Abstract
The Amazon rainforest has experienced rapid land-use changes, including extensive deforestation of riparian areas, putting at risk among the most diverse freshwater fish assemblages on the planet. At nine study reaches distributed among three Colombian blackwater streams, we described and quantified how variability in riparian vegetation structure—during both dry and rainy seasons—influenced the taxonomic and functional diversity of fish assemblages. We observed 117 species across our study reaches, but taxonomic measures such as species richness and evenness were not different among low, intermediate, or high levels of riparian vegetation coverage. Fish assemblage composition was also not different by riparian vegetation, although we observed significant seasonal shifts—only 16% of species were observed in both rainy and dry seasons. Evenness and effective number of species derived from Simpson’s Index were 7 and 38% greater in the dry season than in the rainy season, respectively. Diet varied by riparian zone condition: 56% of fish stomach contents were of allochthonous origin in stream reaches with intact vegetation versus 37% in streams with altered riparian vegetation. We also observed that the relative proportion of detritivorous fishes was 13% greater in the rainy season, while the proportion of invertivores was 26% greater in the dry season. Both functional divergence and dispersion—measures of the variability of species’ traits—were higher in reaches with more intact vegetation during the rainy season. Our findings highlight the importance of the interaction of riparian vegetation and seasonality, and both longitudinal and lateral hydrological connectivity, for fish functional diversity of Amazonian streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture, grazing, hydropower, and human settlement have transformed > 1.4 million km2 of the Amazon basin (~ 20% of total area; Hansen et al. 2013; Arantes et al. 2018). Changes to land cover have altered up to 50% of riparian forests in some regions of the Amazon (Renó et al. 2011), with implications for stream ecosystems including loss or impairment of aquatic habitat, increased erosion, and altered aquatic primary productivity, as well as shifts in flooding regimes leading to changes in species composition and biogeochemical cycling (reviewed in Castello and Macedo 2016). Collectively, these perturbations have put at risk among the most diverse freshwater fish assemblages on the planet: ∼2,500 fish species are found in the Amazon basin—with approximately half endemic to streams (Junk et al. 2007; Winemiller et al. 2008). Despite human activities posing increasing threats to Amazonian streams and the relatively ineffective legislation in place to protect them (Leal et al. 2018), these systems have received limited research attention (but see, e.g., Lorion and Kennedy 2009; Leitão et al. 2018).
Streams are tightly connected to their adjacent riparian zones through exchanges of energy and material (Hynes 1975; Vannote et al. 1980; Junk 1989). Terrestrially derived organic matter inputs from riparian zones can strongly influence the diversity and biomass of fish assemblages (Wondzell and Bisson 2003; Kail et al. 2015; Thompson et al. 2018). Arthropods, for example, provide important nutritional subsidies that support aquatic food webs (Nakano et al. 1999; Nakano and Murakami 2001; reviewed in Baxter et al. 2005; Kautza and Sullivan 2015). Wood entrained into streams from riparian zones provides refuge from predators such as birds and piscivorous fishes (Everett and Ruiz 1993; Koehn et al. 1994; Crook and Robertson 1999), and many fishes exhibit positive associations with large wood for spawning (egg attachment, nest materials), nursery habitat, or feeding (Dolloff and Warren 2003; Gurnell et al. 2005). In addition, the composition and structure of riparian vegetation influence stream ecosystems by controlling light incidence and temperature (Osborne and Kovacic 1993; Abell and Allan 2002; Pusey and Arthington 2003), regulating nutrient concentrations and export (Dodds and Oakes 2008; Larson et al. 2019), and mediating aquatic-terrestrial connectivity (Naiman et al. 1993; Alberts et al. 2013; Alberts and Sullivan 2016). These factors, alone and in concert, influence fish physiology, assemblage composition, and trophic dynamics (Pusey and Arthington 2003; Robinson et al. 2010; Blevins et al. 2013).
In Amazonian streams, environmental heterogeneity is a critical variable related to the diversity of fish assemblages (Ramírez et al. 2015). Environmental characteristics, including variability in riparian vegetation, have been shown to affect both the taxonomic and functional diversity of fish assemblages (Ilha et al. 2018, 2019; Santos et al. 2019). Several studies have observed negative impacts on taxonomic diversity associated with changes in riparian forest cover and land use. For example, streams with grasslands or plantation agriculture in their riparian zones exhibit more homogenous fish assemblages due to lower species diversity and higher relative abundance of generalist species (Casatti et al. 2009; Leite et al. 2015; Juen et al. 2016). Leitão et al. (2018) report decreases in functional uniformity of fish assemblages associated with deforestation, and Brejão et al. (2018) and Bordignon et al. (2016) observed an increase of generalist and a decrease of specialist species as instream habitats are simplified and homogenized. However, higher taxonomic diversity of fishes has also been associated with deforested areas (Burcham 1988; Toham and Teugels 1999; Bojsen and Barriga 2002). Overall, although these findings suggest that the relationship between riparian zones and stream fish assemblages in the Amazon is driven by multiple processes and factors operating across spatiotemporal scales (Montag et al. 2019). However, additional research is needed in order to generate broader patterns on the effects of changes in riparian vegetation on fish assemblages.
Riparian zones are inundated during the Amazon rainy season, increasing the water-mediated availability of terrestrial organic matter and allochthonous energy to stream ecosystems (Pringle 2001; Freeman et al. 2007). This seasonal flooding is especially relevant in streams (Wantzen and Junk 2006; Dos Anjos and Zuanon 2007) such as blackwater ecosystems (sensu Sioli 1950). These blackwater streams are characterized by a high concentration of fulvic and humic acids and a low concentration of dissolved solids and nutrients (oligotrophic systems) that limit the productivity of aquatic primary producers (Lowe-McConnell 1987; Junk 2013; Mendonça et al. 2005). Indeed, a high proportion of fish biomass in blackwater streams of the Colombian Amazon is supported by fruits, arthropods, and plant detritus (Arbeláez et al. 2004; Ramírez et al. 2015; Correa and Winemiller 2018).
Our aim was to describe and quantify how variability in riparian vegetation structure (i.e., canopy cover) and seasonality affects: (i) assemblage structure, (ii) trophic structure, and (iii) taxonomic and functional diversity of fishes in blackwater streams of the Colombian Amazon. We hypothesized that stream reaches where riparian forest vegetation has been altered—leading to reduced canopy cover and altered instream conditions (as described above)—would exhibit more homogeneous fish assemblages. In addition, under these conditions, we anticipated that generalist species would dominate the assemblage. Relative to trophic structure, we expected that altered stream reaches exhibit reduced allochthonous inputs, and therefore, a high percentage of fish diet would be of autochthonous origin. We also hypothesized that fish assemblages in altered reaches would show reduced functional dispersion, functional divergence, and functional evenness. In contrast, we predicted a more even assemblage, including specialist species (invertivores, piscivores), at sites with more intact riparian forest vegetation. We also predicted that the taxonomic and trophic structure of fish assemblages would shift seasonally as a result of changes in resources availability (e.g., those associated with flooded riparian forests) and environmental conditions (e.g., water depth, transparency, dissolved oxygen). Specifically, we expected to find a higher proportion of detritivorous fishes during the rainy season as well as seasonal differences in the taxonomic composition of fish assemblages (e.g., migratory and other species entering streams due to increased connectivity with floodplain lakes and the Amazon River in the rainy season) mediated by changes in environmental conditions.
Materials and methods
Study area and sampling design
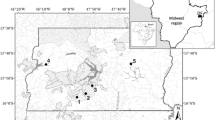
Sampling was performed in three blackwater streams (Yahuarcaca, Arenosa, Pichuna) in the Department of Amazonas, southern Colombia (Fig. 1) during the rainy and dry seasons of 2018. The region is characterized by a monthly temperature range between 25–27 °C (1978–2016), with a 0.7 °C mean increase over the last 40 years (Duque et al. 2018). Annual rainfall averages 3,315 ± 410 mm (1969–2016), with a rainy season from November to May (monthly mean precipitation = 335.4 ± 31.0 mm), followed by a drier season from June to October (monthly mean precipitation = 201.9 ± 34.9 mm; data from Aeropuerto Vázquez Cobo weather station, 04°11' S, 69°56' W). Yahuarcaca (4° 9′ 42.5″ S, 69° 58′ 0.6″ W) is a third-order stream of approximately 16.8 km length (Duque et al. 2018). This stream is characterized by a muddy-to-sandy substrate flowing over Upper Amazonian Tertiary soils (Galvis et al. 1979) and emptying into Lake Yahuarcaca in the floodplain of the Amazon River. Arenosa (4° 7′ 39.9″ S, 69° 57′ 12.8′ W) is a first-order, sandy-bottom stream with a length of 8.5 km, draining over nutrient-poor soils from alluvial plains of tertiary Amazonian blackwater rivers (Duque et al. 1997). Pichuna (4° 5′ 39″ S, 70° 1′ 5.4″ W), also a first-order stream, flows through soils of the Upper Amazonian Tertiary and empties directly into the Amazon River with a drainage area of 4.6 km2 (Sinchi 2017), a length of 12 km (Duque et al. 1997), and a muddy-to-sandy substrate.
