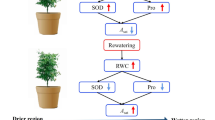
Abstract
Artemisia selengensis is a typical wetland plant with valuable nutritional and medical purposes, and its growth and field distribution is highly dependent on water conditions. However, wetland hydrology is becoming more complex due to global climate change, and the future response of A. selengensis to water deficits and rehydration is uncertain. We here conducted simulations to investigate physiological variations in A. selengensis in response to varying degree of soil moisture (85–90%, 60–65%, 45–50%, and 30–35%) and re-watering. Results show that drought boosted root vigor and increased water and soil nutrient absorption in A. selengensis. As drought conditions progressed, the superoxide anion (\({\text{O}}_{2}^{ - }\)) and malondialdehyde content significantly increased. This led to increased activity of antioxidant enzymes, which significantly inhibited \({\text{O}}_{2}^{ - }\) content. Root vigor and peroxidase (POD) activity were fully recovered after rehydration. The \({\text{O}}_{2}^{ - }\) and MDA content, and catalase (CAT) activity also fully recovered under moderate and mild drought, although full recovery can take longer under severe drought. Superoxide dismutase (SOD) activity decreased significantly after rehydration, but SOD activity in drought conditions has not yet recovered to the control level. A. selengensis is drought tolerant due to its high root vigor and the regulation of antioxidant enzyme system. Our results may provide guidance for the future population dynamics of wetland ecosystems under climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic and biotic stresses are common challenges in the life history of plants (Gechev and Petrov 2020), such as drought, flooding, salinity, temperature extremes, light conditions, chemical toxicity, and pathogen attacks (Gill and Tuteja 2010; Atapaththu and Asaeda 2015; Garssen et al. 2015). Among these stress factors, drought is one of the most severe abiotic challenges that plants face (Mahajan and Tuteja 2005). Drought causes water loss in plant cells, leading to major changes in plant morphology (Eziz et al. 2017), physiology and biochemistry (Zhanassova et al. 2021), and limits the ecological niche and survival space of many plants (Gaviria et al. 2017). Various evidence shows that global warming and climate change have been exacerbated by the impact of human activities, and subsequently has increased the amplitude of climate system variability (IPCC 2021). This can be observed in the increased frequency and intensity of extreme heat events, extreme precipitation events, and agricultural and ecological droughts in some regions (Ummenhofer and Meehl 2017). Water within these regions is unevenly distributed both spatially and temporally, where plants usually undergo cycles of drought and rehydration throughout their life cycle. Therefore, the study of plant adaptation and tolerance mechanisms to drought, as well as recovery ability after re-watering, is critical in predicting the impacts of ongoing climate change (John et al. 2018).
There have been many studies on plant response mechanisms to abiotic stress (Bornette and Puijalon 2011; Li et al. 2013; Das et al. 2016), and part of those results show that drought stress induces excess reactive oxygen species (ROS) and causes damage to the membrane system of plant cells (Voss et al. 2013; Guan et al. 2015). ROS are also produced continuously as by-products of various metabolic pathways that are localized in different cellular organelles such as chloroplast, mitochondria and peroxisomes (Navrot et al. 2007). Under stress, the balance of intracellular ROS production and scavenging is disrupted, and excessive accumulation of ROS can directly or indirectly trigger membrane lipid peroxidation, of which malondialdehyde (MDA) is the final product (Cuin and Shabala 2008). Thus, ROS concentration can be used as an important indicator to monitor the physiological state of plants and to quantify the plant response to the intensity of environmental stress (Asaeda et al. 2022). Superoxide anion (\({\text{O}}_{2}^{ - }\)) is a major reactive oxygen species, and its accumulation seriously damages plants through lipid peroxidation, protein degradation, breakage of DNA and cell death (Gill and Tuteja 2010). To cope with the increased ROS level, plants possess well-developed antioxidative systems which are composed of non-enzymic defense and enzymatic antioxidants to scavenge overproduced ROS (Mansoor et al. 2022), thereby avoiding deleterious effects. Superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) are major components of the antioxidant protective enzyme system, which can reduce the toxic effect of ROS on cells (Bhaskaran and Panneerselvam 2013); therefore, they are often used as physiological biomarkers of crop stress resistance. The root is the first affected part of a plant directly faced with drought stress (Brunner et al. 2015) and the major organ for the uptake of water and nutrients (Kim et al. 2020). It is widely acknowledged that changes in root architecture and metabolism can influence aboveground characteristics, such as seed yield and photosynthesis (Cui et al. 2016). Therefore, root development and activity are inhibited when plants are grown in a water-deficit environment, which causes a series of depressive effects on the physiological functions of above-ground parts. In addition, root vigor is considered an essential physiological characteristic for measuring the growth of terrestrial plants, which can reflect the absorptive, synthetic, oxidative, and reducing capacities of the root system (Kajikawa et al. 2010). Therefore, a sufficient level of root vigor is crucial for plants to adapt to drought and to recover normal metabolism after the stress is relieved.
In the middle and lower reaches of the Yangtze River in China, A. selengensis is a herbaceous perennial plant of the Compositae family that is widely distributed in wetlands, marshes, wet meadows, and freshwater lake meadows (Zhang et al. 2018). A. selengensis has been used as wild vegetable and medicinal herb in China, and a variety of antioxidants and anticarcinogenic substances can be extracted from the plant (Zhang et al. 2015; Wang et al. 2016). Most of the current research on the effects of drought stress on plants has focused on crops (Voronin et al. 2019; Auler et al. 2021) and medicinal herbs (Yan et al. 2015). Other studies considered the physiological response of plants to re-watering after drought stress, suggesting that re-watering has a compensatory effect on the growth and physiological characteristics of plants (Liu et al. 2021). Most of these previous studies focused on arid plants distributed in arid and semi-arid regions. However, compared with arid plants, wetland plants are endangered by drought stress and also face the situation of rising water tables, and these stresses have magnified under the background of climate change and frequent extreme weather (Deng et al. 2022). When the water level in a wetland exceeds or is below the ecological amplitude of wetland plants, it will lead to poor growth and possibly mortality (Luan et al. 2013; Yan et al. 2020). However, less attention has been paid to drought stress in wetland plants growing in wet areas over time, and no studies have been reported on the physiological characteristics of A. selengensis in response to drought stress and rehydration.
