Abstract
The influence of drought stress on barley (Hordeum vulgare) has been investigated. The experiments were conducted with seedlings of barley—by the application of combined drought and temperature stresses. Here we showed that combination of drought and high temperature inflicted more severe damage to plants than the drought and low temperature stress. The temperature stress triggered more drastic changes in plant morphology/physiology and biochemistry than the drought stress. Interestingly, plants exposed to high temperature exhibited significant reduction of shoot and root aldehyde oxidase (AO) activity. Moreover, increased temperature resulted in lower levels of reactive oxygen species (ROS), while drought stress had an opposite effect on ROS accumulation and AO activity. This is the first demonstration of inhibition of plant AO and ROS in response to heat stress. The combined heat and drought stresses resulted in increased activity of catalase (CAT) and superoxide dismutase (SOD) in roots but not in shoots. Our findings indicate that heat and drought stresses may induce activation of different antioxidant enzymatic defense mechanisms and heat stress significantly affects enzymes responsible for the ROS accumulation in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop plants are constantly influenced by a variety of abiotic factors under natural environment conditions throughout their lifetime, resulting in the yield reduction (Hussain et al. 2019). Plants have evolved specific mechanisms at morphological, physiological and biochemical levels to escape, adapt or tolerate harsh environmental circumstances. Sometimes plants might simultaneously be exposed to two or more stress factors. As an example, high temperatures are frequently accompanied by drought due to the ongoing climate change. This combination may result in biological response disturbances of plants such as inhibition of growth and photosynthesis, stomatal closure, discoloration and accumulation of toxic ROS species (Farooq et al. 2009). Numerous studies have been reported regarding the adaptation of plants to individual stress conditions; however, to date, very little is known about the influence of combined stress factors. The plant responses under combination of drought and high temperature may differ from the response to the individual stress and sometimes they might be even antagonistic (Mittler 2006). For instance, an exposure of tobacco to combined drought and heat suppressed the activity of photosynthesis, enhanced the respiration rate and the leaf temperature compared with single stress application (Rizhsky et al. 2002). Another study demonstrated that the combination of salinity and water deficit resulted in a lower photosynthetic capacity, stomatal closure and increased ROS levels in Hordeum spontaneum (Ahmed et al. 2013). Moreover, several studies detected an overexpression of heat shock proteins (HSP) in wheat and wild barley as a result of the combined effect of drought and heat (Ashoub et al. 2015). Overall, these studies demonstrate the vulnerability of cereal crops to an integrated stress application.
As mentioned above, an exposure to drought and high temperature stresses induces the overproduction of ROS in plants (Suzuki et al. 2012). However, elimination of ROS may vary from plant to plant and might depend on the growth conditions, plant stress tolerant level, and applied stress duration. In addition, coordinated down-regulation of ROS by activating scavenging enzymes during plant stress may be involved in signaling mechanisms (del Rio 2015). Examples of ROS are singlet oxygen (1O2), hydroxyl radical (OH·), superoxide anion (O2−·), and hydrogen peroxide (H2O2). At low concentrations these molecules are not deleterious and may operate as intracellular signaling agents to trigger plant defense responses (Dat et al. 2000). However, the excessively accumulated ROS can ultimately cause oxidative damage of biological molecules such as proteins, lipids and nucleic acids (Sarker and Oba 2018; Mittler et al. 2011). Plants have developed enzymatic and non-enzymatic antioxidant defense machinery to scavenge toxic ROS for the prevention of an oxidative damage (Choudhury et al. 2017). The antioxidative enzymes such as SOD, CAT and POD either scavenge the toxic ROS molecules or defend plants by activating a non-enzymatic antioxidant system (Anjum et al. 2012). Plants with increased levels of antioxidant enzymes were shown to have greater tolerance to oxidative injury caused by ROS (Gapinska et al. 2008).
Superoxide dismutase (SOD)—is the multigene family and the main defense metalloprotein with metal cofactor that acts against oxidative stress by converting superoxide radicals into O2 and H2O2 in mitochondria and chloroplast (Davies 2000). SOD is categorized as Fe-SOD, Cu/Zn-SOD, Ni-SOD and Mn-SOD isozymes depending on the metal ion cofactor that binds to the active site (Wu 2016). Recently, two independent works have shown that transgenic plant Puccinellia tenuiflora and tobacco which possess SOD isomers such as Cu/Zn-SOD were more susceptible to drought and salt stress than wild type Puccinellia tenuiflora and tobacco plants (Wu 2016; Negi et al. 2015). The overexpression of the Cu/Zn-SOD isomer in plants may play a crucial role in ameliorating the oxidative damages caused by abiotic stresses. Another study reported that Cu/Zn-SOD and Mn-SOD activities were enhanced under chilling stress in cucumber (Cucumis sativus L.) (Lee and Lee 2000). Overall, SOD acts as the hydrogen peroxide scavenging agent during oxidative stress caused by various environmental conditions.