Location of the nine study reaches on the Yahuarcaca (4° 9′ 42.5″ S, 69° 58′ 0.6″ W), Arenosa (4° 7′ 39.9″ S, 69° 57′ 12.8′ W), and Pichuna (4° 5′ 39″ S, 70° 1′ 5.4″ W) streams in the Colombian, Amazon, along with the level of canopy cover (low canopy cover LCC, intermediate canopy cover ICC, high canopy cover HCC) for each. For Yahuarcaca, the sequence of treatment (upstream to downstream) was: high, intermediate, low; for Arenosa: high, low, intermediate; and for Pichuna: low, intermediate, high
Three 400–450 m-long reaches in each stream were selected to represent three different levels of riparian alteration: low canopy cover (LCC), intermediate canopy cover (ICC), and high canopy cover (HCC; n = 9 study reaches). Despite that there were multiple study sites (n = 3) per stream, there is considerable distance among them: the minimum distance among reaches was 2,275 m and the mean distance was 3,642 m, thus maximizing independence among study reaches to the degree possible. Although riparian canopy cover was primarily determined based on the percentage of canopy cover (owing to the influence of riparian canopy cover in the structuring of fish fauna in relation to longitudinal distribution and deforestation; Jones et al. 1999; Marsh-Matthews and Matthews 2000), proximity to roads or other built infrastructure (e.g., houses) and the width of the riparian zone (Silveira and Izzo 2015) were also factors considered. Thus, low canopy cover represented highly altered and deforested (< 40% canopy cover) stream reaches with low-to-absent riparian forest, a predominance of herbaceous vegetation, and closer proximity to roads and houses. Intermediate canopy cover (40–60%) represented moderate riparian alteration with riparian forest > 10 m wide. High canopy cover (> 70% canopy cover; i.e., forested) streams exhibited riparian-zone widths > 25 m and represented our control sites. Canopy cover (%) was estimated and analyzed with the Gap Light Analysis Mobile Application GLAMA V3.0 software (Tichý 2015) using canopy photographs taken looking upward through a fisheye lens at six points spaced uniformly along the center of each stream reach (Bojsen and Barriga 2002).
Physicochemical properties and riparian vegetation
At each study reach, three lateral transects across the stream were established at upstream, mid, and downstream locations. At each transect, physicochemical characteristics were measured at three locations (left, midstream, right) during both the rainy (May) and dry (October) seasons (n = 27 sampling points per study reach) of 2018. Water-column transparency (cm) was estimated with a Secchi disk. Water depth (m) was estimated using a manual echo-sounder (Hondex Digital Depth Sounder PS7, Honda Electronix Company, Toyohashi, Japan). Temperature (°C), conductivity (μS cm−1), total dissolved solids (TDS), pH, and dissolved oxygen (DO) were measured with a YSI 556 multiparameter sonde (Model 556, Yellow Springs, Ohio, USA). Streamflow velocity (m s−1) was estimated from measurements of the distance traveled by a floating plastic jar along a transect with known length following APHA methodology (1998). Channel width (m) was measured as the transverse distance between stream margins. Proportional substratum cover (sand, clay, mud and leaf litter) was estimated every 50 cm along the wetted width (m) of every study reach.
Riparian habitat structure was characterized following James and Shugart (1970) with slight modifications. Three representative 10-m × 10-m quadrats were established per study reach, and vegetation structure was measured once during the dry season (27 quadrats in total). In each quadrat, the height of five random trees (m), diameter at breast height (DBH; m) of standing trees, density of shrubs (number of shrubs m−2), and the percentage of ground cover using 20 measurements of an ocular tube were also recorded.
Fish assemblages
Fish were collected at each study reach once during the wet season and once during the dry season using active seining (12 m × 1.5 m with 2.5-cm mesh) and passive gill netting (10 m × 2.5 cm between nodes). Our sampling protocols followed those proposed by Dos Anjos and Zuanon (2007), which have shown that ~ 240 m is a sufficient reach length to adequately represent fish richness in small Amazonian streams. Consistent with this protocol, our sampling effort included 45–60-min collections over ~ 400 m2 at each study reach. Overall, we were confident that our sampling scheme was appropriate for a relative comparison of fish assemblages among study reaches.
All individuals captured were identified to species using taxonomic keys and reviews (Caspers 1980; Kullander 1982; Galvis et al. 2006; Kullander and Ferreira 2006), as well as expert assistance when necessary. Further, species names and taxonomic classification were verified using FishBase and the Fish Catalog website of the California Academy of Science (Fricke et al. 2019). We also used the literature as our primary means of assigning fish species to trophic guilds (see below). In addition to FishBase (Fricke et al. 2019), we reviewed theses and the published literature representing studies conducted in same study streams (Prieto-Piraquive 2000; Gutiérrez 2003; Ramírez 2004; Bolivar 2006; Galvis et al. 2006; Castellanos 2012; Prieto-Piraquive 2012; Ramírez et al. 2015). To confirm trophic guilds and to estimate dietary sources, a small subset of individuals caught (three individuals on average from each of the 117 species found for both seasons) were anesthetized with clove essence (Fernandes et al. 2017) and frozen. Later, the specimens were fixed with 10% concentration formalin and then transferred to a 70%-ethanol solution for subsequent gut-content analysis.
Each fish species was classified according to its trophic guild based both on life-history data available in FishBase (Froese and Pauly 2019) and on analysis of the percentage of items consumed. Stomach contents were identified to the lowest possible taxonomic level, and the percentage of each dietary item was estimated using a visual estimation method (Manko 2016). Fish species were classified into trophic guilds using a polar coordinates analysis following the methodology of Guisande et al. (2013). This analysis allows representing each species along nine axes (based on diet) of coordinates in a two-dimensional graph. The relative location of each species on the graph allowed us to determine the trophic guild: omnivore/generalist (center of the graph), detritivore (consumes allochthonous and autochthonous origin items), allochthonous herbivore (fruits, seeds, flowers), autochthonous herbivore (algae, macrophytes), generalist invertivore (terrestrial and aquatic arthropods), autochthonous invertivore (aquatic insects such as larvae of Diptera, Coleoptera, and Trichoptera), allochthonous invertivore (terrestrial arthropods such as Isoptera, Formicidae, adults of Lepidoptera and Hymenoptera), generalist carnivore (consumes larvae of insects, terrestrial arthropods, fish, among others), and piscivore. In addition, dietary items were classified according to their origin: allochthonous/terrestrial or autochthonous/aquatic.
Taxonomic and functional diversity indices
Taxonomic diversity of fish assemblages was estimated using species richness independently of sample size (number of individuals). Simpson’s index (Simpson 1949) and evenness based on species relative abundances were also calculated for every stream reach (Lomolino and Rosenzweig 1996). Furthermore, we converted Simpson’s index into effective number of species, which informs true diversity and facilitates the interpretation and comparison of the values (Jost 2006).
A suite of traits was used to estimate functional diversity (Mason et al. 2005). Functional traits included body size (maximum standard length [mm]), migration (absent, short: < 100 km, long: > 100 km), fertilization (internal or external), parental care (present, absent), mouth position (lower, sub-lower, sub-terminal, upper, terminal, protrusible and ventral terminal), habitat use (benthonic, nektonic and nekton-benthonic), tooth shape (absent, sawn, caniniform, conical, spatula, incisive, mamilla-like teeth, molariform, multicuspid, pentacuspid, plaques, tiny plaques, tricuspid, and villiform; Géry 1977) and diet/trophic guild: omnivore, detritivore, allochthonous herbivore (terrestrial plant material), autochthonous herbivore (algae, macrophytes), generalist invertivore, autochthonous invertivore, allochthonous invertivore, generalist carnivore, and piscivore. See Online Resource 1: Table ESM_1 for details. Most traits were determined for each species from the literature and available information from FishBase (Froese and Pauly 2019). However, tooth shape, diet, and mouth position were observed from fish collected and analyzed in the laboratory.
To estimate functional diversity, three complementary indices were used: functional dispersion (FDis), functional divergence (FDiv), and functional evenness (FEve). Indices were computed in a space built from a principal coordinate analysis (PCoA) computed on Gower distance. We included the number of species and traits considered when calculating the dissimilarity matrix [dimensions n × p, n = samples or localities (nine) and p = traits (eight)]. Functional dispersion is the mean distance of individual species in multidimensional trait space relative to the centroid of all species (i.e., the spread of species in trait space; Laliberté and Legendre 2010). Functional divergence measures the extent to which the abundances of a community are skewed toward the extremes of functional trait space, so that high FDiv values are associated with a marked niche differentiation among dominant species (Mouchet et al. 2010). Functional evenness represents the regularity of the distribution of abundances in trait space. High FEve values are expected when abundances are homogeneously distributed across trait space. Taxonomic and functional indices were calculated in the R environment (R Development Core Team 2011) using the vegan (Oksanen et al. 2019), BiodiversityR (Kindt and Kindt 2019), and FD (Laliberté and Legendre 2010; Laliberté et al. 2015) packages.