We here investigate the physiological response of A. selengensis to different drought levels and re-watering through simulation experiments to reveal the adaptation mechanism of A. selengensis to soil water content changes.
Materials and methods
Plant materials
A. selengensis seedlings were used as our primary experimental materials. On January 13, 2021, we collected thriving, homogeneous-sized wild A. selengensis from Poyang Lake National Wetland Park, Jiangxi Province, China (Fig. 1). Poyang Lake is the largest freshwater lake in China with a natural connection to the Yangtze River. The lake is coupled to the river flow and stage, often experiencing dramatic water-level fluctuations and backflow, and these processes are being exacerbated by climate change and human activities. Thus, the plants in the wetland are subjected to intermittent droughts and floods. The collected seedlings were brought back to the laboratory and cut into small sections about 5 cm long, each section having one or two lateral buds. The stems were planted in plastic pots (35 × 26 × 13 cm3) with the bottom part buried in the soil for about 2 cm, and 30 plants were planted in each pot. We precultured the A. selengensis seedlings for subsequent experimental treatments. The substrate soil used for preculture was extracted from Aixi Lake National Wetland Park, Nanchang City, Jiangxi Province, China. The basic properties of the soil are shown in Table 1.
Experimental design
The experiment was conducted in the plant sunlight culture room at the Key Laboratory of Poyang Lake Wetland and Watershed Research, Ministry of Education, Jiangxi Province, China, from March to June 2021. The test seedlings were exposed to four soil water contents during the drought treatment: Plants were watered to 85–90% of soil saturated water content (control group, CK), 60–65% of soil saturated water content (mild drought, MID), 45–50% of soil saturated water content (moderate drought, MD) and 30–35% of soil saturated water content (severe drought, SD). In the rehydration stage, all treatment groups were watered to 95–100% of soil saturated water content. Three pots of A. selengensis seedlings were placed in each test group, totaling 12 pots. Before the experiment, soil water content was measured using an HH2 soil moisture meter (Delta-T, UK) and controlled to the setting drought levels by adjusting the watering rate and natural evaporation. To maintain the soil water content at the set levels in all treatment groups during the experiment, it was measured daily at 17:00 with soil moisture meter, followed by quantitative water replenishment. The drought experiment began on March 23 and ended on June 1, 2021, for 70 days. All treatment groups were rehydrated to SWC after 70 days and rehydrated for 28 days. The overall duration of the experiment was 98 days. During the experiment, the culture room was naturally illuminated with an average air temperature of 26.1 ± 5.9 °C and average air humidity of 77.09 ± 14.08%.
Analyzed parameters
Sampling
On days 0, 14, 28, 42, 56, and 70 after initiation of the drought treatment and days 14 and 28 after re-watering, we measured the physiological parameters of plants in each pot. On the sampling day, we chose all-green functional leaves except for the top three leaves that were used as samples, because this could reflect the function of plant leaves without affecting photosynthesis. The root of a part of the seedlings was excavated and washed to measure root vigor. Three samples were collected per potted plant (n = 3), and all samples were immediately frozen.
Root vigor assay
The TTC method (Zhang et al. 2019) was used to measure the root vigor of plants. Dehydrogenases in plant roots reduce TTC and produce red, water-insoluble trithizone. Therefore, the amount of TTC reduction can indicate dehydrogenase activity and serve as an indicator of root vigor. We next weighed 200 mg of fresh root sample in a test tube, added 5 mL each of 0.4% TTC solution and 1/15 mM phosphate buffer solution (PBS) and soaked for 3 h. The reaction was then terminated by adding sulfuric acid. The root sample was taken, ground with ethyl acetate and fixed to 5 mL. The absorbance was measured by spectrophotometer at 485 nm (Jinghong Experimental Equipment Co., Ltd., Shanghai, China), and the amount of TTC reduction was obtained from the standard curve.
Determination of MDA and \({\text{O}}_{2}^{ - }\) content
MDA content was determined by the thiobarbituric acid (TBA) method (Dong et al. 2019). In total, 500 mg of fresh leaf samples was ground with 5% trichloroacetic acid (TCA), and the ground homogenate was centrifuged at 3000 r/min for 10 min. Two milliliters of upper supernatant was added with 2 mL of 0.67% TBA, mixed thoroughly, placed in a boiling water bath for 30 min, cooled rapidly and centrifuged again. The absorbance of the extracts was measured at 450 nm, 532 nm and 600 nm, respectively. The concentration of MDA in the extract can be used to determine MDA content in the sample using:
where C represents the MDA concentration in the extract; A450, A532 and A600 represent the absorbance values at 450 nm, 532 nm and 600 nm, respectively.
The \({\text{O}}_{2}^{ - }\) content was determined using the hydroxylamine oxidation method (Li et al. 2010). The fresh leaf tissue (200 mg) was ground in 5 mL of PBS (pH 7.8), and the homogenate was centrifuged at 3000 r/min for 10 min. After adding 0.5 mL of 50 mM PBS (pH 7.8) and 1 mL of 10 mM hydroxylamine hydrochloride to the supernatant (0.5 mL), the mixture was incubated at 25 °C for 1 h and then adding 1 mL of 17 mM sulfanilamide and 1 mL of 7 mM naphthylamine. After standing for 20 min at 25 ℃, the absorbance of the solution was measured at 530 nm. The \({\text{O}}_{2}^{ - }\) content was calculated using the nitrite radical (N\({\text{O}}_{2}^{ - }\)) standard curve.