Catalase (CAT) is an enzyme containing heme with the atomic mass of about 250 kDa (Mhamdi et al. 2012). Catalase was found primarily in chloroplasts, peroxisomes, mitochondria and cytoplasm. The primary function of CAT is to prevent the plant peroxidation by reducing the harmful intracellular hydrogen peroxide to water and oxygen without cell energy utilization. Catalase is the multigene family and has a few isoforms, for example, CAT1, CAT2, and CAT3 (Sharma and Ahmad 2014). It has been reported that plants lacking the enzyme CAT were more delicate to saltiness and ozone stress contrasted with the wild plants (Abid et al. 2018). Moreover, it has been reported that CAT activity declined under drought stress in the drought-sensitive rice cultivar SJ6 and it prompts the gathering of hydrogen peroxide (Lee and Lee 2000).
AO is a member of molybdenum-containing hydroxylases multigene family with a prosthetic group that includes FAD, Fe–S and molybdenum cofactor (Moco) (Koshiba et al. 1996). AO oxidizes a wide range of aldehydes such as indole-3-acetaldehyde (IAAld) and abscisic aldehyde (ABAld) to the indole-acetic acid (IAA) and abscisic acid (ABA) (Koshiba et al. 1996; Omarov et al. 1999). It is well known that AO plays an essential role in the plant adaptation mechanisms with respect to abiotic stress factors (Omarov et al. 1999; Sagi et al. 1998; Yesbergenova et al. 2005). An earlier study with ryegrass suggested a possible link between an increased activity of AO under salinity and ammonium treatment with the adaptation of plants to the applied stress conditions (Sagi et al. 1998). A more recent study on crested wheatgrass also reported an enhanced activity of AO under the high salinity stress which led to the increased tolerance and inhibited an oxidative damage (Babenko et al. 2015). Moreover, it has been reported that AO is not only involved in the generation of hydrogen peroxide (H2O2) (Yesbergenova et al. 2005), but it also can produce superoxide anion (O2−·) using NADH as a substrate (Zarepour et al. 2012).
Individual abiotic stresses have been well-investigated, but much less is known about the effect of combined, co-occurring stress factors, despite the fact that combined stresses are probably dominating under natural conditions (Holopainen and Gershenzon 2010). Therefore, the main goal of the present study was to elucidate physiological antioxidant enzymatic defense mechanisms involved in ROS detoxification and tolerance of H. vulgare under combined application of drought and/or temperature stresses.
Materials and methods
Plant material and growth conditions
The research conducted on the seedlings of barley (H. vulgare) in the naturally illuminated greenhouse under controlled conditions of the Plant Biotechnology Laboratory based in Eurasian National University, Kazakhstan.
As experimental material was selected the uniform seeds of barley (H. vulgare) from a cultivar of “Astana-2000”. Prior to sowing, seeds were sterilized with 50% of aqueous solution of sodium hypochlorite (NaClO) for 10 min, and then they were incubated in 90% ethanol for 1 min. After that, seeds were rinsed three times with distilled water and dried. Thirty seeds were sown in per pots with pre-watered soil. Each plastic pot was filled with 150 g of pre sterilized soil, 10 g of vermiculite and moistened with 40 ml of distilled water. The soil was slightly acidic with pH 6.0–6.5 and contained the main nutrients like nitrogen (NH4 + NO3)—150 mg/l, phosphorus (P2O5)—270 mg/l, potassium (K2O)—300 mg/l. Plants were grown in growth room conditions (+ 25 day/night temperature, 16/8 h light/dark photoperiod, 20–22% relative humidity) for 2 days till the seedlings development. The lamps with a spectrum of 2700 K and 6400 K were used for lighting the growth room.
Application of drought and temperature stress treatments
Two days after emergence of seedlings, plants were divided into six groups depending on the condition of their growth, as shown in Table 1.
Barley plants were grown 5 days in conditions of long-day photoperiod (16-h light/8-h dark) and 20–22% air relative humidity in LCC 500 M growth cabinets (Daihan Labtech Co, LTD., S. Korean) at the temperatures 10 and 40 °C. Control samples were grown in the greenhouse at + 25 °C temperature. For drought stress application, watering to pots was withheld. All plants with normal water conditions were supplied with 40 ml of water every day at the set time of day. For lighting of growth room lamps with 2700 K and 6400 K spectrum were used.
Determination of relative water content (RWC)
The relative water content was determined according to the method of Barr’s and Weatherly’s with some modifications (Barrs and Weatherly 1962). The leaves were clipped and then immediately weighed to measure fresh weight (FW). Then samples were immersed into the tubes with water and placed in a refrigerator at + 10 °C for 24 h to obtain a turgid mass (TW). Thereafter, the samples were removed from the excess water with the filter paper and measured to obtain a turgid mass (TW). To get dry weight (DW), samples were dried in an oven at + 80 °C for 24 h.