Statistical analyses
To check for independence of fish samples within study reaches in the same stream, we analyzed the effect of reach distances on the dissimilarities in fish species composition. To do this, we tested for correlations between the Bray–Curtis dissimilarity (based on fish species composition) among study reaches from each stream and their corresponding distances along the stream. A positive correlation (i.e., the higher the distance, the higher the differences in community composition) would imply that samples were not independent. We did not find any significant relationships, neither for the dry season (r = 0.39, n = 9, p = 0.30) nor the rainy season (r = 0.09, n = 9, p = 0.81).
We assessed the effects of riparian canopy cover (low, intermediate, high), season (rainy, dry), and the interaction among both factors on fish assemblage composition using non-metric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA). To this end, we first obtained the Bray–Curtis dissimilarities among fish assemblages based on species abundance and the number of species of each trophic guild for each study reach. The 95% confidence ellipses (Fox and Weisberg 2011) were plotted on NMDS ordinations when the PERMANOVA was significant. Permutations were constrained by stream (Yahuarcaca, Arenosa, Pichuna) to account for nestedness in our sampling design. Additionally, SIMPER analysis was performed to evaluate the relative contribution of each species to the dissimilarity in fish assemblages between seasons and among streams. All multivariate analyses were performed using vegan package in R (Oksanen et al. 2019).
We tested for the effect of the riparian canopy cover and season on taxonomic and functional diversity measurements, the number of species of the trophic guilds that contributed the most to the differences between seasons or riparian canopy-cover levels, and the origin of fish diet (% terrestrial/aquatic items) using analysis of variance (ANOVA) in a linear mixed-effects model. Riparian canopy cover and season (the main factors of interest) were considered as fixed effects. Stream, which in our sampling design represented a block that grouped the different experimental units (i.e., canopy cover), was considered as a random effect. We additionally performed pairwise comparisons to test for differences among the levels of the two fixed factors using the emmeans R package (Lenth et al. 2019). The p-values from pairwise comparisons were corrected following Benjamini and Hochberg (2000). We also used mixed-model ANOVA to test for the effect of riparian canopy cover and season on physicochemical (temperature, pH, conductivity, dissolved oxygen, transparency, total dissolved solids, channel depth, and channel width) and vegetation variables (ground cover, tree height, diameter at breast height, and density of shrubs). In this case, because these variables were measured at three lateral transects (i.e., locations) at every reach, location was included in the model as a random effect nested within stream.
Normality and homoscedasticity assumptions were checked visually with normal quantile–quantile plots of residuals and plots representing standardized residuals on the y-axis and fitted values on the x-axis, respectively. To fulfill methodological assumptions, richness, effective number of species, conductivity, TDS, channel depth, and DBH data were transformed using natural logarithms. We considered different variances for each season for evenness, functional dispersion, and functional evenness. All models were fitted using the nlme R package (Pinheiro et al. 2019) and R computing software (R Development Core Team, 2011). All plots were performed using ggplot2 R package (Wickham and Winston 2019).
Results
Physicochemical properties and riparian vegetation
Consistent with our experimental design, canopy cover in riparian forest and shrub cover represented a range of riparian vegetation conditions at our study reaches (Table 1). Water temperature, conductivity, and TDS showed higher values at low riparian cover reaches (mixed model ANOVA: p < 0.01; Table 2). All physicochemical properties analyzed except temperature were different between seasons (mixed-model ANOVA: p < 0.05; Table 2). Both channel depth (rainy 1.6 ± 1.55 m; dry 0.3 ± 0.18 m) and width (rainy 6.7 ± 2.09 m; dry 4.6 ± 2.17 m) were higher in the rainy season than in the dry season (Table 1; mixed-model ANOVA: p < 0.05; Table 2). Sandy substrate dominated low-canopy stream reaches, whereas coarse benthic organic matter (leaf litter and woody material) was typical in forested reaches.
Fish assemblage composition and diet
Across all study reaches, we collected 371 individual fish belonging to 117 species, 32 families, and 9 orders (Online Resource 1: Table ESM_2). Characiformes was the dominant order in terms of both richness and abundance (Fig. 2), with 84 species and 272 individuals representing 56.3% of the total number of fish collected. Assemblage composition differed by season (PERMANOVA: R2 = 0.12, p < 0.01), but not by riparian canopy cover (PERMANOVA: p > 0.20; Fig. 3; Online Resource 1: Table ESM_3). Cyphocharax spirulopsis (detritivore), Pyrrhulina laeta (allochthonous invertivore), Astyanax sp., and Moenkhausia lepidura (allochthonous invertivore) contributed the most to the observed differences between seasons (SIMPER analysis; Online Resource 1: Table ESM_4). In addition, the composition of the community was more dissimilar between streams in the dry season than in the rainy season (PERMANOVA: R2 = 0.12, p < 0.01; Fig. 3).
Differences among streams in the rainy season were mainly driven by changes in the abundances of Cyphocharax spirulopsis (detritivore), Pimelodus blochii (generalist/omnivore), Potamorhina altamazonica (detritivore), Bujurquina moriorum (autochthonous invertivore), and Astianax sp. (allochthonous invertivore; SIMPER analysis; Online Resource 1: Table ESM_4). In fact, when samples from the different reaches were pooled by stream, we did not find any species overlap among all three streams during the rainy season. In contrast, Moenkhausia comma (allochthonous invertivore), Bryconops melanurus (autochthonous invertivore), Ctenobrycon hawxwellianus (generalist/omnivore), Farlowella amazonum (detritivore), Iguanodectes spilurus (autochthonous herbivore), and Pyrrhulina laeta (allochthonous invertivore) occurred in all three streams during the dry season. M. comma was the most ubiquitous species and found in five of the nine stream reaches in both seasons.
Trophic guild structure of fish assemblages was not affected by season (PERMANOVA: p = 0.06) or by riparian canopy cover (PERMANOVA: p > 0.20; Online Resource 1: Figure ESM_5 and Table ESM _6). Nevertheless, the number of invertivores and detritivores was higher in the dry and rainy season, respectively (Fig. 4; ANOVA: F1,10 = 7.0, p = 0.02; ANOVA: F1,10 = 15.07, p = 0.003; Online Resource 1: Table ESM_7).
Average number of species per trophic guild by riparian canopy cover (low, intermediate, high), season (dry, rainy), and stream (Yahuarcaca, Arenosa, Pichuna). For a better graphic representation, we summed the three guilds of invertivores and the two guilds of herbivores. Thus, "invertivore" is the sum of generalist invertivore, autochthonous invertivore, and allochthonous invertivore. "Herbivore" is the sum of allochthonous herbivore and autochthonous herbivore. Error bars indicate ± SD. See Online Resource 1: Table ESM_7 for ANOVA results
Based on gut-content analyses, the diet of fishes was composed of both aquatic (autochthonous origin) and terrestrial items (allochthonous origin). Terrestrial items—present in 32.4% of all species analyzed—mainly consisted of fruits, seeds, flowers, and invertebrates (predominantly Formicidae [ants]; Online Resource 2: Table ESM_8), whereas items of aquatic origin were mainly Diptera. The contribution of each food source to the diet of fish was influenced by both season and riparian cover, which was revealed by the significant interaction between both factors (ANOVA season × canopy cover: F2,10 = 4.22, p = 0.04; Online Resource 1: Table ESM_9; Fig. 5). However, we did not find significant pairwise differences between season and riparian canopy cover (pairwise contrast, p > 0.05; Online Resource 1: Table ESM_10).
Mean contribution percentage of aquatic and terrestrial sources to fish diet by riparian canopy cover (low, intermediate, high) and season (dry, rainy). Error bars represent standard deviation (SD is the same for aquatic and terrestrial items). See Online Resource 1: Table ESM_9 for ANOVA results for diet and Online Resource 1: Table ESM_10 for pairwise treatment differences in diet
Taxonomic and functional diversity
The influence of riparian canopy cover and season was not uniform across taxonomic and functional diversity indices (Fig. 6; Online Resource 1: Table ESM_11). Species richness was not different by riparian cover or season (ANOVA: p > 0.05; Online Resource 1: Table ESM_11). Evenness and effective number of species were higher in the dry than in the rainy season (ANOVA: p < 0.05; online resource 11). Functional dispersion was affected by canopy cover (ANOVA: F2,10 = 22.29, p < 0.001; Online Resource 1: Table ESM_11); in the rainy season, we observed higher FDis in reaches with intermediate and high riparian canopy cover than with low canopy cover (pairwise contrast: p < 0.05; Online Resource 1: Table ESM_12). Functional divergence was also affected by canopy cover (intermediate) in the rainy season (pairwise contrast: p = 0.02; Online Resource 1: Table ESM_12). On the contrary, functional evenness was not affected by riparian canopy cover or season (ANOVA: p > 0.05; Online Resource 1: Table ESM_11). Notably, we also observed greater variability in evenness, functional divergence, and functional evenness in the rainy season. In contrast, species richness and functional dispersion displayed greater variability in the dry season (Online Resource 1: Table ESM_12).
