doi.org/10.18632/oncotarget.19804
Sena IFG, Paiva AE, Prazeres P, Azevedo PO, Lousado L, Bhutia SK, Salmina AB, Mintz A, Birbrair A (2018) Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. https://doi.org/10.1002/cam4.1375
Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307(1):C25–C38. https://doi.org/10.1152/ajpcell.00084.2014
Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto H, Gu C, De Palma M, Kalluri R, LeBleu VS (2015) Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell Rep 10(7):1066–1081. https://doi.org/10.1016/j.celrep.2015.01.035
Hainsworth JD, Spigel DR, Sosman JA, Burris HA 3rd, Farley C, Cucullu H, Yost K, Hart LL, Sylvester L, Waterhouse DM, Greco FA (2007) Treatment of advanced renal cell carcinoma with the combination bevacizumab/erlotinib/imatinib: a phase I/II trial. Clin Genitourin Cancer 5(7):427–432. https://doi.org/10.3816/CGC.2007.n.030
Nisancioglu MH, Betsholtz C, Genove G (2010) The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res 70(12):5109–5115. https://doi.org/10.1158/0008-5472.CAN-09-4245
Mezheyeuski A, Bradic Lindh M, Guren TK, Dragomir A, Pfeiffer P, Kure EH, Ikdahl T, Skovlund E, Corvigno S, Strell C, Pietras K, Ponten F, Mulder J, Qvortrup C, Portyanko A, Tveit KM, Glimelius B, Sorbye H, Ostman A (2016) Survival-associated heterogeneity of marker-defined perivascular cells in colorectal cancer. Oncotarget 7(27):41948–41958. https://doi.org/10.18632/oncotarget.9632
Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H (2006) Pericytes limit tumor cell metastasis. J Clin Investig 116(3):642–651. https://doi.org/10.1172/JCI25705
Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T, Imamura M (2005) Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology 69(2):159–166. https://doi.org/10.1159/000087840
Hong J, Tobin NP, Rundqvist H, Li T, Lavergne M, Garcia-Ibanez Y, Qin H, Paulsson J, Zeitelhofer M, Adzemovic MZ, Nilsson I, Roswall P, Hartman J, Johnson RS, Ostman A, Bergh J, Poljakovic M, Genove G (2015) Role of tumor pericytes in the recruitment of myeloid-derived suppressor cells. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv209
Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer cell 21(1):66–81. https://doi.org/10.1016/j.ccr.2011.11.024
Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O (2017) How plastic are pericytes? Stem cells development 26(14):1013–1019. https://doi.org/10.1089/scd.2017.0044
Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351(6269):176–180. https://doi.org/10.1126/science.aad0084
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2013) Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 305(11):C1098–C1113. https://doi.org/10.1152/ajpcell.00171.2013
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22(16):2298–2314. https://doi.org/10.1089/scd.2012.0647
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O (2014) Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 5(6):122. https://doi.org/10.1186/scrt512
Dore-Duffy P, Katychev A, Wang X, Van Buren E (2006) CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 26(5):613–624. https://doi.org/10.1038/sj.jcbfm.9600272
Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renstrom E, Svensson M, Haegerstrand A, Brundin P (2012) The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS ONE 7(4):e35577. https://doi.org/10.1371/journal.pone.0035577
Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B (2012) Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 11(4):471–476. https://doi.org/10.1016/j.stem.2012.07.007
Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsuyama T (2015) Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 33(6):1962–1974. https://doi.org/10.1002/stem.1977
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319(1):45–63. https://doi.org/10.1016/j.yexcr.2012.09.008
Birbrair A, Delbono O (2015) Pericytes are Essential for Skeletal Muscle Formation. Stem Cell Rev Rep 11(4):547–548
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2014) Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00245
Pereira LX, Viana CTR, Orellano LAA, Almeida SA, Vasconcelos AC, de Miranda Goes A, Birbrair A, Andrade SP, Campos PP (2017) Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life Sci 176:67–74
Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, Santos GSP, Goncalves R, Mintz A, Birbrair A (2018) Pericytes in the premetastatic niche. Cancer Res (in press)
Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, Santos G, Guerra D, Prazeres P, Mesquita LL, de Barros Silva LS, Leonel C, Mintz A, Birbrair A (2017) Lung as a Niche for Hematopoietic Progenitors. Stem Cell Rev Rep 13(5):567–574
Birbrair A (2017) Stem cell microenvironments and beyond. Adv Exp Med Biol 1041:1–3. https://doi.org/10.1007/978-3-319-69194-7_1
Birbrair A, Frenette PS (2016) Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 1370(1):82–96. https://doi.org/10.1111/nyas.13016
Lucas D (2017) The bone marrow microenvironment for hematopoietic stem cells. Adv Exp Med Biol 1041:5–18. https://doi.org/10.1007/978-3-319-69194-7_2
Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I (2006) Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci 9(3):331–339. https://doi.org/10.1038/nn1657
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11(1):69–82. https://doi.org/10.1016/j.ccr.2006.11.020
Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Santos GSP, Guerra DAP, Prazeres PHDM, Souto L, Mintz A, Birbrair A (2017) LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle 16(21):2018–2022
Sena IFG, Prazeres PHDM, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Guerra DAP, Lousado L, Souto L, Mintz A, Birbrair A (2017) Identity of Gli1 + cells in the bone marrow. Experimental Hematology 54:12–16
Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R (2001) Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 14(10):1651–1658
Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leuko Biol 75(3):388–397. https://doi.org/10.1189/jlb.0303114
Silva WN, Prazeres P, Paiva AE, Lousado L, Turquetti AOM, Barreto RSN, de Alvarenga EC, Miglino MA, Goncalves R, Mintz A, Birbrair A (2018) Macrophage-derived GPNMB accelerates skin healing. Exp dermatol. https://doi.org/10.1111/exd.13524
Crisan M, Corselli M, Chen WC, Peault B (2012) Perivascular cells for regenerative medicine. J Cell Mol Med. https://doi.org/10.1111/j.1582-4934.2012.01617.x
Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33(34):13882–13887. https://doi.org/10.1523/JNEUROSCI.2524-13.2013
Lousado L, Prazeres PHDM, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A (2017) Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis 8(10):e3072
Dahl D, Zapatka S, Bignami A (1986) Heterogeneity of desmin, the muscle-type intermediate filament protein, in blood vessels and astrocytes. Histochemistry 84(2):145–150
Choi JH, Riew TR, Kim HL, Jin X, Lee MY (2017) Desmin expression profile in reactive astrocytes in the 3-nitropropionic acid-lesioned striatum of rat: characterization and comparison with glial fibrillary acidic protein and nestin. Acta Histochem 119(8):795–803. https://doi.org/10.1016/j.acthis.2017.10.003
Andreotti JP, Prazeres PHDM, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A (2018) Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci 67:33–36
Pruimboom-Brees IM, Brees DJ, Shen AC, Ibebunjo C (2004) Malignant astrocytoma with binucleated granular cells in a Sprague-Dawley rat. Vet Pathol 41(3):287–290. https://doi.org/10.1354/vp.41-3-287
Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, Delbono O, Birbrair A (2017) Pericytes are heterogeneous in their origin within the same tissue. Dev Biol 427(1):6–11. https://doi.org/10.1016/j.ydbio.2017.05.001
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502(7473):637–643. https://doi.org/10.1038/nature12612
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160(3):985–1000. https://doi.org/10.1016/S0002-9440(10)64920-6
Nehls V, Denzer K, Drenckhahn D (1992) Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res 270(3):469–474
Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O, Rota M (2011) Nestin-GFP Transgene Reveals Neural Precursor Cells in Adult Skeletal Muscle. PLoS ONE 6(2):e16816
Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J (2011) A pericyte origin of spinal cord scar tissue. Science 333(6039):238–242. https://doi.org/10.1126/science.1203165
Kaukonen J, Lahtinen I, Laine S, Alitalo K, Palotie A (1996) BMX tyrosine kinase gene is expressed in granulocytes and myeloid leukaemias. Br J Haematol 94(3):455–460
Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, Shou W, Hambardzumyan D, Lee J, Hjelmeland AB, Sloan AE, Bredel M, Stark GR, Rich JN, Bao S (2011) Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 19(4):498–511. https://doi.org/10.1016/j.ccr.2011.03.004
Andreotti JP, Lousado L, Magno LAV, Birbrair A (2017) Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell 21(3):293–294. https://doi.org/10.1016/j.stem.2017.08.005
Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML Jr, Mintz A, Birbrair A (2017) Adipocytes role in the bone marrow niche. Cytom Part A. https://doi.org/10.1002/cyto.a.23301
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ (2010) The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 107(29):13075–13080. https://doi.org/10.1073/pnas.1004594107
Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, Siegal D (2016) The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood 128(1):138–140. https://doi.org/10.1182/blood-2016-05-712828
Brown JR, Hillmen P, O’Brien S, Barrientos JC, Reddy NM, Coutre SE, Tam CS, Mulligan SP, Jaeger U, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Thornton P, Caligaris-Cappio F, Delgado J, Montillo M, DeVos S, Moreno C, Pagel JM, Munir T, Burger JA, Chung D, Lin J, Gau L, Chang B, Cole G, Hsu E, James DF, Byrd JC (2018) Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 32(1):83–91. https://doi.org/10.1038/leu.2017.175
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O’Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ, Investigators R- (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373(25):2425–2437. https://doi.org/10.1056/NEJMoa1509388
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P, Investigators R (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371(3):213–223. https://doi.org/10.1056/NEJMoa1400376
O’Brien S, Jones JA, Coutre SE, Mato AR, Hillmen P, Tam C, Osterborg A, Siddiqi T, Thirman MJ, Furman RR, Ilhan O, Keating MJ, Call TG, Brown JR, Stevens-Brogan M, Li Y, Clow F, James DF, Chu AD, Hallek M, Stilgenbauer S (2016) Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol 17(10):1409–1418. https://doi.org/10.1016/S1470-2045(16)30212-1
Acknowledgements
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708-15285, a Grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); a Grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570-16)], and a Grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313-16)]; Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar Grant (124443-MRSG-13-121-01-CDD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Guerra, D.A.P., Paiva, A.E., Sena, I.F.G. et al. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis 21, 667–675 (2018). https://doi.org/10.1007/s10456-018-9621-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9621-x