The relative water content of samples were calculated by the following formula:
Determination of chlorophyll content
The total chlorophyll content was measured according to the method of Arnon (1949). The 1 g of raw plant leaves were homogenized with 20 ml of prechilled 80% ethyl alcohol and centrifuged at 10,000 rpm for 10 min. After that, 80 ml of the 80% ethanol was added to the tube, and then the remaining chlorophyll content in the leaves was measured spectrophotometrically at 664 nm against 80% ethanol blank.
Determination of malondialdehyde (MDA) content
MDA levels in the leaves were measured according to Srivastava (2017) with slight changes. At the beginning, 100 mg of fresh leaves was pestle grounded in a chilled phosphate–saline buffer containing 10% trichloroacetic acid (TCA). Then, the mixture was centrifuged at 3000 rpm for 10 min and the supernatant was incubated with an equal volume of 0.25% solution of 2-thiobarbituric acid (TBA) in a water bath (95 °C) for 45 min. After that, the tubes with the solution were cooled in ice and centrifuged at 10,000 rpm for 15 min. Furthermore, supernatants were selected for spectrophotometric analysis. Optical density (OD) was measured at wavelengths of 532 and 600 nm to determine MDA content on a fresh weight basis. The experimental values were determined against the MDA standard curve.
Determination of total soluble proteins
Soluble proteins were extracted in 25 mM phosphate buffer (pH 7.5) with 0.25 M sucrose, 0.025% Triton X-100 and 0.05 μM phenylmethylsulfonyl fluoride (PMSF). The homogenized plant material was centrifuged at 3000 rpm for 10 min, stirred by shaking, incubated on ice for 30 min and centrifuged at 10,000 rpm for 20 min. The resulting supernatant was incubated in a water bath at 60 °C for 90 s and centrifuged at 10,000 rpm for 5 min. To desalinate the proteins, ion exchange chromatography was used preliminarily equilibrated with 10 mM Tris–HCl buffer (pH 7.5). For this, 500 μl of the resulting solution was passed through a 2.8 cm length Sephadex G-25 column, and then 1000 μl of 25 mM phosphate buffer (pH 7.5) was eluted.
The total soluble protein content was evaluated using the Bradford method with crystalline bovine serum albumin as a standard (Bradford 1976). Briefly, protein extracts from plant samples were diluted 1:25 with DDW and mixed with diluted Protein assay at a ratio 1:10 (w/v). Absorption for each sample was measured at 595 nm on a spectrophotometer. Protein concentrations were calculated using the following formula:
Protein extraction and fractionation for in gel activities
The preparation of samples for native PAGE was carried out in accordance with (Batyrshina et al. 2018). In ice-cold extraction buffers containing 250 mM Tris–HCl (pH 7.5), 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid (EDTA), 4 mM 1,4-dithiothreitol (DTT), 5 mM l-cysteine, 0.001 mM aprotinin, 0.1 mM phenylene diamine tetraacetic fluoride (PMSF) and 0.001 mM pepstatin, fresh tissues were homogenized. The ratio of extraction buffer and tissue was 1:2 for leaves and 1:3 for roots. The plant tissue extracts were then centrifuged for 20 min at 10,000 rpm (+ 4 °C), then the supernatants were transferred to the new tubes and carried out at 4 °C until 7.5% polyacrylamide gel electrophoresis in the absence of SDS (Sagi et al. 1998). The amount of soluble protein in the samples was calculated by Bradford assay, with BSA as standard (Bradford 1976). All samples had been recalculated with the aim of loading 20 μg of soluble protein per line.
Determination of superoxide dismutase (SOD) in gel activity
The activity of SOD was determined according to the method previously described by Yergaliyev et al. (2016). After electrophoresis, the gels were washed three times with distilled water and incubated in 0.1% NBT solution on the shaker at 80 rpm for 15 min in the dark. After that, the gel was rinsed three times with distilled water and equilibrated in riboflavin solution (28 μM riboflavin and 28 mM TEMED in 0.1 M potassium phosphate buffer, pH 7.0) for 15 min. Then, the gel was washed with distilled water and irradiated under UV light until the appearance of bands.
Determination of catalase (CAT) in gel activity
CAT activity was performed following the method of Aebi (1984). The reaction mixture contained 10 µl of sample supernatant, 50 mM potassium phosphate buffer (pH 6.9) and 0.03% H2O2 mixed thoroughly for 15 min. After that, for visualization of catalase isozymes, the gel was incubated in 0.003% hydrogen peroxide solution for 10 min. Then, specific staining for catalase activity was performed in a solution containing 1% potassium hexacyanoferrate (III) and 1% iron (III) chloride.
Determination of aldehyde oxidase (AO) in gel activity
AO activity was determined following the method described by Sagi et al. (1998) with slight changes. After electrophoresis, the gels were washed with distilled water and immersed in a staining solution that contained 1 mM indole-3-carboxaldehyde as substrate, 1 mM MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) as indicator, 0.1 mM phenazine methosulfate as electron carrier in 0.1 M TRIS–HCl, pH 7.4, at + 37 °C for 40 min.