Discussion
Impairment of riparian zones in the Amazon is largely driven by crop agriculture and livestock production, which are rapidly increasing in the tropics (Zimbres et al. 2018). These activities have been shown to contribute to deforestation and loss of biodiversity in tropical watersheds (Wohl et al. 2012) such as the Amazon basin (Castello and Macedo 2016). In our study, we found that alterations to riparian zones affected diet and functional divergence. We did not observe differences in assemblage structure, trophic structure, and taxonomic diversity mediated by riparian canopy cover. However, fish assemblages were more dissimilar, more even, and composed of a higher number of invertivores during the dry season than in the rainy season. On the other hand, detritivores were more common in the rainy season. Functional diversity was not affected by season, but functional dispersion was higher in streams with high and intermediate riparian canopy cover compared to low cover only in the rainy season. Moreover, functional divergence was higher in streams reaches with intermediate canopy cover compared to those with low cover.
Taxonomic Diversity
Among the unexpected findings of this study were the high levels of richness recorded in the three oligotrophic study streams (117 species in total), compared with other similar-sized streams of the Amazon basin (19 species, Castro and Casatti 1997; 35 species, Bührnheim and Cox Fernandes 2003; 49 species, Mendonça et al. 2005; 53 species, Espírito‐Santo et al. 2009; 13 species; Espírito-Santo and Zuanon 2017). High species richness in nutrient-poor Amazon streams is related to adaptive processes such as a wide range of feeding and reproductive strategies, color diversification, and miniaturization (reduction of body size; Weitzman and Vari 1988) mediated by geographic isolation (Oberdorff et al. 2019). Some of these adaptations (e.g., miniaturization) reduce energetic demands by fishes that inhabit resource-limited environments (Zorro 2018), thereby minimizing inter- and intraspecific competition and allowing for a greater overlap of niche breadths (Mortillaro et al. 2015).
Inconsistent with our hypotheses, species richness and diversity (e.g., effective number of species derived from Simpson’s Index) were not significantly different among canopy cover levels (Fig. 2, Online Resource 1: Table ESM_11). These findings suggest that the effect of vegetation structure on the taxonomic diversity of fish assemblages may be context dependent, influenced by other factors like chemical water quality (Mendonça et al. 2005) or varying by spatial scale (Tuanmu and Jetz 2015). For example, we observed that, at broader spatial extents (e.g., 1 km2), the amount of forest cover surrounding the study streams was high (Yahuarcaca 68%, Arenosa 72%, Pichuna 70%), yet within the immediate riparian zone, stream reaches may not exhibit intact riparian forests. In that sense, the forest matrix of a stream could be a ‘source’ of species to the ‘sink’ of deforested stream reaches (Noss 1990; Zeni et al. 2019). Stream network connectivity could allow species with high dispersal capacity to persist in impaired habitats via mass effects (i.e., source-sink models of metapopulation theory; Gido et al. 2016). Indeed, Orlinskiy et al. (2015) observed the potential of forests as recolonization sources for vulnerable aquatic invertebrates in agricultural streams. Except for very small-bodied species with small home ranges (Woolnough et al. 2009), many fish species commonly move from deforested to forested area; thus, diversity in stream reaches with low riparian vegetation cover can be maintained.
Despite some studies having illustrated the negative effects of removing riparian forests on fish assemblage diversity—e.g., more homogenous fish assemblages due to lower species diversity and higher relative abundances of generalist species (Casatti et al. 2009; Leite et al. 2015; Juen et al. 2016)—others have shown results similar to those found in our work. Working in Amazonian streams in Ecuador and Brazil, Bojsen and Barriga (2002) and Fernandes et al. (2013), respectively, observed an overall increase in abundance and species richness in streams where the riparian forest was partially removed. The investigators implicate the presence of fishes that can better exploit certain conditions (e.g., higher primary productivity of deforested stream reaches) as a likely explanation. Indeed, stream reaches with low canopy cover in our study sites showed significantly higher conductivities and temperatures compared to forested reaches (Table 1). In turn, higher conductivity and temperatures are often associated with higher nutrient and light availability, respectively (Paul and Meyer 2001), promoting an overall increase in primary productivity (Pusey and Arthington 2003). In fact, we observed numerically higher primary productivity in streams reaches with low canopy cover (x̄ = 0.137 ± 0.14 mg m−3) than those with intermediate (x̄ = 0.016 ± 0.015 mg m−3) or high (x̄ = 0.07 ± 0.0038 mg m−3; unpublished data) canopy cover. Thus, many fish species inhabiting areas with low riparian cover may benefit from greater food availability, such as secondary production of macroinvertebrates (Bojsen and Barriga 2002; Bojsen and Jacobsen 2003).
Fish assemblage composition was also not significantly different across levels of canopy cover, although we observed significant shifts by season (Fig. 3), implying high beta diversity among streams. We observed that only 16.2% of species were shared among seasons including, for example, Moenkhausia comma, Pyrrhulina laeta, and Astyanax sp. Several studies suggest that fish assemblages in streams are usually subject to regional (e.g., stream order and annual rainfall (Hoeinghaus et al. 2007; Pinto et al. 2009) or local (e.g., riparian vegetation, depth, width and water temperature; Grossman et al. 1998; Bryce et al. 1999; Fernandes et al. 2013) factors. Events such as colonization, migration, and species displacements (Winemiller et al. 2008) can also influence species turnover among Amazonian streams (Mojica et al. 2009).
In particular, seasonal effects can be promoted by changes in physical and chemical properties associated with rainfall variability (Junk et al. 1989). The greater channel depths and widths found in the rainy season increase hydrological connectivity between streams (and stream reaches) and, consequently, fish migration (Tockner et al. 2000; Torres-Bejarano et al. 2013). In this regard, Cyphocharax spirulopsis, Pimelodus blochii, and Potamorhina altamazonica were the migratory species that most contributed to the observed seasonal differences. The latter two species perform short (Gil-Manrique et al. 2011) and medium distance (between 100 and 500 km; Usma et al. 2013) migrations, respectively, from whitewater rivers (Amazon River) to blackwater streams when water levels rise (Galvis et al. 2006). Moreover, the differences in water transparency, oxygen saturation, and pH can affect habitat quality and therefore the distribution of fish along streams (Mendonça et al. 2005; Espírito-Santo et al. 2009; Winemiller and Jepsen 1998). However, other studies conducted in small Amazonian streams have reported little seasonal change in fish assemblages (Bührnheim and Cox-Fernandes 2001; Arbeláez et al. 2004).
Work in remote environments such as the Colombian Amazon poses many challenges, including logistical, infrastructure, and “off-limits” areas (Fernández 2011; Kintisch 2014). These challenges can alter experimental designs, collection methods, and other field protocols and may contribute to the wide variety of collection methods employed in studies of fish in tropical streams (e.g., hand and seine nets: Espíritu-Santo et al. 2009, Ferreira et al. 2018; electrofishing: Lorion and Kennedy 2009, Zeni et al. 2017; dip nets: Montag et al. 2018; circular hand nets: Santos et al. 2019). Although we are confident that our sampling design was robust, we urge interpreting our abundance estimates as relative (among experimental treatments) vs. absolute.
Diet, trophic structure, and functional diversity
The acute cyclic changes associated with seasonality in tropical streams (Lowe-McConnell 1987) affects habitat conditions that frequently result in differences in the availability of food resources for fishes (Prance and Goulding 1981). For example, in lowland streams, many periphyton grazers shift to feeding on detritus and sediments rich in organic matter when periphyton stocks are reduced (as may happen on a seasonal basis; Winemiller et al. 2008). During the rainy season, we observed that 23% of fish species were detritivores, followed by generalists (17% of species) and primarily allochthonous invertivores (16% of the species). During the dry season, 9% of species were detritivores, and the majority of species consumed allochthonous and autochthonous invertebrates (32% and 30% of species, respectively).
The presence and distribution of riparian vegetation mediate the input of allochthonous material into streams (Chan et al. 2008) and explain the pattern we observed, wherein 56% of fish stomach contents were of allochthonous origin in stream reaches with high versus 37% with low canopy cover (Fig. 5). Multiple investigations have reported similar findings (e.g., Lowe-McConnell 1999; Alvim and Peret 2004; Cardoso and Couceiro 2017). Additionally, the consumption of allochthonous resources might be associated with dietary and morphological specializations of fishes (Winemiller et al. 2008). Indeed, allochthonous invertivores with a superior mouth position in our study exhibited higher quantities of allochthonous resources in their stomachs (e.g., Carnegiella strigata: 100% Formicidae; C., sternicla: 50% Formicidae, 30% other terrestrial insects, 20% terrestrial Coleoptera; Anablepsoides ornatus: 65% terrestrial Coleoptera, 35% Formicidae). This pattern has been confirmed in other studies (e.g., Galvis et al. 2006; Castellanos 2012).
