Abstract
Root vigor is an important trait for the growth of terrestrial plants, especially in water-deficit environments. Although deserts plants are known for their highly developed root architecture, the molecular mechanism responsible for this trait has not been determined. Here we established an efficient protocol for the genetic manipulation of two varieties of watermelon plants: a desert-grown wild watermelon that shows vigorous root growth under drought, and a domesticated cultivar showing retardation of root growth under drought stress. Agrobacterium rhizogenes-mediated transgenic hairy roots were efficiently induced and selected from the hypocotyls of these plants. Transgenic GUS expression was detected in the roots by RT-PCR and histochemical GUS staining. Moreover, a liquid culture system for evaluating their root growth was also established. Interestingly, growth of the hairy roots derived from domesticated variety of watermelon strongly inhibited under high osmotic condition, whereas the hairy roots derived from wild variety of watermelon retained substantial growth rates under the stress condition. The new protocol presented here offers a powerful tool for the comparative study of the molecular mechanism underlying drought-induced root growth in desert plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild watermelon (Citrullus lanatus sp.), which inhabit the Kalahari Desert in Botswana, exhibit strong resistance to drought compared with domesticated watermelon cultivar (Citrullus lanatus cv.) (Kawasaki et al. 2000; Takahara et al. 2005; Akashi et al. 2008; Kohzuma et al. 2009). Wild watermelon extends its highly developed root system into deep soil layers, which contributes not only to the efficient water uptake but also during water storage (Larcher 1995). Interestingly, it was demonstrated that the onset of drought stimulated vigorous root growth in wild watermelon, whereas the same treatment contrastingly resulted in the suppression of root growth in domesticated watermelon (Yoshimura et al. 2008). These observations suggested that the wild watermelon is equipped with unique molecular mechanisms that promote root development under drought conditions.
Recently, our group reported a proteome analysis of wild watermelon roots under drought, which revealed that the expression of a unique set of proteins was differentially modulated in a temporally regulated manner (Yoshimura et al. 2008). To understand the function of these drought-responsive proteins in detail, a comparative study between wild and domesticated watermelon via genetic manipulation of the gene in question will be a powerful approach. Although the Agrobacterium tumefaciens-mediated transformation and regeneration of wild and domesticated watermelon plants has been reported (Choi et al. 1994; Ellul et al. 2003; Akashi et al. 2005), the protocol involves time-consuming processes to obtain the next generation of transgenic plants.
Transgenic hairy root in vitro culture system induced by A. rhizogenes was proposed as an effective and rapid transformation system for studying gene function in plant roots (Guillon et al. 2006a, b). A. rhizogenes is a common soil organism capable of infecting plants through a wound site and causing a proliferation of secondary roots, referred to as hairy roots. The underlying mechanism of hairy root formation is the transfer of several bacterial genes from Ri (root-inducing) plasmid in the bacterium to the genome of the infected plant cells (Guillon et al. 2006a, b). The hairy root system thus offers tremendous potential for delivering transgenes, and evaluating gene functions in plant roots.
This study reports the establishment of a system of A. rhizogenes-mediated hairy root transformation in both wild and domesticated varieties of watermelon. Genetic manipulation using this protocol is rapid, highly efficient, and reproducible. Factors influencing the efficiency of genetic transformation, such as strains of A. rhizogenes and tissue types of watermelon, are also described. Furthermore, we examined effect of osmotic stress on the growth of hairy roots derived from these watermelon varieties.
Materials and methods
Plant materials
A wild variety of watermelon (Citrullus lanatus sp. No.101117-1), which grows naturally in the Kalahari Desert, has been described previously (Kawasaki et al. 2000). As a reference, we also used a domesticated watermelon cultivar (Citrullus lanatus cv. Sanki) (Marutane Breeding, Kyoto, Japan) for hairy root induction. After removing the outer seed coat, these seeds were surface-sterilized for 10 min with 5% (v/v) sodium hypochlorite and 0.05% (v/v) Tween-20, and rinsed five times with sterile water. The sterilized seeds were germinated in vitro and grown for 4 days on hormone-free Murashige and Skoog (MS) medium (Murashige and Skoog 1962) containing 0.3% gellan gum, at 28°C in the dark.