ROS determination
For detecting O2−· and H2O2, samples were extracted in 50 mM phosphate buffer (pH 7.5) at a ratio of 1:8 (w/v) and centrifuged twice at 18,000 g. for 20 min. The reaction mixture for detecting O2¯ consisted of 4 mM epinephrine as an electron acceptor in 100 mM Tris–HCl buffer (pH 7.8) in the presence or absence of 2100 U/ml CuZn-SOD as previously described Yesbergenova et al. (2005). Absorbance was measured at 480 nm by employing a Biochrom Asys Expert 96 Microplate Spectrophotometer supported by Kim software.
The reaction mixture for detecting H2O2 consisted of 0.85 mm 4-aminoantipyrine, 3.4 mm 3,5-dichloro-2-hydroxybenzene sulfonate, 4.5 U/ml HRP in 2 ml of 50 mM phosphate buffer (pH 7.5) as previously described (Yesbergenova et al. 2005). Absorbance was measured after 5 min at 515 nm.
The nitroblue tetrazolium (NBT) for superoxide radical staining in shoots were done as previously described by Rao and Davis (1999) with slight modifications. Shoots of barley were immersed and infiltrated under vacuum with 3.5 mg/ml NBT staining solution in potassium phosphate buffer (10 mM) containing 10 mM NaN3. After infiltration, stained shoots were bleached in acetic acid–glycerol–ethanol (1/1/3) (v/v/v) solution at + 100 °C during 5 min. Samples were then stored in a glycerol–ethanol (1/4) (v/v) solution until photographs were taken. O2−· was visualized as a blue color produced by NBT precipitation.
Statistical analyses
Two-tailed unpaired Student’s t test (two-sample unequal variance) was employed to show differences between pairs of samples. ANOVA (Tukey–Kramer honestly significant difference [HSD]) was used to compare multiple groups of samples (JMP 15.1.0) software; http://www.jmp.com/). The NBT staining and bands on AO, SOD and CAT in gel activities were analyzed with ImageJ software (http://rsbweb.nih.gov/ij/). All experiments with determination of enzymes in gel activities were carried out at least 4.
Results
The combined effects of temperature and drought stresses on morphological and physiological parameters of barley
Comparison of morphological parameters of experimental plants allows to estimate the influence of the temperature during the exposure to both factors (temperature and drought). Profound morphological changes in plant growth and development were observed under heat and cold stresses. This was evident by observed reduction the shoot and root lengths (Fig. 1b, c). The most negative effect was observed due to heat stress with a severe deformation and twisting of the stems and the change of plant color to brown. No significant differences were detected between the plants exposed to drought and watered plants at the low temperature conditions. Such treatments also did not affect the root and shoot length; however, at room and high temperature, plants without watering exhibited significantly reduced height and root length (Fig. 1a–c).
Combined effects of temperature and drought stresses on morphology (a), shoot (b) and root length (c), relative water contents (d) and chlorophyll level (e). The values are means ± SE (n = 4). Values denoted with different letters are significantly different according to the Tukey–Kramer HSD and T test analyses, P < 0.05 (JMP 15.1.0) software, http://www.jmp.com/). Different uppercase letters indicate significant differences between temperature and drought stressed plants. Asterisks indicate significant differences within the temperature stress in response to drought stress
Similarly to plant growth the relative water contents and photosynthetic pigments content were also significantly inhibited during the exposure to low and high temperature stresses compared with control plants with optimal temperature (+ 25℃) (Fig. 1b, c).
The combined effects of temperature and drought stresses on ROS
Oxidative damage naturally occurs in plants as a result of aerobic respiration and this effect can be enhanced by a plethora of environmental factors, including temperature and drought stresses, which in turn may lead to the elevated accumulation of ROS. Therefore, levels of superoxide (O2−·) and hydrogen peroxide (H2O2) radicals were tested to examine whether severe stress at + 40 ℃ is accompanied by the increased accumulation of ROS.
Superoxide quantification was carried out using NBT staining in the shoots and determined spectrophotometrically in the roots (Fig. 2a, b). Interestingly, plants grown under the optimal conditions showed the highest levels of O2−· radicals both in the roots and shoots. Moreover, in spite of severe stress factors shoots of plants exposed to heat stress demonstrated the weak staining indicating low levels of ROS content. Thus, lower levels of O2−·radicals under the heat stress in the shoots might be the result of their either decreased production by ROS-producing enzymes or increased levels of ROS detoxification.
Combined effects of temperature and drought stresses on the levels of superoxide radicals (a and b) and hydrogen peroxide (c and d) in shoots and roots of barley. Superoxide in the shoots were detected using the nitroblue tetrazolium (NBT) staining method. The values are means ± SE (n = 4). Values denoted with different letters are significantly different according to the Tukey–Kramer HSD and T test analyses, P < 0.05 (JMP 15.1.0 software, http://www.jmp.com/). Different uppercase letters indicate significant differences between temperature and drought stressed plants. Asterisks indicate significant differences within the temperature stress in response to drought stress
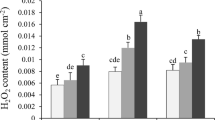
The effect of H2O2 accumulation was stronger in the roots compared to the shoots under the temperature stress. Hydrogen peroxide concentration under the high temperature stress increased in the roots (Fig. 2c, d).