Although our dietary results generally coincide with findings of other studies in tropical stream ecosystems (Ramírez et al. 2015; Correa and Winemiller 2018), they should be interpreted with care owing to the low sample size (n = 3 per species) of stomach-content analysis. Typically, dietary studies based on gut-content analysis include substantially more individuals per species in order to account for individual variability in foraging as well as other factors (e.g., Bonato et al. 2012; Cardoso and Couceiro 2017; Souza et al. 2020). Nonetheless, our empirical findings—coupled with our literature review of diet and trophic guilds—underscore the contributions of terrestrial insects, fruits, and other plant material to the diet of multiple fish species in Amazonian streams. This observation implicates the importance of maintaining the riparian forest and can explain, at least in part, the paradox of high species richness in these oligotrophic systems.
Our findings did not provide compelling evidence of a strong relationship between species number per trophic guild and changes in vegetation structure. This may be related to the high trophic plasticity of fish communities in Neotropical streams (Winemiller et al. 2008). For example, Melo et al. (2019) observed that the diet of Mylossoma duriventre was based on fruits and seeds before the construction of a dam on the Río Madeira, Brazil, but shifted to a carnivorous diet primarily consisting of aquatic insects after dam construction. However, this result must be considered in the context of other studies that have shown negative changes in fish guild structure (e.g., Casatti et al. 2009; Leite et al. 2015; Juen et al. 2016), with specialists tending to disappear under large-scale changes in riparian vegetation.
The numbers of species of detritivores and invertivores were higher during the rainy and dry seasons, respectively. Observed differences could be attributed to changes in habitat conditions that resulted in differences in the availability of food resources (e.g., in the rainy season the availability of organic matter can increase; Prance & Goulding 1981) as hydrological connectivity between streams and their adjacent ecosystems increase (i.e., here, Yahuarcaca lakes and the Amazon River). In the rainy season, many detritivorous species of our study migrate seasonally (8 of the 16 detritivores species observed), with abundant species such as Potamorhina altamazonica and Psectrogaster amazonica that migrate on a local scale moving from the Amazon river to the blackwater streams as water levels rise (Galvis et al. 2006). In this way, migratory fishes—such as 30 of the species we observed in our study—can transport nutrients from nutrient-rich whitewater river ecosystems to nutrient-poor blackwater streams, thus linking spatially distant food webs (Winemiler and Jepsen 2002).
During the dry season, we found a higher number of invertivorous species (46 species: allochthonous invertivore 32%, autochthonous invertivore 30%, generalist invertivore 4% of total number of species). Invertebrates were the main food resource consumed in the dry season, supported by our observation of a 26% greater relative proportion of invertivorous fishes in the dry season than in the wet season. Several studies reference the importance of invertebrates for fish assemblages of blackwater streams (e.g., Prance & Goulding 1981; Correa and Winemiller 2018), especially during the dry season (Peterson and Winemiller 1997, Rueda-Delgado et al. 2006). Multiple mechanisms may drive increased invertivore abundance during the dry season and conversely, lower density during the rainy season. For example, hydrological variability during the rainy season may create unsuitably harsh conditions for many fish species, restricting them to leaf litter packs that provide invertebrate food and shelter (Richardson 1992). More stochastic and unstable hydrological conditions can reduce both food availability and capture efficiency (Uieda 1995). Similarly, Henderson and Walker (1986) suggested that less turbulent conditions during the dry season would increase the influence of macroconsumer (e.g., fish, shrimp) in controlling the abundance of invertebrates associated with leaf litter in Amazonian blackwater streams.
Contrary to our predictions, we found variability in functional divergence (i.e., increased niche differentiation) among species in reaches of intermediate canopy cover during the rainy season. Stream reaches under these conditions are commonly characterized by high lateral hydrological connectivity, patchy and dynamic habitats, and multiple forms of refugia such as variable water depths and vegetation coverage (e.g., Galacatos et al. 2004; Espírito-Santo et al. 2009; Pazin et al. 2006) that might be expected to promote increased niche diversity. Structural complexity has been linked to functional divergence in coral reef fishes, where it is thought to act as an environmental filter that governs the distribution and abundance of associated traits (Richardson et al. 2017). Barbosa et al. (2020) report that agricultural disturbance was related to an increase in fish functional diversity, implicating water-quality changes and implying that disturbance may lead to greater functional divergence. Teresa and Casatti (2012) suggest that stream deforestation can positively affect fish functional diversity in Neotropical lowland streams mainly via decreases in species turnover among mesohabitat patches. However, this latter line of evidence only partly supports our observations for functional dispersion, where we observed a greater breadth of trait combinations in reaches with intermediate and high riparian canopy cover, also only in the rainy season.
Amazonian streams can have extremely high fish richness, representing ecosystems of great relevance for the maintenance of biodiversity both globally and in the basin. At least half of Amazonian fish species occur in complex networks of small streams (Mcclain and Elsenbeer 2001; Junk et al. 2007; Albert and Reis 2011). Yet, these small streams have been less studied compared to larger rivers (Beltrão et al. 2019), and their rate of alteration by human activities is increasing rapidly (Castello and Macedo 2016). Their critical roles as reservoirs of biodiversity coupled with their precarious conservation status makes increased research with clear management implications urgent for these imperiled streams and their fish assemblages.
Although we have highlighted challenges and caveats to this study, our findings contribute important information to conservation planning in Colombia, a biodiversity hotspot that has not received sufficient ecological research attention (e.g., Negret et al. 2017). Our findings support specific management considerations. Firstly, hydrological connectivity between small Amazonian streams and their adjacent floodplains will be critical in sustaining fish assemblages and stream ecosystem functioning (Gido et al. 2016). For example, we observed that ~ 56% of stomach contents were of allochthonous origin in stream reaches with high canopy cover vs 37% in streams with low canopy cover. Both climate change (e.g., alteration of rainfall patterns) and modification of riparian vegetation cover can interrupt hydrological connectivity and impair streams through multiple synergistic effects (Brando et al. 2012). Although we did not observe reduced taxonomic richness in reaches with low canopy cover, we did observe alterations in functional measures of fish assemblages, implying that alterations to riparian vegetation can affect the mechanisms by which fish interact with their environment. Further, the maintenance of fish assemblage diversity across reaches with various degrees of canopy cover was likely enabled by fish movement and longitudinal connectivity, highlighting the need to maintain free-flowing streams (i.e., not fragmented, for example, by dams; Sousa et al 2018).
We recommend that future studies incorporate broader temporal and spatial scales. The timing of studies, for instance, can be an important consideration as biological responses of fish assemblages may exhibit a lag following alterations in riparian zones or instream habitat (Zeni et al. 2017; Brejão et al. 2018). Studies would also benefit from incorporating the spatial extent at which landscape attributes are measured. Broader landscape variables can exert strong impacts on inferred species–landscape relationships (e.g., the scale of effect or the scale of ecological response; Jackson and Fahrig 2015). For example, the percentage of forest cover at the landscape scale may have a significant effect on fish assemblage at the local, reach scale. Therefore, we also recommend including multi-scale approaches in future studies.