Agrobacterium strains and binary vector
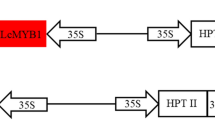
Two A. rhizogenes strains, ATCC43056 (R1000) and ATCC15834, harboring a binary vector pIG121-Hm (Akama et al. 1992) were used for co-cultivation experiments. The T-DNA of the binary vector contains a neomycin phosphotransferase II (NPTII) cassette for kanamycin resistance and a hygromycin phosphotransferase (hpt) gene cassette for hygromycin resistance, which are located adjacent to the left and right borders, respectively. The vector also contains an intron-GUS gene (encoding β-glucuronidase) expression cassette (Ohta et al. 1990) between the NPTII and hpt gene cassettes. The binary vector was introduced into each A. rhizogenes strain via electroporation.
A single colony of the transformed bacteria was used to inoculate liquid YEB medium supplemented with kanamycin (50 mg/l) and hygromycin (50 mg/l). Bacterial cultures were grown overnight at 28°C. Agrobacterium cells were collected by centrifugation at 10,000 × g for 1 min at room temperature. The cells was resuspended in liquid MS medium and adjusted to an OD600 of 0.4–0.5, prior to transformation.
Co-cultivation
The 4-day-old cotyledon, hypocotyl, and root tissues were cut into pieces of 5 × 5 mm (cotyledons) or 5 mm long (hypocotyls and roots), and incubated with the Agrobacterium cells harboring pIG121-Hm in liquid MS medium for 10 min at 28°C. The explants were transferred to MS medium, and co-cultured for 2 days at 28°C in the dark. Following co-cultivation, the explants were washed five times with sterile water containing 500 mg/l cefotaxime to remove the Agrobacteria. They were transferred to selective root induction medium (MS medium supplemented with 50 mg/l kanamycin or 5 mg/l hygromycin) for transgenic hairy root induction. The medium also contained 250 mg/l cefotaxime for the elimination of Agrobacteria. The selection and induction steps were conducted at 26°C under continuous light from white fluorescent lamps (50–100 μmol photons m−2 s−1).
Genomic DNA extraction and polymerase chain reaction (PCR) amplification
Total genomic DNA was extracted from the hairy roots by a Plant DNeasy mini kit (Qiagen, Hilden, Germany) and subjected to genomic PCR analysis. Approximately 50 ng of genomic DNA was used as a template. The primer pair used for the detection of the intron-GUS gene expression cassette was: 35S-F, 5′-ACCCTTCCTCTATATAAGGAAG-3′, and GUS-01R, 5′-CGTGACATCGGCTTCAAATGG-3′. The amplification conditions were: a 2-min melting step at 94°C followed by 30 cycles of a 30-s melting step at 94°C, a 30-s annealing step at 55°C, and a 30-s elongation step at 72°C using 1 unit of Ex Taq DNA polymerase (Takara Bio, Tokyo, Japan). PCR products were analyzed by electrophoretic separation on 1% agarose gels and stained with ethidium bromide.
Total RNA extraction and reverse transcription (RT)-PCR amplification
RT-PCR was performed as described previously (Kajikawa et al. 2009) with some modifications. Total RNA was extracted from the hairy root by a Plant RNA Isolation Mini kit (Agilent, Wilmington, DE) and subjected to DNase (Qiagen) treatment for 15 min at 25°C. Subsequently, the RNA was purified by an RNeasy spin column (Qiagen). One microgram of total RNA was used for reverse transcription using ReverTra Ace-α- (Toyobo, Osaka, Japan). One microliter of the resulting cDNA was added to 20 μl PCR reactions containing 1 unit of Ex Taq DNA polymerase. The primers for the detection of the intron-spliced GUS gene transcript were: GUS-exon-F, 5′-TCTAGAACATGGATCCCTACAGG-3′, and GUS-01R as described above. The sequences for GUS-exon-F and GUS-01R primers correspond to the upstream and downstream regions, respectively, of the intron in the GUS coding region. The primers for the detection of the endogenous actin gene transcript that was used as a RT-PCR control were: CLactin2/7-RT-F, 5′-CATTCTCCGTTTGGACCTTGCT-3′, and CLactin2/7-RT-R, 5′-TCGTAGTTTTCTCAATGGAGGAACTG-3′. The amplification conditions for the GUS and actin fragments were the same as those for the genomic PCR described above. PCR products were analyzed by electrophoretic separation on 1% agarose gels and stained with ethidium bromide.