The combined effects of temperature and drought stresses on AO activity
Since our results revealed decreased levels of superoxide radicals in plants under the high temperature treatment, it was seemed logical to test activity of ROS-producing enzymes, such as AO, because many studies showed the involvement of MoCo-containing AO in superoxide production in plants and animals (Bittner and Mendel 2006).
Effects of combined stress conditions in barley on AO were examined using in gel enzyme activity assay. Through the use of indole-3-carboxaldehyde as an AO substrate, one leaf isoform and three root isoforms were observed (Fig. 3a, b). Low temperature did not significantly affect AO activity in shoots of barley. However, the combination of low temperature and drought stress resulted in notable reduction of the enzyme activity. Drought and high temperature stresses (combined or separately) resulted in significantly reduced levels of AO activity in the shoots (Fig. 3a). In roots, at high temperature conditions the enzyme activity significantly decreased but response to drought stress AO activity slightly elevated (Fig. 3b). Strikingly, similarly to O2−· radicals accumulation, under the high temperature stress AO activity in the shoots was barely detectable in spite of the severe impact of these conditions on plant morphological parameters.
Combined effects of temperature and drought stresses on AO activity in shoots (a) and roots (b) of barley. The data are from three different experiments that yielded similar results. The bands were evaluated by employing ImageJ software (http://rsbweb.nih.gov/ij/)
The combined effects of temperature and drought stresses on SOD activity
To investigate how combined stresses affect the SOD activity in barley, an enzymatic staining assay after non-denaturing electrophoresis was performed. Samples extracted from the shoots of plants exposed to drought stress revealed significantly reduced levels of SOD, in comparison with extracts obtained from control plants of each temperature conditions (Fig. 4a). The decrease of SOD activity was also detected in roots of plants exposed drought at low and optimal temperature. However, at high-temperature root SOD was slightly activated by the condition of water shortage. Moreover, in roots two distinct SOD isoforms were detected (Fig. 4b).
Combined effects of temperature and drought stresses on SOD activity level in shoots (a) and roots (b) of barley. The data are from three different experiments that yielded similar results. The bands were evaluated by employing ImageJ software (http://rsbweb.nih.gov/ij/)
The combined effects of temperature and drought stresses on CAT activity
Since our results revealed distinct levels of SOD activity in roots and shoots under the temperature stress, we performed experiments to detect CAT in gel activity. Similarly to SOD activity, CAT showed reduced activity in the shoots in response to drought stress in comparison to the control plants. In addition, in response to temperature stress CAT activity also followed the SOD activity pattern with reduction of activity compared to the plants grown under + 25 ℃ (Fig. 5a).
Combined effects of temperature and drought stresses on CAT activity level in shoots (a) and roots (b) of barley. The data are from three different experiments that yielded similar results. The bands were evaluated by employing ImageJ software (http://rsbweb.nih.gov/ij/)
H2O2-producing CAT activity was almost undetectable in the roots under the low temperature stress compared to the control plants and the effect of drought stress also was not detectable under the control and low temperature conditions (Fig. 5b). Significantly, higher levels of CAT were detected in the roots of plants grown under the low temperature compared to the non-stressed plants. Furthermore, 4 distinct CAT isoforms were detected in the roots under the high temperature stress. This effect was most noticeable especially when high temperature was combined with drought.
Discussion
Drought and high/low temperatures are unfavorable environmental circumstances that inflict significant damage to crop yield. In the past decades scientists focused much attention to individual stress factors such as, drought, salinity, low and high temperature, UV radiation and so on. However, obviously in the natural field conditions plants are exposed to a combination of a plethora of factors. The goal of present study was to elucidate physiological, antioxidant enzymatic defense mechanisms involved in ROS detoxification and tolerance of barley (H. vulgare) under separate and combined application of drought/temperature stresses.
Our results showed that drought and high/low temperatures remarkably reduced the growth of leaves, roots and caused decline in water retention capacity (Fig. 1). Moreover, water deficit and high temperature induced the stem twisting and shrinking of leaf plates. Recently, a number of studies have reported the adverse influence of an integrated drought and high temperature stresses on the growth and yield quality of various crops (Barnabás et al. 2008; Gooding et al. 2003; Fahad et al. 2017). In the case of Hordeum Vulgare, combination of drought and low temperature significantly influenced physiological responses. This indicates that drought and low temperature treated plants respond to the applied stress in quite different manner than when plants exposed to the drought and heat stress. As anticipated, the integration of drought and temperature stress remarkably decreased the water conservation and chlorophyll content in barley. The RWC declined under thermal stress (Fig. 1d), thus suggesting that plants reduced the growth and induced the stomatal closure to prevent water loss due to temperature stresses. It was previously reported that in Arabidopsis thaliana the combination of drought and water deficit induce the closure of stomatal pores to minimize the water loss (Rizhsky et al. 2002; Prasch and Sonnewald 2013). Chlorophyll is an essential pigment of photosynthesis. The total chlorophyll content is an indicative marker to depict the photosynthesis level in plants. According to our results, chlorophyll content in the shoots of barley was drastically downregulated under thermal stress (+ 40 ℃) treatment (Fig. 1e). This suggests that, combination of drought and high temperature caused more damage than the drought and low temperature stress to the photosynthetic machinery. Limitation in photosynthesis under the influence of those combined stresses can restrict the growth of plants and subsequently lead to the reduction of crop yield. Our results are in agreement with the previous studies conducted on chickpea (Awasthi et al. 2014), winter wheat (Ristic et al. 2007), cotton (Carmo-Silva et al. 2012), sorghum (Djanaguiraman et al. 2010). These works revealed the negative influence of interactive drought and high temperature stress on chlorophyll content which caused damage in the structural integrity of chloroplasts.