Determination of SOD activity
The 200 mg fresh leaf samples were ground with PBS (pH 7.8, contains 1% polyvinylpyrrolidone) and fixed to 5 mL and then centrifuged (4000 r/min) to obtain the supernatant. SOD activity was measured based on the inhibition of the reduction of nitrogen blue tetrazolium (NBT) by SOD under light conditions. The 50 mM PBS (pH 7.8), 130 mM methionine, 100 μM EDTA, 750 μM NBT and 20 μM riboflavin were added to 0.05 mL supernatant, and the mixture was reacted under light (4000 Lx) for 20 min, and then the absorbance was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme that inhibits 50% NBT photoreduction.
Determination of POD activity
The POD activity was measured by guaiacol method. Two hundred milligrams of fresh leaves was chopped, ground in a mortar with PBS (pH 5.5, contains 1% polyvinylpyrrolidone) and fixed at 5 mL to obtain homogenate, which was then centrifuged (3000 r/min) for 10 min to obtain the supernatant, then refrigerated and stored. The reaction system for the POD assay included 50 mM PBS (2.9 mL), 2% H2O2 (1 mL), 50 mM guaiacol (1 mL) and supernatant (0.1 mL). The mixture was held at 37 °C for 15 min, and then the reaction was terminated by adding 20% trichloroacetic acid (2 mL). The boiled enzyme supernatant was used as a control. The absorbance was measured at 470 nm after centrifugation and read once per minute for 4 min, and the change in absorbance of 0.01 per minute was used as one POD activity unit.
Determination of CAT activity
The UV absorption method was used to assay CAT activity. First, 200 mg of fresh leaves was ground and fixed with PBS (contains 1% polyvinylpyrrolidone) of pH 7.8 to obtain a homogenate, which was then centrifuged at 4000 r/min for 15 min, and the supernatant was the crude CAT extract. The extracts (0.2 mL), 0.2 M PBS (1.5 mL) and 0.1 M H2O2 (0.3 mL) were mixed, and then the absorbance was measured at 240 nm and read once per minute for four minutes. The amount of enzyme decreasing by 0.1 per minute of absorbance was used as one CAT activity unit. The determination of SOD, POD and CAT activity was based on the Principles and Techniques of Plant Physiological and Biochemical Experiments (Wang 2006).
Statistical analysis
At least three replicates were used in this study, and all data are presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA) software, and the effects of drought and rehydration on A. selengensis were analyzed by one-way variance analysis (ANOVA) using Duncan’s multiple range tests (p < 0.05). Two-way variance analysis (two-way ANOVA) was used to analyze the treatment × time interaction with the physiology of A. selengensis. Pearson’s correlations were calculated to measure the relation of the variables. All figures in this paper were produced by Origin 2021b.
Results
Effects of drought and re-watering on root vigor
Root vigor showed different behavior depending on the soil moisture (Fig. 2), and the effects of soil moisture, duration and their interactive effect on root vigor were highly significant as shown in Table 2 (p < 0.001). The trends of root vigor in different drought groups were consistent during the drought period. Root vigor in the drought groups increased rapidly with treatment time for days 0–28 days, and each group’s root vigor was ranked as: SS > MD > MID > CK. For days 42–70, root vigor decreased and then increased and reached a maximum at 70 days, and root vigor in the drought groups was significantly higher than that of the CK group (p < 0.001). After 14 days of re-watering, the root vigor increased in all treatments as compared to 70 days, and the SD, MD, MID, and CK groups increased by 14.00, 20.06, 13.65, and 1.97 mg.g−1.h−1, respectively, with significant differences (p < 0.001). After 28 days of rehydration, root vigor decreased in all groups, and the root vigor in drought groups was significantly different than pre-rehydration (p < 0.01). The root vigor under SD was significantly higher than that of the CK group (p < 0.001), the MD group was significantly lower than that of the CK group (p < 0.001), and the MID group was not significantly different from the CK group.
Effects of different levels of drought and re-watering on root vigor. RW14: 14th day of re-watering, RW28: 28th day of re-watering, different capital letters stand for significant differences between different experimental times (p < 0.05), and different lowercase letters stand for significant differences between different drought groups (p < 0.05)
Effects of drought and re-watering on MDA content
The MDA content of drought groups increased for days 0–28, decreasing for days 28–42 and increasing again for days 42–70, while the MDA content of the CK group was relatively stable in 0–56 days (Fig. 3). During the drought period, the MDA content in the SD, MD and MID groups was generally significantly higher than that of the CK group (p < 0.05). Longer drought treatment time increased the difference in MDA content between drought groups, and that of the SD group was significantly higher than that of other treatment groups at 70 days (p < 0.05). The change of MDA content was influenced by the drought degree, but drought duration also affected it significantly (p < 0.001; Table 2). After 14 days of re-watering, each group showed different changes, and MDA content increased in the MD, MID and CK groups, while it decreased in the SD group. The MDA content of the CK group was significantly higher than those of drought groups, and the SD group was significantly lower than the MD group and the MID group (p < 0.001), while those of MD and MID were closer and the difference was not significant. After 28 days of rehydration, the MDA content of each group decreased, and that of drought groups was even significantly lower (p < 0.05) than pre-rehydration. The content of MDA in the SD, MD and MID groups was closer and did not show significant differences. However, they were significantly lower than the CK group (p < 0.001).