Histochemical analysis
Transient histochemical GUS assays were conducted on tissue explants 10 days after co-cultivation with A. rhizogenes harboring pIG121-Hm. Tissues were incubated overnight at 37°C in a GUS-staining solution containing 50 mM potassium phosphate buffer, pH 7.0, 5 mM DTT, 0.1% Tween-20, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 20% methanol, and 1 mM 5-bromo-4-chloro-3-indoyl-β-d-glucuronide (X-gluc). The tissues were then soaked in 70% ethanol for several hours to remove chlorophyll. Quantification of GUS-expressing units was performed by counting the number of blue spots on the tissues. For the induced hairy root lines, the histochemical GUS assay was performed 3 weeks after infection, using the GUS-staining solution as described above.
Liquid culture of watermelon hairy roots
Tissues of the watermelon hairy roots (approximately 0.2–0.5 g) were inoculated into 100 ml of B5 liquid medium (Gamborg et al. 1968) containing 30 g/l sucrose, and incubated at 26°C in the dark on an orbital shaker at 80 rpm. Root growth was assayed essentially as described by Dunlop and Curtis (1991). Briefly, the root tissues were aseptically removed from the liquid culture, and liquid medium on their surface was blotted thoroughly by sterilized absorbent paper towels. The blotted tissues were weighed in a sterile petri dish aseptically, and then transferred back into the liquid medium.
Growth analysis of watermelon hairy roots under high osmotic stress condition
To analyze the hairy root growth under high osmotic stress, approximately 5 cm of the watermelon hairy roots were placed on a solid half-strength MS medium supplemented with 250 mg/l cefotaxime and 10% polyethylene glycol (PEG) 6,000 (a reagent grade for molecular biology; Nakalai tesque, Kyoto, Japan), and cultured for 4 days in the dark. The rate of hairy root elongation was measured every 24 h. As a control, the same transgenic lines were placed on a solid half-strength MS medium supplemented with 250 mg/l cefotaxime without PEG 6,000, and the elongation of the hairy toots was monitored.
Results and discussion
Optimization of the transformation efficiency by transient GUS spot assay
To determine the optimal condition for transgene expression, transient GUS spot assays were carried out using different tissues of wild watermelon and two A. rhizogenes strains. A GUS gene expression vector pIG121-Hm was used for transformation. This vector has a GUS gene containing a modified intron from the castor bean catalase gene within the coding sequence; therefore, this intron-GUS reporter gene does not give rise to detectable GUS activity in Agrobacterium cells, but produces the functional protein after gene transfer into plant cells (Ohta et al. 1990). Four-day-old cotyledons, hypocotyls, and the basal and apical parts of the roots from wild watermelon were used as target tissues for transformation. Two A. rhizogenes strains, ATCC43056 and ATCC15834, harboring the pIG121-Hm vector, were used for transformation (Table 1). Ten days after co-culture of each tissue with the A. rhizogenes strain, the tissue segments were subjected to the GUS spot assay. Consequently, the tissues infected with ATCC15834 showed more GUS spots than those infected with ATCC43056 (Table 1). Among tissues infected with ATCC15834, hypocotyls gave rise to the highest number of GUS spots; GUS spots were observed in 19 out of 20 explants (95%) tested in this assay, and the average number of GUS spots was 5.9 per GUS-positive explant, which was markedly higher than the other tissues examined in this assay. This result suggests that hypocotyl fragments and the ATCC15834 strain were the optimal combination of target tissue and A. rhizogenes strain for watermelon transformation. Therefore, this combination was used for the induction of the transgenic hairy roots in the following analyses.
Isolation and characterization of transgenic hairy roots
To isolate transgenic hairy roots carrying the intron-GUS reporter gene, co-cultured and washed hypocotyl fragments were incubated on selective root induction medium. Figure 1 shows representative images for the kanamycin selection of hairy roots derived from wild and domestic varieties of watermelon. After 2 weeks, many roots were induced from the hypocotyl fragments (Fig. 1a–d). The well-elongated lines (>3 cm) on the selective medium (50 mg/L kanamycin or 5 mg/L hygromycin) were transferred onto fresh selective medium. After 1 week, more than 10 hairy root lines that showed further elongation (Fig. 1e, f) were generated from both varieties of watermelon. Hereafter, hairy root lines induced from the wild variety of watermelon were referred to as “wk” (kanamycin-resistant) and “wh” (hygromycin-resistant) lines. Similarly, the kanamycin- and hygromycin-resistant lines induced from the domesticated variety of watermelon were referred to as “dk” and “dh” lines.