Plants have evolved an antioxidative defense machinery to prevent the oxidative damages caused by ROS (Hussain et al. 2019). Unfavorable environmental factors induce biochemical enzymatic and non-enzymatic defense mechanisms of plants (Ashraf 2009). Plants with elevated levels of antioxidant enzymes were more tolerant to oxidative stresses.
ROS such as superoxide anion, hydrogen peroxide and singlet oxygen are considered to be extremely reactive and to be able to interact with cell compartments. Lipid peroxidation, in which the lipid membranes are degraded, is one of the negative processes of ROS, eventually contributing to structure destruction and damage to the cell (Mittler 2006). Our results showed that the combination of drought and temperature stresses caused enhancement of MDA content in test plants with respect to control plants (Fig. S), suggesting the generation of ROS. The overproduction of MDA under adverse environmental conditions leads to the lipid peroxidation (Gills and Tuteja 2010). Considerable higher accumulation of hydrogen peroxide H2O2 was detected in roots under the thermal and water deficit stress. In contrast, it was downregulated in shoots and thus indicate that the ROS scavenging machinery was not enough to defense the roots against oxidative injury, especially those induced by integration of water deficit and thermal stresses, as established by the enhanced accumulation of MDA and H2O2).
The plant soluble proteins are the one of the main biomolecules that play an essential role in osmoregulation (Slama et al. 2015). The present study revealed that the total soluble proteins decreased under all stress circumstances; however, under thermal stress conditions (40 °C), it was substantially declined (Fig. S). Our results showed that thermal stressed plants had higher soluble protein content in shoots than in roots and this might mean that drought and heat stresses combination activate biosynthesis of soluble proteins to increase the resistance of plants towards the applied stress conditions.
The aldehyde oxidase (AO) is an essential enzyme that produce hydrogen peroxide (H2O2) and superoxide (Yergaliyev et al. 2016) and play crucial role in the adaptation of plants toward the abiotic stresses (Omarov et al. 1999). It has been established that the AO is an essential final link in ABA biosynthesis from abscisic aldehyde (Schwartz et al. 1997; Seo et al. 2000; Sekimoto et al. 1997). At an elevated concentration of exogenous ABA, the growth of plants slows down, and this happens primarily during drought (Lata and Prasad 2011). Under drought stress, stomata closed with the help of ABA to control transpiration rate, providing a plant defense mechanism (Guajardo et al. 2016). As regards our results, drought and high temperature stress showed an increase in AO activity; however, this activity decreased in response to stress caused by high temperature (Fig. 3). At high temperatures and adequate watering, the plant does not require ABA synthesis on such a broad scale as under combined drought and high temperature stress, and hence, AO activity is less than under combined stress. The above pattern is observed at both 25 °C of drought and control and 10 °C of drought and control. We assumed that during the drought, the plant attempts to form large quantities of ABA to survive by controlling transpiration. Taken together, it is reasonable to hypothesize that AO may act as a key player in the quenching of ROS molecules under combined stress applications.
The occurrence of comparatively low AO activity at high temperatures and combined stress compared to samples grown at 25 °C and 10 °C may be because of modifications in the structural configuration of proteins and/or degradation.
However, the activity of AO in barley leaves was distinct from that of roots. It is likely that AO may perform other metabolic functions in the leaves, in addition to phytohormone synthesis, since AO is capable of catalyzing different substrates. It seems that the production of free radicals resulting in the presence of AO and xanthine dehydrogenase can be used as a signaling pathway in plants to protect itself. Moreover, these results indicate that high temperature resulted in inhibited activity of AO and reduced production of superoxide radicals (O2−·) in barley.