Effects of different levels of drought and re-watering on MDA content. RW14: 14th day of re-watering, RW28: 28th day of re-watering, different capital letters stand for significant differences between different experimental times (p < 0.05), and different lowercase letters stand for significant differences between different drought groups (p < 0.05)
Effects of drought and re-watering on \({\text{O}}_{2}^{ - }\) content
During the drought period, the \({\text{O}}_{2}^{ - }\) content fluctuated significantly among drought groups, which first increased and then decreased (Fig. 4). For days 0–28 days of drought treatment, the \({\text{O}}_{2}^{ - }\) content was not significantly different among the SD, MD and MID groups, and was generally higher than that of the CK group (p < 0.001). For days 42–70, the \({\text{O}}_{2}^{ - }\) content in drought groups was gradually lower than that of the CK group, and the \({\text{O}}_{2}^{ - }\) content was lower with deeper drought. As shown in Table 2, the \({\text{O}}_{2}^{ - }\) content was significantly (p < 0.001) affected by the drought level and treatment duration; thus, it varied with drought time and level. After re-watering, the \({\text{O}}_{2}^{ - }\) content increased in all groups and the difference was highly significant (p < 0.001) compared to 70 days. The \({\text{O}}_{2}^{ - }\) content of each group was ranked as MD > MID > SD > CK, and there was a highly significant difference between these groups (p < 0.001). After 28 days of rehydration, the \({\text{O}}_{2}^{ - }\) content in all groups decreased, and that of the MD group and MID group was significantly lower (p < 0.05) than pre-rehydration. In this case, the \({\text{O}}_{2}^{ - }\) content differed significantly among the different treatment groups, which were higher in the SD group and lower in the MD and MID groups compared to the CK group. In general, the \({\text{O}}_{2}^{ - }\) content trend was consistent with MDA under drought and rehydration.
Effects of different levels of drought and re-watering on \({\text{O}}_{2}^{ - }\) content. RW14: 14th day of re-watering, RW28: 28th day of re-watering, different capital letters stand for significant differences between different experimental times (p < 0.05), and different lowercase letters stand for significant differences between different drought groups (p < 0.05)
Effects of drought and re-watering on antioxidative enzyme activity
SOD activity was significantly (p < 0.001) influenced by drought level, treatment time and their interaction effects (Table 2). The SOD activity was significantly higher during days 0–28 (Fig. 5a), and the SOD activity in drought groups was higher than that of the CK group at 28 days (p < 0.01). At 42 days, the SOD activity in the MID group was lower than that of other groups (Table 3A), and that of the SD group and MD group was significantly different from the CK group (p < 0.001). At 70 days, the SOD activity of the MID group reached the maximum value followed by SD, MD and CK, respectively, exhibiting significant differences among treatments (p < 0.001). After re-watering, the SOD activity significantly decreased in all treatments compared to that of 70 days (p < 0.001). The comparison between groups revealed that the SOD activity of the MD group was significantly higher than that of the CK group at 14 days after rehydration (p < 0.001), while that of the SD group and the MID group was statistically similar to the CK group. The SOD activity of the SD group and MID group changed slightly from 14 to 28 days after re-watering, and that of drought groups was significantly higher than that of the CK group (p < 0.05).
POD activity was also significantly (p < 0.001) influenced by the drought level, treatment time and their interaction effects (Table 2). The POD activity of all groups showed an increase followed by a decrease during the drought treatment stage (Fig. 5b). For days 0–28, the POD activities of all drought groups were significantly lower than that of the CK group (p < 0.001) (Table 3B). For days 42 to 56, the POD activity of the MD and MID groups was significantly higher than that of the CK group, while that of the SD group was lower (p < 0.001). At 70 days, the maximum POD activity was found in the CK treatment followed by MD, MID and SD, respectively, with significant differences seen among treatments at the p = 0.01 level. After 14 days of re-watering, POD activity significantly increased as compared with 70 d (p < 0.001), and the difference between groups was significant (p < 0.001). After 28 days of rehydration, the POD activity of the SD group continued to increase, while other groups gradually decreased. The POD activity of the MD group and MID group was significantly lower than that of the CK group (p < 0.001), but the POD activity of SD group was recovered to the control level.
The activity of CAT was similar to SOD and POD activities, being significantly (p < 0.001) influenced by the drought level, treatment time and their interaction effects (Table 2). Similar to POD, the CAT activity showed an increase followed by a decrease during the drought treatment (Fig. 5c). During this period, the CAT activity of the drought groups was always lower than that of the CK group. In general, the stronger the drought level, the higher the CAT activity. At 70 days, the order of the CAT activity in the different groups was: CK > SD > MD > MID, and the drought groups differed significantly (p < 0.01) from the CK group (Table 3C). After 14 days of re-watering, CAT activity increased significantly in the drought groups, which was significantly different from that of 70 days (p < 0.001), and CAT activity in the SD group showed the largest growth. The CAT activity of drought groups was significantly higher than that of the CK group (p < 0.01) at this time. After 28 days of rehydration, the CAT activity of each group gradually decreased. The CAT activity of the SD group was significantly higher than that of the CK group (p < 0.001), while the MD and MID groups recovered to the control level.
Correlation analysis of physiological parameters of A. selengensis leaves
The correlations between root vigor, MDA, \({\text{O}}_{2}^{ - }\), SOD, POD and CAT were next analyzed in more detail (Table 4). MDA and SOD showed a significant positive correlation with root vigor (p < 0.01). The responses of MDA, SOD and root vigor to water changes were consistent. MDA was positively correlated with SOD, and their relationship was statistically significant (p < 0.01). In addition, there is a strong relationship between antioxidant enzymes, SOD is positively correlated with CAT (p < 0.05), and POD is positively correlated with CAT (p < 0.01). It is thus highly suggested that multiple antioxidant enzymes in plants during drought stress work together to eliminate cell damage caused by ROS.