a Rhizogenes-mediated transformation of watermelon carrying the intron-GUS reporter gene. After co-incubation with A.rhizogenes, hypocotyl fragments from wild (a) and domesticated (b) varieties of watermelon were placed on MS medium containing kanamycin. Formation of kanamycin-resistant hairy roots from the wild (c) and domesticated (d) varieties was observed 2 weeks after co-incubation. Well-elongated lines were transferred onto the fresh selective medium. After 1 week, hairy roots lines that showed further elongation (indicated by arrows in e and f for wild and domesticated varieties, respectively) were harvested for analyses by genomic PCR, RT-PCR, and histochemical GUS staining. Scale bar 1 cm
The wk and dk lines were subjected to genomic PCR analysis to detect the fragment of the GUS gene expression cassette. Among ten wk lines examined, six showed an amplified DNA band with the expected size for the transgene (Table 2). In the case of dk lines, nine out of ten lines showed the expected signal (Table 2). These observations suggested that the transgene was transmitted into the hairy root lines with high frequency.
Subsequently, the expression of the GUS gene in the genomic PCR-positive lines was detected by RT-PCR. A band corresponding to the intron-spliced GUS transcript (389 bp) was detected from all the 8 wk lines, and 8 out of the 11 dk lines (Fig. 2a, c). A band corresponding to the intron-unspliced GUS transcript (579 bp) was also clearly detected in most of the lines (Fig. 2a, b), suggesting that the castor bean intron was not efficiently spliced from the GUS gene transcript in the watermelon cells. The histochemical GUS assays showed that all the RT-PCR-positive lines in wk lines showed GUS staining (Fig. 2a). The intensity of the GUS staining was relatively weak in the four of the lines (lines wk-4, wk-5, wk-11 and wk-12), in which the intron-unspliced GUS transcript was detected. Among the eight RT-PCR-positive lines in domesticated watermelon, four lines showed strong GUS staining (dk-1, dk-3, dk-7 and dk-10), one line showed partial GUS staining (dk-6), two lines showed weak GUS signal (dk-11 and dk-12) and the other three lines did not show any detectable GUS staining (dk-4, dk-8 and dk-9) (Fig. 2b). The dk-2 line, which had no detectable RT-PCR product, also did not show any GUS staining. In the genomic PCR-negative wk and dk lines, neither RT-PCR nor GUS staining was detected (data not shown). These results demonstrated that the transgenic hairy roots from both wild and domesticated watermelon plants with high expression of the GUS transgene were isolated efficiently in this screening.
RT-PCR and histochemical analyses of GUS gene expression in the transformed hairy roots. The expression of GUS reporter genes was analyzed in the hairy root lines derived from wild (a and b) and domesticated (c and d) varieties of watermelon. The hairy root lines were selected by kanamycin (a and c) or hygromycin (c and d). The sizes for the RT-PCR products correspond to the intron-spliced (389 bp) and -unspliced (579 bp) GUS transcripts are indicated by bars on the right side of each gel image. The watermelon Actin gene was used as the RT-PCR control. The pIG121-Hm vector DNA solution was used as the PCR positive control (vc vector control) for the amplification of the intron-containing GUS fragment. In the images of GUS staining, scale bar 1 cm
In the hygromycin selection, nine and eight of the 10 wh and dh lines, respectively, were genomic PCR-positive (Table 2). RT-PCR experiments showed that the intron-spliced GUS transcript was detected in all the genomic PCR-positive wh and dh lines (Fig. 2b, d). Similar to the kanamycin selection, the intron-remaining GUS transcript was detected in five out of nine wh RT-PCR positive-lines and all the dh RT-PCR-positive lines (Fig. 2b, d). In the histochemical GUS assays, strong GUS staining was detected in four wh lines (wh-1, wh-3, wh-7 and wh-10), in which only the intron-spliced GUS transcript was detected. The remaining five wh lines that accumulated the intron-unspliced GUS transcript showed either weak (wh-2, wh-5 and wh-9), or no GUS staining (wh-6 and wh-8). In the case of dh lines, two (dh-3 and dh-7), three (dh-2, dh-4 and dh-10), and two lines (dh-5 and dh-8) showed strong, weak and no GUS staining, respectively, among the eight RT-PCR-positive lines.