References
Abell R, Allan JD (2002) Riparian shade and stream temperatures in an agricultural catchment, Michigan, USA. SIL Proceedings. https://doi.org/10.1080/03680770.2001.11902578
Albert JS, Reis RE (eds) (2011) Historical biogeography of neotropical freshwater fishes. University of California Press, Oakland, California
Alberts JM, Sullivan SMP (2016) Factors influencing aquatic-to-terrestrial contaminant transport to terrestrial arthropod consumers in a multiuse river system. Environ Pollut 213:53–62. https://doi.org/10.1016/j.envpol.2016.02.003
Alberts JM, Sullivan SMP, Kautza A (2013) Riparian swallows as integrators of landscape change in a multiuse river system: Implications for aquatic-to-terrestrial transfers of contaminants. Sci Total Environ 463–464:42–50. https://doi.org/10.1016/j.scitotenv.2013.05.065
Alvim MC, Peret AC (2004) Food resources sustaining the fish fauna in a section of the upper São Francisco River in Três Marias, MG, Brazil. Braz J Biol 64:195–202. https://doi.org/10.1590/S1519-69842004000200003
Dos Anjos MB, Zuanon J (2007) Sampling effort and fish species richness in small terra firme forest streams of central Amazonia, Brazil. Neotrop Ichthyol. https://doi.org/10.1590/s1679-62252007000100006
Arantes CC, Winemiller KO, Petrere M et al (2018) Relationships between forest cover and fish diversity in the Amazon River floodplain. J Appl Ecol. https://doi.org/10.1111/1365-2664.12967
Arbeláez F, Gálvis G, Mojica JI, Duque S (2004) Composition and richness of the ichthyofauna in a terra firme forest stream of the Colombian Amazonia. Amazoniana 18:95–107
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220. https://doi.org/10.1111/j.1365-2427.2004.01328.x
Beltrão H, Zuanon J, Ferreira E (2019) Checklist of the ichthyofauna of the Rio Negro basin in the Brazilian Amazon. Zookeys. https://doi.org/10.3897/zookeys.881.32055
Benjamini Y, Hochberg Y (2000) On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. https://doi.org/10.2307/1165312
Blevins ZW, Effert EL, Wahl DH, Suski CD (2013) Land use drives the physiological properties of a stream fish. Ecol Indic 24:224–235. https://doi.org/10.1016/j.ecolind.2012.06.016
Bojsen BH, Barriga R (2002) Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshw Biol 47:2246–2260. https://doi.org/10.1046/j.1365-2427.2002.00956.x
Bojsen BH, Jacobsen D (2003) Effects of deforestation on macroinvertebrate diversity and assemblage structure in Ecuadorian Amazon streams. Arch Fur Hydrobiol 158:317–342. https://doi.org/10.1127/0003-9136/2003/0158-0317
Bolivar A (2006) Estudio de la comunidad de peces de dos quebradas de aguas Negras (Amazónicas): Ecología y bases genéticas para su entendimiento. Universidad Nacional de Colombia sede Amazonia, p 103
Bonato KO, Delariva RL, Silva JCD (2012) Diet and trophic guilds of fish assemblages in two streams with different anthropic impacts in the northwest of Paraná. Brazil Zoologia (curitiba) 29(1):27–38
Bordignon CR, Casatti L, Brejão GL (2016) Taxonomic and functional responses of stream fish assemblages from Amazon. In: II International symposium of ecology. “Ecology in the Anthropocene”
Brando PM, Nepstad DC, Balch JK et al (2012) Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob Chang Biol. https://doi.org/10.1111/j.1365-2486.2011.02533.x
Brejão GL, Hoeinghaus DJ, Pérez-Mayorga MA et al (2018) Threshold responses of Amazonian stream fishes to timing and extent of deforestation. Conserv Biol. https://doi.org/10.1111/cobi.13061
Bryce SA, Larsen DP, Hughes RM, Kaufmann PR (1999) Assessing relative risks to aquatic ecosystems: A mid-Appalachian case study. J Am Water Resour Assoc. https://doi.org/10.1111/j.1752-1688.1999.tb05449.x
Bührnheim CM, Cox Fernandes C (2003) Structure of fish assemblages in Amazonian rain-forest streams: effects of habitats and locality. Copeia. https://doi.org/10.1643/0045-8511(2003)003[0255:SOFAIA]2.0.CO;2
Burcham J (1988) Fish communities and environmental characteristics of two lowland streams in Costa Rica. Rev Biol Trop 36(2A):273–285
Cardoso AC, Couceiro SRM (2017) Insects in the diet of fish from Amazonian streams, in western Pará, Brazil. Mar Freshw Res. https://doi.org/10.1071/MF16173
Casatti L, de Ferreira CP, Carvalho FR (2009) Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: An assessment of two tropical pasture river basins. Hydrobiologia 632:273–283. https://doi.org/10.1007/s10750-009-9849-y
Caspers H (1980) Jacques Géry: Characoids of the World. TFH Publications, lnc. Ltd., Neptune City, NJ, 672 pp, 1977. ISBN 0–87666–483–3. https://doi.org/10.1002/iroh.19800650616
Castellanos C (2012) Disponibilidad y uso de los recursos alimenticios de las especies de peces que habitan la columna de agua en un río amazónico de Terra firme. Tesis Doctoral. Universidad Complutense de Madrid, Facultad de Ciencias Biológicas Departamento de Zoología y Antropología Física. 128 p.
Castello L, Macedo MN (2016) Large-scale degradation of Amazonian freshwater ecosystems. Glob Chang Biol 22(3):990–1007
Castro RM, Casatti L (1997) The fish fauna from a small forest stream of the upper Parana River basin, southeastern Brazil. Ichthyol Explor Freshwaters 7(4):337–352
Catarino MF, Zuanon J (2010) Feeding ecology of the leaf fish Monocirrhus polyacanthus (Perciformes: Polycentridae) in a terrafirme stream in the Brazilian Amazon. Neotrop Ichthyol. https://doi.org/10.1590/s1679-62252010000100022
Chan E, Zhang Y, Dudgeon D (2008) Arthropod ‘ rain ’ into tropical streams: the importance of intact riparian forest and influences on fish diets. Mar Freshw Res 59:653–660
Correa SB, Winemiller K (2018) Terrestrial–aquatic trophic linkages support fish production in a tropical oligotrophic river. Oecologia. https://doi.org/10.1007/s00442-018-4093-7
Crook DA, Robertson AI (1999) Relationships between riverine fish and woody debris: implications for lowland rivers. Mar Freshw Res 50(8):941–953
Dodds WK, Oakes RM (2008) Headwater influences on downstream water quality. Environ Manage. https://doi.org/10.1007/s00267-007-9033-y
Dolloff CA, Warren ML (2003) Fish relationships with large wood in small streams. Am Fish Soc Symp 37:179–193
Duque S, Ruiz J, Gómez J, Roessler E (1997) Tipificación ecológica de ambientes acuáticos en el área del eje Apaporis - Tabatinga, Amazonia Colombiana. In: Zonificación ambiental del eje Apaporis - Tabatinga (Amazonia colombiana)
Espírito-Santo HMV, Zuanon J (2017) Temporary pools provide stability to fish assemblages in Amazon headwater streams. Ecol Freshw Fish. https://doi.org/10.1111/eff.12292
Espírito-Santo HMV, Magnusson WE, Zuanon J et al (2009) Seasonal variation in the composition of fish assemblages in small Amazonian forest streams: evidence for predictable changes. Freshw Biol 54(3):536–548
Everett RA, Ruiz GM (1993) Coarse woody debris as a refuge from predation in aquatic communities - An experimental test. Oecologia. https://doi.org/10.1007/BF00328954
Fernandes IM, Lourenço LS, Ota RP et al (2013) Effects of local and regional factors on the fish assemblage structure in Meridional Amazonian streams. Environ Biol Fishes. https://doi.org/10.1007/s10641-012-0079-1
Fernández F (2011) The greatest impediment to the study of biodiversity in Colombia. Caldasia 33(2):iii-v. http://www.icn.unal.edu.co/
Ferreira MC, Begot TO, da Silveira PB, Juen L, de Assis Montag LF (2018) Effects of oil palm plantations on habitat structure and fish assemblages in Amazon streams. Environ Biol Fishes 101(4):547–562
Fox J, Weisberg S (2011) Multivariate Linear Models in R. R Dev. Core Team
Freeman MC, Pringle CM, Jackson CR (2007) Hydrologic connectivity and the contribution of stream headwaters to ecological integrity at regional scales. J Am Water Resour Assoc. https://doi.org/10.1111/j.1752-1688.2007.00002.x
Fricke, R., Eschmeyer, W. N. & Van der Laan R (2019) Eschmeyer’s catalog of fishes: genera, species, references. In: Calif. Acad. Sci. (Electronic version)
Froese R, Pauly D (2019) Fishbase. World Wide Web electronic publication. In: FishBase
Galacatos K, Barriga-Salazar R, Stewart DJ (2004) Seasonal and habitat influences on fish communitieswithinthelowerYasuniRiverbasinofEcuadorian Amazon. Environ Biol Fishes 71:33–51
Galvis J, Huguett A, Ruge P, De Boorder H (1979) La Amazonía Colombiana y Sus Recursos Escala 1(500):000
Galvis G, Mojica JI, Duque SR, Castellanos C, Sánchez-Duarte P, Arce M, Leiva M (2006) Peces del medio Amazonas región de Leticia. Panamericana, formas e impresos SA. 546 p.