Discussion
Drought is a key factor limiting plant growth and development. Root vigor has been proven to be a reliable and sensitive indicator for assessing drought resistance (Lan et al. 2022) and reflects root metabolic activity (Liu et al. 2015). Previous studies have found that drought stress greatly inhibits the root growth and vigor, such as the root vigor of the aquatic plants Arundo donax var. versicolor and Typha orientalis decreases with the increase of groundwater depth (Chen et al. 2021a, b; Huang et al. 2021). However, we demonstrated here that different levels of drought can increase the root vigor of A. selengensis, similar to terrestrial plants. For example, studies comparing oxidative damage and antioxidant responses of two cotton cultivars found that drought-resistant cotton showed increased root length and vigor for better productivity under water-deficit conditions (Zhang et al. 2014). Increased root vigor in potatoes can also accelerate phosphorus acquisition and thus increases yield (White et al. 2018). It has been suggested that drought-tolerant plant species promote aboveground biomass accumulation by maintaining higher root vigor under drought conditions, which is a physiological mechanism for their higher drought tolerance (Li et al. 2020). Similarly, A. selengensis seedlings may transport more photosynthetic products to the roots under drought conditions, enhancing the roots in up-taking soil nutrients and water by increasing root vigor. In addition, A. selengensis adapts to soil water deficit by increasing root vigor and also shows a compensatory effect after re-watering, which increases with the drought degree and duration (Li et al. 2021).
Cell membranes are one of the main targets of environmental pressure. Lipids are important membrane components that contribute to membrane integrity and keep cell compartmentalization during drought stress (Gigon et al. 2004). MDA is the end product of membrane lipid peroxidation in plants and represents the degree of damage to cell membranes by ROS. Here, we found that the MDA content under drought treatment was higher than that of the CK group. It indicates that peroxidative damage occurred in the cell membrane of A. selengensis, which led to an accumulation of an excess level of MDA in cell tissues. In addition, the MDA content of the drought groups was substantially reduced at 42 days, indicating that A. selengensis can adapt to drought after sufficient time, and changes in MDA under drought conditions are generally considered to be related to the drought tolerance and antioxidant system activity of the plants (Shao et al. 2007). However, the regulatory capacity of antioxidant system was clearly limited, as evidenced by the re-increase of MDA content at the later stages of drought. The observation of MDA after re-watering is coherent with studies of wheat seedlings, in which MDA content first increased and then decreased (Maevskaya and Nikolaeva 2013). In those results, the initial elevation in MDA content is clearly related to the activation of oxidative processes, but plant metabolism normalizes with the extension of rehydration time and so MDA content begins to decrease. Interestingly, the MDA content of control group in our study increased at a later part of the experiment. A possible explanation for this could be that saturated soil moisture is not optimal for the survival of A. selengensis, which can be verified by inhibited root vigor and elevated \({\text{O}}_{2}^{ - }\).
Plants produce reactive oxygen species such as \({\text{O}}_{2}^{ - }\) and H2O2 when subjected to stress. ROS are well known to cause lipid peroxidation and oxidative damage, which leads to the disruption of metabolic functions and loss of cellular integrity at their accumulation sites (Foyer et al. 1997). Previous work found increased \({\text{O}}_{2}^{ - }\) content in Chrysanthemum under drought stress (Sun et al. 2013), and similar changes were observed in A. selengensis. Additionally, we observed that the \({\text{O}}_{2}^{ - }\) content of A. selengensis gradually decreased with the duration of drought. These results suggest that water deficit leads to excessive accumulation of ROS, with serious negative effects for plants. This is consistent with studies on tea trees, purslane and other species (Hrishikesh et al. 2008; Jin et al. 2015). However, contrary results showed that A. selengensis can effectively scavenge ROS within a limited range by way of antioxidant enzymes, etc. This scavenging capacity is not limitless, however, and the antioxidant system of A. selengensis can be damaged by persistent drought so that \({\text{O}}_{2}^{ - }\) content is increased again in the later stages of drought. After 14 days of rehydration, \({\text{O}}_{2}^{ - }\) production and scavenging had not yet reached balance. It is thought that the increase in \({\text{O}}_{2}^{ - }\) content may be attributed to two reasons (Hura et al. 2015): One is the cumulative and lagged response of plant physiology to soil drought; the other is the negative effect of excessively rapid rehydration on plants under drought stress. The disruptive effects of rapid rehydration are expressed in the intensification of physiological processes associated with ROS production. It is suggested that the key reason for the over-production of ROS after rehydration could be the electron leakage due to the overloading of the electron transport chain (ETC) in PSI and PSII (Edreva 2005), leading to the reduction of molecular oxygen to superoxide radicals. Rapid rehydration may have further activated the peroxidation process (Maevskaya and Nikolaeva 2013). After 28 days of rehydration, with the exception of the SD group, the \({\text{O}}_{2}^{ - }\) content in drought groups was significantly lower than the control level. One possibility is that the plant’s antioxidant enzyme systems under severe drought were so seriously disrupted that they required a longer time than our study allowed to return to normal levels, or were unable to recover at all.