These results demonstrated that hygromycin selection of the transgenic hairy roots is practicable, in contrast to a previous report that transgenic plants were not obtained by hygromycin selection in A. tumefaciens-mediated transformation of wild variety of watermelon (Akashi et al. 2005). In the present study, a positive correlation was observed between the efficiency of splicing of the intron in the GUS transgene, and the strength of the GUS signals (Fig. 2). Interestingly, several transgenic lines with detectable intron-spliced GUS transcript showed no GUS staining (Fig. 2), suggesting that the GUS expression was suppressed post-transcriptionally in these hairy root lines. Although further investigations are necessary to reveal the mechanism of post-transcriptional silencing of the heterologous gene expression in these transgenic watermelon plants, the present study highlights the importance of examining protein expression and/or protein activity to establish the transgenic hairy root lines.
Proliferation of the transgenic hairy roots in the liquid culture
A liquid culture system provides rapid and effective production of the transgenic hairy roots (Giri and Narasu 2000; Triplett et al. 2008); therefore, it might offer a powerful tool for the physiological study of watermelon root tissues. To establish liquid cultures of the transgenic watermelon hairy roots, several lines with relatively rapid growth rates in the kanamycin-resistant lines (wk-11 and wk-12 lines from the wild variety, and dk-11 and dk-12 lines from the domesticated variety) were transferred into 100 ml of B5 liquid medium without the antibiotic (Fig. 3). The hairy root lines grew rapidly in the liquid medium. After 14 days, the fresh weight of the hairy roots in each culture increased by 8- to 18-fold in comparison to their starting weight (Fig. 3). The average growth rates (g/day) were 0.81 ± 0.36 and 1.25 ± 0.33 for wk-11 and wk-12, respectively, and 0.46 ± 0.18 and 0.52 ± 0.08 for dk-11 and dk-12, respectively. The average growth rate of the wk-12 line was different significantly from those of the dk-11 (t test, P < 0.05) and dk-12 lines (t test, P < 0.05), whereas the average growth rate of wk-11 line was statistically insignificant compared to those of the dk lines (t test, P > 0.05). The high growth rate of these hairy root lines was maintained for several passages of the liquid culture (data not shown).
Time course analysis of the hairy root growth in liquid culture. The growth of transformed kanamycin-resistant hairy root cultures of watermelon. In a, the hairy root cultures at the beginning and 14 days after the inoculation are shown for hairy root lines wk-11 and wk-12 derived from wild variety of watermelon, and hairy root lines dk-11 and dk-12 from the domesticated variety. At regular intervals, hairy roots were recovered from the medium and weighed aseptically. In the graph of b, filled circle and diamond designate fresh weight of the hairy roots for wk-11 and wk-12 lines, respectively, whereas open circle and diamond designate those for dk-11 and dk-12 lines, respectively. Data indicate the mean values ± SD from three replicates
Growth of the transgenic hairy roots under high osmotic condition
To examine the effect of environmental stress on the growth of watermelon hairy roots, two hairy root lines derived from wild watermelon (wk-11 and wk-12), and two lines from domesticated watermelon (dk-11 and dk-12) were grown under the high osmotic condition. Osmotic treatment was administrated using PEG 6,000 at a final concentration of 10% on the solid MS medium for 4 days. Differential response was found between the wk and dk lines (Fig. 4). In the dk lines derived from domesticated variety of watermelon, rate of root growth elongation strongly dropped from 0.22 ± 0.01 and 0.28 ± 0.07 cm/day on control medium, to 0.05 ± 0.001 (23% of the control medium; t test, P < 0.01) and 0.03 ± 0.03 cm/day (9%; t test, P < 0.01) on 10% PEG medium for dk-11 and dk-12 lines, respectively. These observations demonstrated very strong inhibition of root growth under high osmotic condition in the dk lines. By contrast, degree of inhibition was markedly small in the wk lines derived from the wild variety of watermelon. Growth rates of wk-11 and wk-12 lines on 10% PEG medium were 0.25 ± 0.08 (51% of the control medium; t test, P < 0.05) and 0.32 ± 0.06 cm/day (75% of the control; t test, P > 0.05), respectively, which were modestly slower or statistically insignificant compared to those in the control medium (0.50 ± 0.06 and 0.43 ± 0.10 cm/day for the wk-11 and wk-12 lines, respectively).