Gido KB, Whitney JE, Perkin JS, Turner TF (2016) Fragmentation, connectivity and fish species persistence in freshwater ecosystems. In: Closs GP, Krkosek M, Olden DD (eds) Conservation of freshwater fishes. Cambridge, Cambridge, pp 292–323
Gil-Manrique B, Agudelo E, Acosta-Santos A, Villa-Navarro F (2011) Pimelodus blochii Capítulo 7. In: Lasso, C A, Córdoba A, Jiménez-Segura L, et al. (eds) I Catálogo de los recursos pesqueros continentales de Colombia Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH), Bogotá, pp 464–465
Géry J (1977) Characoids of the World. TFH Publications, Neptune City, 315 p
Grossman GD, Ratajczak RE, Crawford M, Freeman MC (1998) Assemblage organization in stream fishes: effects of environmental variation and interspecific interactions. Ecol Monogr. https://doi.org/10.1890/0012-9615(1998)068[0395:AOISFE]2.0.CO;2
Guisande CG, Lise A V, Felpeto AB (2013) Tratamiento de datos con R, Statistica y SPSS. Ediciones Díaz de Santos
Gurnell A, Tockner K, Edwards P, Petts G (2005) Effects of deposited wood on biocomplexity of river corridors. Front Ecol Environ 3(7):377–382
Gutiérrez AL (2003) Análisis de algunos aspectos tróficos y reproductivos de la comunidad de peces de un caño de aguas negras en cercanías de Leticia, Amazonas. Departamento de Biología, Universidad Nacional de Colombia, Colombia. Tesis de grado, p 142
Hansen MC, Potapov PVMR et al (2013) High-resolution global maps of 21st century forest cover change. Science 342:850–853
Henderson PA, Walker I (1986) On the leaf litter community of the Amazonian blackwater stream Tarumazinho. J Trop Ecol 2(1):1–16
Hoeinghaus DJ, Winemiller KO, Birnbaum JS (2007) Local and regional determinants of stream fish assemblage structure: inferences based on taxonomic vs. functional groups. J Biogeogr 34:324–338. https://doi.org/10.1111/j.1365-2699.2006.01587.x
Hynes HBN (1975) Edgardo Baldi memorial lecture. The stream and its valley. Verhandlungen der Int Vereinigung fur Theor und Angew Limnol. Verhandlungen 19(1):1–15
Ilha P, Schiesari L, Yanagawa FI et al (2018) Deforestation and stream warming affect body size of Amazonian fishes. PLoS ONE 13:1–20. https://doi.org/10.1371/journal.pone.0196560
Ilha P, Rosso S, Schiesari L (2019) Effects of deforestation on headwater stream fish assemblages in the Upper Xingu River Basin, Southeastern Amazonia. Neotrop Ichthyol 17:1–12. https://doi.org/10.1590/1982-0224-20180099
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr. https://doi.org/10.1111/geb.12233
James FCFC, Shugart HHJ (1970) A quantitative method of habitat description. Audubon Field Notes 24(6):727–736
Jones EB, Helfman GS, Harper JO, Bolstad PV (1999) Effects of riparian forest removal on fish assemblages in Southern Appalachian streams. Conserv Biol 13:1454–1465
Jost L (2006) Entropy and diversity. Oikos 113(2):363–375
Juen L, Cunha EJ, Carvalho FG et al (2016) Effects of oil palm plantations on the habitat structure and biota of streams in eastern Amazon. River Res Appl 32:2081–2094. https://doi.org/10.1002/rra.3050
Junk WJ (1989) Flood tolerance and tree distribution in central Amazonian floodplains. Trop for Bot Dyn Speciat Divers. https://doi.org/10.1016/b978-0-12-353550-4.50012-5
Junk WJ (ed) (2013) The central Amazon floodplain: ecology of a pulsing system, vol 126. Springer Science & Business Media
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci 106(1):110–127
Junk WJ, Soares MGM, Bayley PB (2007) Freshwater fishes of the Amazon River basin: their biodiversity, fisheries, and habitats. Aquat Ecosyst Heal Manag 10:153–173
Kail J, Brabec K, Poppe M, Januschke K (2015) The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: a meta-analysis. Ecol Indic 58:311–321
Kautza A, Sullivan SMP (2015) Shifts in reciprocal river-riparian arthropod fluxes along an urban-rural landscape gradient. Freshw Biol 60:2156–2168. https://doi.org/10.1111/fwb.12642
Kindt R, Kindt MR (2019) Package ‘BiodiversityR’. Package Community Ecol Suitabil Anal 2:11–12
Kintisch E (2014) The joys and hazards of fieldwork. Science. https://doi.org/10.1126/science.caredit.a1400224
Koehn JD, O’Connor NA, Jackson PD (1994) Seasonal and size-related variation in microhabitat use by a southern victorian stream fish ssemblage. Mar Freshw Res 45:1353–1366. https://doi.org/10.1071/MF9941353
Kullander SO (1982) Cichlid fishes from the La Plata Basin Part IV review of the Apistogramma species with description of a new species (Teleostei Cichlidae). Zool Scr. https://doi.org/10.1111/j.1463-6409.1982.tb00541.x
Kullander SO, Ferreira EJG (2006) A review of the South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyol Explor Freshw 17(4):289–398
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91(1):299–305
Laliberté E, Legendre P, Shipley B (2015) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R Packag
Larson DM, Dodds WK, Veach AM (2019) Removal of woody riparian vegetation substantially altered a stream ecosystem in an otherwise undisturbed grassland watershed. Ecosystems. https://doi.org/10.1007/s10021-018-0252-2
Leal CG, Barlow J, Gardner TA et al (2018) Is environmental legislation conserving tropical stream faunas? a large-scale assessment of local, riparian and catchment-scale influences on Amazonian fish. J Appl Ecol 55:1312–1326. https://doi.org/10.1111/1365-2664.13028
Leitão RP, Zuanon J, Mouillot D et al (2018) Disentangling the pathways of land use impacts on the functional structure of fish assemblages in Amazon streams. Ecography (cop) 41:219–232. https://doi.org/10.1111/ecog.02845
Leite GFM, Silva FTC, Gonçalves JFJ, Salles P (2015) Effects of conservation status of the riparian vegetation on fish assemblage structure in neotropical headwater streams. Hydrobiologia. https://doi.org/10.1007/s10750-015-2351-9
Lenth R, Singmann H, Love J, et al (2019) Package “emmeans”: Estimated marginal means, aka least-squares means. In: Compr. R Arch. Netw.
Lomolino MV, Rosenzweig ML (1996) Species diversity in space and time. J Wildl Manage. https://doi.org/10.2307/3802400
Lorion CM, Kennedy BP (2009) Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecol Appl. https://doi.org/10.1890/08-0050.1
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. Cambridge University Press
Manko P (2016) Stomach content analysis in freshwater fish feeding ecology. University of Prešov, vol 116
Marsh-Matthews E, Matthews WJ (2000) Geographic, terrestrial and aquatic factors: which most influence the structure of stream fish assemblages in the midwestern United States? Ecol Freshw Fish. https://doi.org/10.1034/j.1600-0633.2000.90103.x
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness functional evenness and functionaldivergence: the primary components of functional diversity. Oikos 111:112–118
Mcclain ME, Elsenbeer H (2001) Terrestrial inputs to Amazon streams and internal biogeochemical processing. The biogeochemistry of the Amazon Basin, pp 185–208
Melo T, Torrente-Vilara G, Röpke CP (2019) Flipped reducetarianism: a vegan fish subordinated to carnivory by suppression of the flooded forest in the Amazon. For Ecol Manage 435:138–143
Mendonça FP, Magnusson WE, Zuanon J (2005) Relationships between habitat characteristics and fish assemblages in small streams of Central Amazonia. Copeia 2005:751–764. https://doi.org/10.1643/0045-8511(2005)005[0751:RBHCAF]2.0.CO;2
Mojica JI, Castellanos C, Lobón-Cerviá J (2009) High temporal species turnover enhances the complexity of fish assemblages in Amazonian Terra firme streams. Ecol Freshw Fish. https://doi.org/10.1111/j.1600-0633.2009.00382.x
Mojica JI, Lobón-Cerviá J, Castellanos C (2014) Quantifying fish species richness and abundance in a mazonian streams: assessment of a multiple gear method suitable for Terra firme stream fish assemblages. Fish Manage Ecol 21(3):220–233
Montag LFA, Winemiller KO, Keppeler FW et al (2019) Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecol Freshw Fish. https://doi.org/10.1111/eff.12455
Mortillaro JM, Pouilly M, Wach M et al (2015) Trophic opportunism of central Amazon floodplain fish. Freshw Biol 60:1659–1670. https://doi.org/10.1111/fwb.12598
Mouchet MA, Villéger S, Mason NWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876. https://doi.org/10.1111/j.1365-2435.2010.01695.x
Naiman RJ, Decamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecol Appl. https://doi.org/10.2307/1941822
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci 98(1):166–170
Nakano S, Miyasaka H, Kuhara N (1999) Terrestrial-aquatic linkages: Riparian arthropod inputs alter trophic cascades in a stream food web. Ecology. https://doi.org/10.1890/0012-9658(1999)080[2435:TALRAI]2.0.CO;2
Negret PJ, Allan J, Braczkowski A, Maron M, Watson JEM (2017) Need for Conservation Planning in Colombia 31(3):499–500
Noss RF (1990) Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv Biol 4:355–364. https://doi.org/10.1111/j.1523-1739.1990.tb00309.x
Oberdorff T, Dias MS, Jézéquel C et al (2019) Unexpected fish diversity gradients in the Amazon basin. Sci Adv 5(9)
Oksanen J, Blanchet FG, Friendly M et al (2019) vegan: Community Ecology Package. R package version 2.5–2. Cran R
Orlinskiy P, Münze R, Beketov M et al (2015) Forested headwaters mitigate pesticide effects on macroinvertebrate communities in streams: mechanisms and quantification. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2015.03.143
Osborne LL, Kovacic DA (1993) Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw Biol 29:243–258. https://doi.org/10.1111/j.1365-2427.1993.tb00761.x
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol Syst 32(1):333–365
Pazin VF, Magnusson WE, Zuanon J, Mendonça FP (2006) Fish assemblages in temporary pondsadjacent to ‘terra-firme’ streams in Central Amazonia. Freshw Biol 51:1025–1037
Pinheiro J, Bates D, DebRoy S, Sarkar D (2019) R Core Team. 2019. nlme: linear and nonlinear mixed effects models. R package version 3.1–141
Pinto BCT, Araujo FG, Rodrigues VD, Hughes RM (2009) Local and ecoregion effects on fish assemblage structure in tributaries of the Rio Paraíba do Sul Brazil. Freshw Biol. https://doi.org/10.1111/j.1365-2427.2009.02269.x
Prance GT, Goulding M (1981) The fishes and the forest: explorations in Amazonian natural history. Brittonia. https://doi.org/10.2307/2806336
Prieto-Piraquive, (2000) estudio ictiologico de un caño de aguas negras de la Amazonia colombiana, Leticia – Amazonas. Departamento de Biología, Universidad Nacional de Colombia, Colombia. Tesis de grado, p 100
Prieto-Piraquive E (2012) Peces de la quebrada Yahuarcaca. Editorial Académica Espanola, 92 p.