Drought disturbs the balance of ROS metabolism in plants. When the concentration of ROS is excessive, the balance of membrane lipid peroxidation and cellular material exchange is also disrupted, leading to a range of physiological and metabolic disorders (Gill and Tuteja 2010). To combat these illnesses, plants have evolved protective enzymes over long evolutionary period. The collaboration (or compensation) between antioxidant enzymes is a decisive factor in the capacity of the antioxidant (Blokhina et al. 2003), and they can eliminate ROS such as \({\text{O}}_{2}^{ - }\) and H2O2 as well as reduce the damage they cause to plants (Smirnoff 1993). Here, SOD and \({\text{O}}_{2}^{ - }\) were significantly positively correlated, both showing a bimodal trend, while POD and CAT increased and then decreased during the drought treatment. The POD and CAT activity reached a maximum in the middle of drought period, and the \({\text{O}}_{2}^{ - }\) and MDA content decreased during this period (Figs. 3, 4). The results indicate that the SOD is effective in scavenging \({\text{O}}_{2}^{ - }\) and protecting cells from peroxidative damage. Since SOD decomposes \({\text{O}}_{2}^{ - }\) to H2O2, which is then converted to H2O by POD and CAT (Blokhina et al. 2003), the increases in POD and CAT may be due to elevated H2O concentration. The physiological mechanism of drought tolerance in A. selengensis is similar to that of the sand plants Agriophyllum squarrosum and Setaria viridis, in that the accumulated ROS stimulates the antioxidant enzyme protection system to continuously increase the enzyme activity, thus maintaining the ROS balance (Chen et al. 2021a, b), showing a strongly ROS control and scavenging ability. However, the antioxidant enzyme system of A. selengensis may have been damaged with the longer drought time, leading to a decrease in enzyme activity and a rise in ROS. During the drought period, the POD and CAT activities of the CK group were quite elevated, indicating that too much soil moisture can be detrimental to A. selengensis and leads to an increase in antioxidant enzyme activity.
After rehydration, the SOD activities were significantly reduced, but these of drought groups were still slightly higher than that of the CK group, indicating that SOD activities were not fully recovered. The POD and CAT activities increased substantially at 14 days of rehydration, and a key reason for the inability of POD and CAT to recover may be that the \({\text{O}}_{2}^{ - }\) content was still increasing (Upadhyaya and Panda 2004) and recovered slowly during subsequent rehydration, which also matched the persistently high MDA and \({\text{O}}_{2}^{ - }\) content after rehydration (Figs. 3, 4). Other studies have also suggested that the lack of recovery of antioxidant enzymes after rehydration may be related to the fact that plant physiological metabolism has not improved immediately, meaning that there is a lag in plant response to soil moisture (Hura et al. 2015). Alternatively, their antioxidant enzyme systems may be disrupted and take longer than our research period allowed to restore to normal levels (Chen et al. 2021a, b). As expected, both POD and CAT activities gradually decreased after 28 days of rehydration, and these of all groups recovered to that of the CK group, except for CAT under severe drought. The synergistic changes in \({\text{O}}_{2}^{ - }\) content and antioxidant enzyme activities suggest that the antioxidant enzyme system also responds to water stress and then scavenges ROS to protect cells from excessive oxidative damage.
Conclusion
The physiological response of plants to environmental stresses was often more sensitive than changes in morphological features. Here, we observed that different drought levels contributed to improved root vigor. The changes in \({\text{O}}_{2}^{ - }\) and MDA content were consistent, indicating that drought induced an excessive accumulation of \({\text{O}}_{2}^{ - }\) and caused peroxidative damage to plant cells. Antioxidant enzymes had a major regulatory effect on \({\text{O}}_{2}^{ - }\), particularly SOD activity, which was positively correlated with \({\text{O}}_{2}^{ - }\) content. The rehydration compensated root vigor, membrane lipid peroxidation and antioxidant enzyme activity in A. selengensis. However, it is remarkable that long-term soil saturation moisture also produced oxidative stress on A. selengensis, which was expressed by the progressively higher MDA and \({\text{O}}_{2}^{ - }\) contents as well as inhibited root vigor in the control group. Thus, A. selengensis was unsuitable for high water conditions and even highly drought tolerant, although it is a wetland plant. The physiological indicators monitored in this study have the potential to predict the distribution of A. selengensis under the context of climate change.
References
Asaeda T, Rahman M, Vamsi-Krishna L, Schoelynck J, Rashid MH (2022) Measurement of foliar H2O2 concentration can be an indicator of riparian vegetation management. Sci Rep 12(1):13803. https://doi.org/10.1038/s41598-022-17658-2
Atapaththu K, Asaeda T (2015) Responses of freshwater macrophytes to fluctuations in abiotic stress vectors. 91–116
Auler PA et al (2021) Stress memory of physiological, biochemical and metabolomic responses in two different rice genotypes under drought stress: the scale matters. Plant Sci. https://doi.org/10.1016/j.plantsci.2021.110994
Bhaskaran J, Panneerselvam R (2013) Accelerated reactive oxygen scavenging system and membrane integrity of two panicum species varying in salt tolerance. Cell Biochem Biophys 67(3):885–892. https://doi.org/10.1007/s12013-013-9576-x
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194. https://doi.org/10.1093/aob/mcf118
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73(1):1–14. https://doi.org/10.1007/s00027-010-0162-7