Time course analysis of the hairy root growth in high osmotic condition. The elongation of hairy roots after transfer on a half-strength MS medium supplemented polyethylene glycol are shown for hairy roots derived from the wild (a) and domesticated (b) variety watermelon. Closed circles and triangles designate for wk-11 and wk-12 (a), and dk-11 and dk-12 (b), respectively, on the medium supplemented 10% PEG 6000. Open circles and triangles designate for wk-11 and wk-12 (a), and dk-11 and dk-12 (b), respectively, on the normal medium as control. Data indicate the mean values ± SD from three replicates
In this study, an efficient protocol for the generation of transgenic hairy roots was established from two different varieties of watermelon plants, the one for drought-resistant wild variety, and the other for drought-susceptible domesticated variety. The new protocol presented in this study will be useful in comparative functional analysis of the genes in wild and domesticated watermelon, which show significant differences in root vigor under water deficits. The future applications of this protocol would include loss-of-function experiments of a gene in question by RNAi or antisense techniques, gain-of-function experiments of a gene by overexpression, and measurement of gene expression with chimeric promoter–reporter systems. It is anticipated that these approaches would yield invaluable insights into the molecular mechanisms of root vigor in the desert plant under drought.
References
Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y (1992) Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep 12:7–11
Akashi K, Morikawa K, Yokota A (2005) Agrobacterium-mediated transformation system for the drought and excess light stress-tolerant wild watermelon (Citrullus lanatus). Plant Biotechnol 22:13–18
Akashi K, Yoshimura K, Nanasato Y, Takahara K, Munekage Y, Yokota A (2008) Wild plant resources for studying molecular mechanisms of drought/strong light stress tolerance. Plant Biotechnol 25:257–263
Choi PS, Soh WY, Kim YS, Yoo OJ, Liu JR (1994) Genetic transformation and plant regeneration of watermelon using Agrobacterium tumefaciens. Plant Cell Rep 13:344–348
Dunlop SD, Curtis WR (1991) Synergistic response of plant hairy-root cultures to phosphate limitation and fungal elicitation. Biotechnol Prog 7:434–438
Ellul P, Rios G, Atares A, Roig LA, Serrano R, Moreno V (2003) The expression of the Saccharomyces cerevisiae HAL1 gene increases salt tolerance in transgenic watermelon [Citrullus lanatus (Thumb.) Matsun & Nakai.]. Theor Appl Genet 107:462–469
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Giri A, Narasu L (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18:1–22
Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P (2006a) Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol 24:403–409
Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P (2006b) Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol 9:341–346
Kajikawa M, Hirai H, Hashimoto T (2009) A PIP-family reductase is required for biosynthesis of tobacco alkaloids. Plant Mol Biol 69:287–298
Kawasaki S, Miyake C, Kohchi T, Fujii S, Uchida M, Yokota A (2000) Responses of wild watermelon to drought stress: accumulation of an ArgE homologue and citrulline in leaves during water deficits. Plant Cell Physiol 41:864–873
Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A, Kramer DM (2009) The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ 32:209–219
Larcher W (1995) Plants under stress. In: Physiological plant ecology. Springer, Berlin, pp 321–448
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Takahara K, Akashi K, Yokota A (2005) Purification and characterization of glutamate N-acetyltransferase involved in citrulline accumulation in wild watermelon. FEBS J 272:5353–5364
Triplett BA, Moss SC, Bland JM, Dowd MK (2008) Induction of hairy root cultures from Gossypium hirsutum and Gossypium barbadense to produce gossypol and related compounds. In Vitro Cell Dev Biol Plant 44:508–517
Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49:226–241
Acknowledgments
The authors gratefully acknowledge Ms. Masayo Inoue for her technical contribution and Dr. Kazuhito Akama (Shimane University) for providing us with the pIG121-Hm vector plasmid. This work was financially supported partly by the Japan Science and Technology Agency (JST), partly by the Nissan Science Foundation, partly by a Grants-in-Aid for Scientific Research, Japan Society for the Promotion of Science, the Ministry of Education, Science, Sports and Culture, and partly by a grant from Foundation for Nara Institute of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato.
Rights and permissions
About this article
Cite this article
Kajikawa, M., Morikawa, K., Abe, Y. et al. Establishment of a transgenic hairy root system in wild and domesticated watermelon (Citrullus lanatus) for studying root vigor under drought. Plant Cell Rep 29, 771–778 (2010). https://doi.org/10.1007/s00299-010-0863-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0863-3