The SOD acts as a front line of defense system against ROS by converting superoxide anion to oxygen and hydrogen peroxide (Jaleel et al. 2009). The previous studies conducted on cotton (Wang et al. 2017) and wheat (Tyagi et al. 2017) showed incremental activity of SOD under various abiotic stresses. The inhomogeneity of SOD with a given pattern (Fig. 4) can be explained by the short viability and high reactivity of the superoxide anion (Fridovich 1995). According to our research, the samples that were exposed to combined drought and temperature stresses showed considerably lower activity of SOD in the shoots and thus indicated that under drought and temperature stresses shoots of barley plants had lower ability to scavenge the superoxide radical (Fig. 4). Interestingly, the SOD activity in the shoots revealed no alterations, while in the roots of barley seedlings the activity of SOD drastically increased under temperature stresses. This may indicate the essential involvement of SOD in overcoming the stresses. Moreover, in the roots two new SOD isoforms were determined and thus may represent the enhancement of the ability of plants to combat drought and thermal stresses. The higher the SOD activity or higher number of isoforms, greater the potential to remove ROS. Over-expressing plants of various SOD isoforms increases enhanced tolerance to oxidative stress and to other environmental stresses (Berwal and Ram 2018).
Information regarding the CAT activity under various abiotic stresses is diverse. CAT is an antioxidant enzyme that decomposes H2O2 to oxygen and water molecules (Mhamdi et al. 2012). In our experiment, a combination of drought and temperature showed a diverse pattern of accumulation of CAT enzymes (Fig. 5). The activity of CAT under interactive influence of drought and low temperature stresses was almost undetectable which indicates the “weak affinity” to hydrogen peroxide. Interestingly, the activity of CAT was upregulated in roots under high temperature treatment compared to shoots. Our results are consistent with other studies conducted on wheat varieties found out the enhanced activity of CAT enzyme under drought and chilling stresses (Guo et al. 2004; Janmohammadi et al. 2012; Devi et al. 2012). Both SOD and CAT enzyme activities were provoked by the thermal stress treatment in roots, but were strongly suppressed in shoots and thus may indicate that heat stimulates the activation of protein isozymes.
Changes in SOD, CAT and AO activity and ROS accumulation in shoots and roots were not equal due to drought and temperature stress treatments, indicating that barley plants use complex mechanisms in response to different stressors. Moreover, the higher activities of SOD, CAT and AO under water deficit and high temperature indicates the ability of barley plants to eliminate ROS effectively and prevent the overaccumulation of ROS.
The results of our research showed that temperature stress has stronger effect in plant growth than drought stress and the roots are more susceptible to the combined effect than the shoots. The heat and drought stresses resulted in increased activity of CAT and SOD in roots and decreased in shoots. Moreover, reduced AO activity with lower ROS level under the heat stress in spite the severe negative effect in plants and increased AO activity in roots with enhanced ROS under the drought stress may indicate distinct antioxidant enzymatic defense mechanisms involved in ROS homeostasis under the heat and drought stresses.
Author contribution statement
Determination of AO, CAT, SOD in gel activities were performed by BG, AK, KZ, RY. Determination of ROS were analyzed by BG, AB, UA and ZT. Determination of MDA and total soluble protein content was performed by IN. Statistical analysis performed by BG. The paper was written by KZ, AK and RO. Principal investigators of the project RO and ZM.
Abbreviations
- AO:
-
Aldehyde oxidase
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- HSP:
-
Heat shock proteins
- ALDH:
-
Aldehyde dehydrogenases
- H2O2 :
-
Hydrogen peroxide
- NBT:
-
Nitroblue tetrazolium
- RWC:
-
Relative water content
- MDA:
-
Malondialdehyde
References
Abid M, Ali S, Qi LK (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep 8(1):4615
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed IM, Cao F, Zhang M, Chen X, Zhang G, Wu F (2013) Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys. PLoS ONE 8(10):e77869
Anjum S, Farooq M, X-yu X, X-jian L, Ijaz MF (2012) Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci Hortic 140:66–73
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol 24(1):1–15
Ashoub A, Baeumlisberger M, Neupert ME, Karas M, Bruggemann W (2015) Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol Biol 87(4–5):459–471
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27(1):84–93
Awasthi R, Kaushal N, Vadez V, Turner N, Berger J (2014) Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct Plant Biol 41(11):1148–1167
Babenko ON, Brychkova G, Sagi M, Alikulov ZA (2015) Molybdenum application enhances adaptation of crested wheatgrass to salinity stress. Acta Physiol Plant 37(14):1–13
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31(1):11–38
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Batyrshina Z, Yergaliyev TM, Nurbekova Z, Moldakimova NA, Masalimov ZK, Sagi M, Omarov RT (2018) Differential influence of molybdenum and tungsten on the growth of barley seedlings and the activity of aldehyde oxidase under salinity. J Plant Physiol 228:189–196
Berwal MK, Ram C (2018) Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In: De Oliveira A (ed) Abiotic and Biotic Stress in Plants. IntechOpen, London, UK
Bittner F, Mendel RR (2006) Cell biology of molybdenum. Biochim Biophys Acta 1763(7):621–635