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00547
Chen J, Zhao X, Li Y, Luo Y, Zhang Y, Liu M, Li Y (2021a) Physiological responses of Agriophyllum squarrosum and Setaria viridis to drought and re-watering. Sci Rep-Uk. https://doi.org/10.1038/s41598-021-98246-8
Chen Y, Cao Y, Tang S, Huang H, Qi J (2021b) Study on water level ecological amplitude of Typha orientalis seedlings. J Freshw Ecol 36(1):293–304. https://doi.org/10.1080/02705060.2021.1975581
Cui X, Dong Y, Gi P, Wang H, Xu K, Zhang Z (2016) Relationship between root vigour, photosynthesis and biomass in soybean cultivars during 87 years of genetic improvement in the northern China. Photosynt 54(1):81–86. https://doi.org/10.1007/s11099-015-0160-z
Cuin TA, Shabala S (2008) Compatible solutes mitigate damaging effects of salt stress by reducing the impact of stress-induced reactive oxygen species. Plant Signal Behav 3(3):207–208. https://doi.org/10.4161/psb.3.3.4966
Das SK, Patra JK, Thatoi H (2016) Antioxidative response to abiotic and biotic stresses in mangrove plants: a review. Int Rev Hydrobiol 101(1–2):3–19. https://doi.org/10.1002/iroh.201401744
Deng KQ, Azorin-Molina C, Yang S, Hu CD, Zhang GF, Minola L, Vicente-Serrano S et al (2022) Shifting of summertime weather extremes in Western Europe during 2012–2020. Adv Clim Chang Res 13(2):218–227. https://doi.org/10.1016/j.accre.2022.01.008
Dong S, Jiang Y, Dong Y, Wang L, Wang W, Ma Z, Yan C et al (2019) A study on soybean responses to drought stress and rehydration. Saudi J Biol Sci 26(8):2006–2017. https://doi.org/10.1016/j.sjbs.2019.08.005
Edreva A (2005) Generation and scavenging of reactive oxygen species in chloroplasts: a submolecular approach. Agr Ecosyst Environ 106(2–3):119–133. https://doi.org/10.1016/j.agee.2004.10.022
Eziz A, Yan Z, Tian D, Han W, Tang Z, Fang J (2017) Drought effect on plant biomass allocation: a meta-analysis. Ecol Evol 7(24):11002–11010. https://doi.org/10.1002/ece3.3630
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plantarum 100:241–254
Garssen AG, Baattrup-Pedersen A, Voesenek L, Verhoeven JTA, Soons MB (2015) Riparian plant community responses to increased flooding: a meta-analysis. Glob Change Biol 21(8):2881–2890. https://doi.org/10.1111/gcb.12921
Gaviria J, Turner BL, Engelbrecht BMJ (2017) Drivers of tree species distribution across a tropical rainfall gradient. Ecosphere 8(2):e01712. https://doi.org/10.1002/ecs2.1712
Gechev T, Petrov V (2020) Reactive Oxygen Species and Abiotic Stress in Plants. Int J Mol Sci 21(20):7433. https://doi.org/10.3390/ijms21207433
Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi AT (2004) Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann Bot-London 94(3):345–351. https://doi.org/10.1093/aob/mch150
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Guan G-F, Wang Y-S, Cheng H, Jiang Z-Y, Fei J (2015) Physiological and biochemical response to drought stress in the leaves of Aegiceras corniculatum and Kandelia obovata. Ecotoxicology 24(7–8):1668–1676. https://doi.org/10.1007/s10646-015-1470-4
Hrishikesh U, Kumar PS, Kumar DB (2008) Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta Physiol Plant 30(4):457–468. https://doi.org/10.1007/s11738-008-0143-9
Huang H, Cao Y, Xu L (2021) The ecological amplitude of giant cane (arundo donax var. versicolor)r under different water level gradients. Appl Ecol Environ Res 19(5):3601–3620. https://doi.org/10.15666/aeer/1905_36013620
Hura T, Hura K, Ostrowska A, Dziurka K (2015) Rapid plant rehydration initiates permanent and adverse changes in the photosynthetic apparatus of triticale. Plant Soil 397(1–2):127–145. https://doi.org/10.1007/s11104-015-2607-1
IPCC (2021) Summary for policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press
Jin R, Shi H, Han C, Zhong B, Wang Q, Chan Z (2015) Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Sci Hortic-Amsterdam 194:215–221. https://doi.org/10.1016/j.scienta.2015.08.023
John GP, Henry C, Sack L (2018) Leaf rehydration capacity: associations with other indices of drought tolerance and environment. Plant Cell Environ 41(11):2638–2653. https://doi.org/10.1111/pce.13390
Kajikawa M, Morikawa K, Abe Y, Yokota A, Akashi K (2010) Establishment of a transgenic hairy root system in wild and domesticated watermelon (Citrullus lanatus) for studying root vigor under drought. Plant Cell Rep 29(7):771–778. https://doi.org/10.1007/s00299-010-0863-3
Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim KH (2020) Root response to drought stress in rice (Oryza sativa L.). Int J Mol Sci 21(4):1513. https://doi.org/10.3390/ijms21041513
Lan Y, Chawade A, Kuktaite R, Johansson E (2022) Climate change impact on wheat performance-effects on vigour, plant traits and yield from early and late drought stress in diverse lines. Int J Mol Sci 23(6):3333. https://doi.org/10.3390/ijms23063333
Li C, Bai T, Ma F, Han M (2010) Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci Hortic 124(2):274–279. https://doi.org/10.1016/j.scienta.2009.12.029
Li F, Qin XY, Xie YH, Chen XS, Hu JY, Liu YY, Hou ZY (2013) Physiological mechanisms for plant distribution pattern: responses to flooding and drought in three wetland plants from Dongting Lake, China. Limnology 14(1):71–76. https://doi.org/10.1007/s10201-012-0386-4
Li J-H, Wang Y-Y, Xia J, Gao H-Y, Shi X-J, Hao X-Z, Luo H-H (2020) Responses of root physiological characteristics of different drought-tolerant cotton varieties to drought. Chin J Appl Ecol 31(10):3453–3460. https://doi.org/10.13287/j.1001-9332.202010.021
Li YQ, Wu XC, Huang YM, Li XY, Shi FZ, Zhao SD, Yang YT et al (2021) Compensation effect of winter snow on larch growth in Northeast China. Clim Change 164(3–4):1–7. https://doi.org/10.1007/s10584-021-02998-1
Liu R, Yang C, Zhang G, Zhang L, Yang F, Guo W (2015) Root recovery development and activity of cotton plants after waterlogging. Agron J 107(6):2038–2046. https://doi.org/10.2134/agronj14.0567
Liu X, Zhang QY, Song MX, Wang N, Fan PX, Wu P, Cui KN et al (2021) Physiological responses of robinia pseudoacacia and quercus acutissima seedlings to repeated drought-rewatering under different planting methods. Front Plant Sci. https://doi.org/10.3389/fpls.2021.760510
Luan Z, Wang Z, Yan D, Liu G, Xu Y (2013) The ecological response of Carex lasiocarpa community in the riparian wetlands to the environmental gradient of water depth in sanjiang plain Northeast China. Sci World J. https://doi.org/10.1155/2013/402067
Maevskaya SN, Nikolaeva MK (2013) Response of antioxidant and osmoprotective systems of wheat seedlings to drought and rehydration. Russ J Plant Physl 60(3):343–350. https://doi.org/10.1134/s1021443713030084
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444(2):139–158. https://doi.org/10.1016/j.abb.2005.10.018
Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, Ahmad A et al (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11(2):225. https://doi.org/10.3390/antiox11020225
Navrot N, Rouhier N, Gelhaye E, Jacquot J-P (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plantarum 129(1):185–195. https://doi.org/10.1111/j.1399-3054.2006.00777.x
Shao HB, Chu LY, Wu G, Zhang JH, Lu ZH, Hu YC (2007) Changes of some anti-oxidative physiological indices under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at tillering stage. Colloid Surface B 54(2):143–149. https://doi.org/10.1016/j.colsurfb.2006.09.004
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125(1):27–58. https://doi.org/10.1111/j.1469-8137.1993.tb03863.x
Sun J, Gu J, Zeng J, Han S, Song A, Chen F, Fang W et al (2013) Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci Hortic-Amsterdam 161:249–258. https://doi.org/10.1016/j.scienta.2013.07.015
Ummenhofer CC, Meehl GA (2017) Extreme weather and climate events with ecological relevance: a review. Philos Trans R Soc B 372(1723):10–1098. https://doi.org/10.1098/rstb.2016.0135
Upadhyaya H, Panda SK (2004) Responses of Camellia sinensis to drought and rehydration. Biol Plantarum 48(4):597–600. https://doi.org/10.1023/b:Biop.0000047158.53482.37
Voronin PY, Maevskaya SN, Nikolaeva MK (2019) Physiological and molecular responses of maize (Zea mays L.) plants to drought and rehydration. Photosynthetica 57(3):850–856. https://doi.org/10.32615/ps.2019.101
Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol 15(4):713–722. https://doi.org/10.1111/j.1438-8677.2012.00710.x
Wang XK (2006) Principles and techniques of plant physiological and biochemical experiments. Higher Education Press, Beijing
Wang J, Lu HD, Muhammad U, Han JZ, Wei ZH, Lu ZX, Bie XM et al (2016) Ultrasound-assisted extraction of polysaccharides from Artemisia selengensis Turcz and its antioxidant and anticancer activities. J Food Sci Tech Mys 53(2):1025–1034. https://doi.org/10.1007/s13197-015-2156-x
White PJ, Bradshaw JE, Brown LK, Dale MFB, Dupuy LX, George TS, Hammond JP et al (2018) Juvenile root vigour improves phosphorus use efficiency of potato. Plant Soil 432(1–2):45–63. https://doi.org/10.1007/s11104-018-3776-5
Yan L, Wang X, Liu H, Tian Y, Lian JM, Yang RJ, Hao SM et al (2015) The genome of dendrobium officinale illuminates the biology of the important traditional Chinese orchid herb. Mol Plant 8(6):922–934. https://doi.org/10.1016/j.molp.2014.12.011
Yan D, Luan Z, Xu D, Xue Y, Shi D (2020) Modeling the spatial distribution of three typical dominant wetland vegetation species’ response to the hydrological gradient in a ramsar wetland, honghe national nature reserve Northeast China. Water 12(7):2041. https://doi.org/10.3390/w12072041
Zhanassova K, Kurmanbayeva A, Gadilgereyeva B, Yermukhambetova R, Iksat N, Amanbayeva U, Bekturova A et al (2021) ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol Plant 43(8):1–12. https://doi.org/10.1007/s11738-021-03281-7
Zhang L, Peng J, Chen TT, Zhao XH, Zhang SP, Liu SD, Dong HL et al (2014) Effect of drought stress on lipid peroxidation and proline content in cotton roots. J Anim Plant Sci 24(6):1729–1736
Zhang L, Tu ZC, Wang H, Fu ZF, Wen QH, Fan D (2015) Metabolic profiling of antioxidants constituents in Artemisia selengensis leaves. Food Chem 186:123–132. https://doi.org/10.1016/j.foodchem.2015.03.068
Zhang G, Yang C, Liu R, Ni W (2019) Effects of three phenolic compounds on mitochondrial function and root vigor of cotton (Gossypium hirsutum L.) seedling roots. Acta Physiol Plant 41(5):1–10. https://doi.org/10.1007/s11738-019-2851-8
Zhang X, Yang CD, Seago JL (2018) Anatomical and histochemical traits of roots and stems of Artemisia lavandulaefolia and A. selengensis (Asteraceae) in the Jianghan floodplain, China. Flora 239:87–97. https://doi.org/10.1016/j.flora.2017.11.009
Funding
This work was supported by Grants from the National Natural Science Foundation of China [42261010, 42061021].
Author information
Authors and Affiliations
Contributions
YCa and HH designed the experiment; HH, YC and JQ conducted the experiment and analyzed the data; HH composed the manuscript; YCa, KX and RL revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The work described here has not been published previously and was not considered for publication elsewhere before a decision was made by the journal. There is no conflict of interest in the submission of this manuscript, and the manuscript is approved by all the authors for publication.
Additional information
Handling Editor: Dr. Asaeda Takashi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Hx., Cao, Y., Xin, Kj. et al. Dynamic responses of root vigor, lipid peroxidation and antioxidant enzymes in Artemisia selengensis to long-term drought and re-watering. Aquat Ecol 57, 321–335 (2023). https://doi.org/10.1007/s10452-023-10012-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-023-10012-2