Bradford M (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carmo-Silva AE, Gore M, Andrade-Sanchez P, French A, Hunsaker D (2012) Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ Exp Bot 83:1–11
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90(5):856–867
Dat J, Vandenabeele S, Van Montagy M, Inze D (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57(5):779–795
Davies K (2000) Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50:279–289
del Río LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66(10):2827–2837
Devi R, Kaur N, Gupta AK (2012) Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.). Indian J Biochem Biophys 49(4):257–265
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48(12):999–1007
Fahad S, Bajwa A, Nazir U, Anjum SA (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Gapinska M, Sklodowska M, Gabara B (2008) Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30:11–18
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gooding MJ, Ellis RH, Shewry PR, Schofield JD (2003) Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J Cereal Sci 37(3):295–309
Guajardo E, Correa JA, Contreras-Porcia L (2016) Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 243(3):767–781
Guo H, Gao S, Zhao F, Li F (2004) Effects of cold acclimation on several enzyme activities in Euonymus radicans ‘emorald and gold’ and its relation to semi-lethal temperature. Forestry Stud China 6:10–17
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15(3):176–184
Hussain HA, Men S, Hussain S (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9(1):3890
Jaleel CA, Riadh K, Ragupathi G, Manivannan P, Jallali I (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Janmohammadi M, Enayati V, Sabaghnia N (2012) Impact of cold acclimation, de-acclimation and re-acclimation on carbohydrate content and antioxidant enzyme activities in spring and winter wheat. Icel Agric Sci 25:3–11
Koshiba T, Saito E, Yamamoto N, Sato M (1996) Purification and properties of flavin- and molybdenum-containing aldehyde oxidase from coleoptiles of maize. Plant Physiol 110(3):781–789
Lata C, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot 62(14):4731–4748
Lee DH, Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159(1):75–85
Mhamdi A, Noctor G, Baker A (2012) Plant catalases: peroxisomal redox guardians. Arch Biochem Biophys 61:4197–4220
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11(1):15–19
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16(6):300–309
Negi NP, Shrivastava DC, Sarin NB (2015) Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Rep 34(7):1109–1126
Omarov RT, Akaba S, Lips SH (1999) Aldehyde oxidase in roots, leaves and seeds of barley (Hordeum vulgare L.). J Exp Bot 50:63–69
Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162(4):1849–1866
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17(6):603–614
Ristic Z, Bukovnik U, Prasad PVV (2007) Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Sci 47:2067–2073
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130(3):1143–1151
Sagi M, Omarov RT, Lips SH (1998) The Mo-hydroxylases xanthine dehydrogenase and aldehyde oxidase in ryegrass as affected by nitrogen and salinity. Plant Sci 135:125–135
Sarker U, Oba S (2018) Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep 8(1):16496
Schwartz SS, Leon-Kloosterzeil KM, Koornneef M, Zeevaart AD (1997) Biochemical characterization of the ab2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114:161–166
Sekimoto H, Seo M, Dohmae N, Takio K, Kamiya Y, Koshiba T (1997) Cloning and molecular characterization of plant aldehyde oxidase. J Biol Chem 272:15280–15285
Seo M, Koiwai H, Akaba S, Komano T, Oritani T, Kamiya Y, Koshiba T (2000) Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana. Plant J 8:23(4):481–488
Sharma I, Ahmad P (2014) Catalase: a versatile antioxidant in plants. In: Oxidative Damage to Plants: Antioxidant Networks and Signalling, pp 131–148
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115(3):433–447
Srivastava S (2017) Aldehyde oxidase 4 plays a critical role in delaying silique senescence by catalyzing aldehyde detoxification. Plant Physiol 173(4):1977–1997
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35(2):259–270
Tyagi S, Sharma S, Taneja M (2017) Superoxide dismutases in bread wheat (Triticum aestivum L.): comprehensive characterization and expression analysis during development and biotic and abiotic stresses. Agric Gene 6:1–13
Wang W, Zhang X, Deng F, Yuan R, Shen F (2017) Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom 18(1):376
Wu J (2016) Identification and characterization of a PutCu/Zn-SOD gene from Puccinellia tenuiflora (Turcz.). Plant Growth Regulat 79:55–64
Yergaliyev TM, Nurbekova Z, Mukiyanova G (2016) The involvement of ROS producing aldehyde oxidase in plant response to Tombusvirus infection. Plant Physiol Biochem 109:36–44
Yesbergenova Z, Yang G, Oron E, Soffer D, Fluhr R, Sagi M (2005) The plant Mo-hydroxylases aldehyde oxidase and xanthine dehydrogenase have distinct reactive oxygen species signatures and are induced by drought and abscisic acid. Plant J 42(6):862–876
Zarepour M, Simon K, Wilch M (2012) Identification of superoxide production by Arabidopsis thaliana aldehyde oxidases AAO1 and AAO3. Plant Mol Biol 80(6):659–671
Funding
This work was supported by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan under Grant nos. BR05236574, AP05135485.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interests regarding the publication of this article.
Additional information
Communicated by G. Bartosz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhanassova, K., Kurmanbayeva, A., Gadilgereyeva, B. et al. ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol Plant 43, 114 (2021). https://doi.org/10.1007/s11738-021-03281-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03281-7