Pringle CM (2001) Hydrologic connectivity and the management of biological reserves: a global perspective. Ecol Appl. https://doi.org/10.1890/1051-0761(2001)011[0981:HCATMO]2.0.CO;2
Pusey BJ, Arthington AH (2003) Importance of the riparian zone to the conservation and management of freshwater fish: a review. Mar Freshw Res 54:1–16. https://doi.org/10.1071/MF02041
R Development Core Team R (2011) R: A Language and Environment for Statistical Computing
Ramírez F (2004) Morfología del aparato bucal y digestivo y su relación con la dieta de algunas especies de peces en una quebrada de aguas negras (Amazonia Colombiana). Pontificia Universidad Javeriana, Bogotá, Tesis de grado. Departamento de Biología, p 124
Ramírez F, Davenport TL, Mojica JI (2015) Dietary-morphological relationships of nineteen fish species from an Amazonian terra firme blackwater stream in Colombia. Limnologica. https://doi.org/10.1016/j.limno.2015.04.002
Renó V, Novo E, Suemitsu C et al (2011) Assessment of deforestation in the Lower Amazon floodplain using historical Landsat MSS/TM imagery. Elsevier
Richardson JS (1992) Food, microhabitat, or both? Macroinvertebrate use of leaf accumulations in a montane stream. Freshw Biol 27:169–176
Richardson LE, Graham NAJ, Pratchett MS, Hoey AS (2017) Structural complexity mediates functional structure of reef fish assemblages among coral habitats. Environ Biol Fishes 100(3):193–207
Robinson JM, Josephson DC, Weidel BC, Kraft CE (2010) Influence of variable interannual summer water temperatures on brook trout growth, consumption, reproduction, and mortality in an unstratified adirondack lake. Trans Am Fish Soc 139:685–699. https://doi.org/10.1577/t08-185.1
Rueda-Delgado G, Wantzen KM, Tolosa MB (2006) Leaf-litter decomposition in an Amazonian floodplain stream: effects of seasonal hydrological changes. J N Am Benthol Soc 25(1):233–249
Santos LL, Benone NL, Soares BE et al (2019) Trait–environment relationships in Amazon stream fish assemblages. Ecol Freshw Fish 28:424–433. https://doi.org/10.1111/eff.12465
Silveira R, Izzo J (2015) Curso de Ecologia de Campo: Programa de Pós-Graduação em Ecologia e Conservação da Biodiversidade, ONF. Brasil
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sioli H (1950) Das Wasser Im Amazonasgebiet Forsch Fortschr 26(21/22):274–279
Sousa R, De Almeida MM, Siqueira-Souza F, Hurd L, De Carvalho FC (2018) Small dams for aquaculture negatively impact fish diversity in Amazonian streams. Aquac Environ Interact 10:89–98. https://doi.org/10.2307/26849944
Souza FB (2020) de Alcantara Santos AC da Silva AT (2020) Trophic structure of ichthyofauna in streams of the contas river basin brazil. Studies on Neotropical Fauna and Environment 10(1080/01650521):1809610
Teresa FB, Casatti L (2012) Influence of forest cover and mesohabitat types on functional and taxonomic diversity of fish communities in Neotropical lowland streams. Ecol Freshw Fish 21:433–442
Tichý L (2015) Gap Light Analysis Mobile Application GLAMA Freeware Scientific Application Dept. of Botany and Zoology, MU Brno, Czech Republic http://www.sci.muni.cz/botany/glama/
Thompson MSA, Brooks SJ, Sayer CD et al (2018) Large woody debris “ rewilding ” rapidly restores biodiversity in riverine food webs. J Appl Ecol. https://doi.org/10.1111/1365-2664.13013
Tockner K, Malard F, Ward JV (2000) An extension of the flood pulse concept. Hydrol Process. https://doi.org/10.1002/1099-1085(200011/12)14:16/17%3c2861::AID-HYP124%3e3.0.CO;2-F
Toham A, Teugels GG (1999) First data on an Index of Biotic Integrity (IBI) based on fish assemblages for the assessment of the impact of deforestation in a tropical West African river system. Hydrobiologia. https://doi.org/10.1023/A:1003605801875
Torres-Bejarano AM, Duque SR, Caraballo-Gracia PR (2013) Heterogeneidad espacial y temporal de las condiciones físicas y químicas de dos lagos de inundación en la Amazonia Colombia. Actual Biol 3598:63–76
Tuanmu MN, Jetz W (2015) A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modelling. Glob Ecol Biogeogr 24(11):1329–1339
Uieda VS (1995) Comunidades de Peixes em um Riacho Litorâneo: Composiçâo, Habitat e Hábitos. PhD Thesis, Universidade Estadual de Campinas, Campinas.
Usma JS, Valderrama M, Escobar MD, et al (2013) Guía de las especies migratorias de la biodiversidad en Colombia. Volumen 2. Peces
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37(1):130–137
Wantzen KM, Junk WJ (2006) Aquatic-terrestrial linkages from streams to rivers: biotic hot spots and hot moments. Arch für Hydrobiol Suppl 158(4)
Weitzman SH, Vari RP (1988) Miniaturization in South American freshwater fishes; an overview and discussion
Wickham H, Winston C (2019) Create Elegant Data Visualisations Using the Grammar of Graphics. Packag “ggplot2.” https://doi.org/10.1093/bioinformatics/btr406
Winemiller KO, Jepsen DB (1998) Effects of seasonality and fish movement on tropical river food webs. J Fish Biol 53:267–296
Winemiller KO, Agostinho AA, Caramaschi ÉP (2008) Fish ecology in tropical streams. In: Tropical stream ecology, pp 107–III. Academic Press
Wohl E, Barros A, Brunsell N et al (2012) The hydrology of the humid tropics. Nat Clim Chang 2(9):655–662
Wondzell S, Bisson P (2003) nfluence of wood and aquatic biodiversity the ecology and management of wood in world rivers. Am Fish Soc Symp 37:249–264
Woolnough DA, Downing JA, Newton TJ (2009) Fish movement and habitat use depends on water body size and shape. Ecol Freshw Fish. https://doi.org/10.1111/j.1600-0633.2008.00326.x
Zeni JO, Hoeinghaus DJ, Casatti L (2017) Effects of pasture conversion to sugarcane for biofuel production on stream fish assemblages in tropical agroecosystems. Freshw Biol. https://doi.org/10.1111/fwb.13047
Zeni JO, Pérez-Mayorga MA, Roa-Fuentes CA et al (2019) How deforestation drives stream habitat changes and the functional structure of fish assemblages in different tropical regions. Aquat Conserv Mar Freshw Ecosyst. https://doi.org/10.1002/aqc.3128
Zimbres B, Machado RB, Peres CA (2018) Land Use Policy Anthropogenic drivers of headwater and riparian forest loss and degradation in a highly fragmented southern Amazonian landscape. Land Use Policy 72:354–363. https://doi.org/10.1016/j.landusepol.2017.12.062
Zorro MC (2018) The fishes and the Igapó forest 30 years after goulding. In: Igapó (Black-water flooded forests) of the Amazon Basin. Springer, Cham, pp 209–227
Acknowledgements
The study was supported by an AMACIENCIAS´ project from Amazon government, managed by Fundación Centro de Estudios Interdisciplinarios Básicos y Aplicados (Interdisciplinary foundation of basic and applied studies). We extend our thanks to the Instituto Amazónico de Investigaciones IMANI (Amazonian Research Institute) of the Universidad Nacional de Colombia (National University of Colombia) and staff of The Schiermeier Olentangy River Wetland Research Park and members of the Stream and River Ecology (STRIVE) Lab, School of Environment and Natural Resources, The Ohio State University. We also want to thank to the laboratory and field team: Antonio Monteiro, Alexis Rufino, Adrian Candre, Francisco Luque, Bibiana Martínez, Ana Kolze, and Scott Glassmeyer. We also thank Eliana Jiménez for her helpful general comments to the manuscript.
Funding
The research leading to these results was funded from an AMACIENCIAS´ project from Amazon Government, managed by Fundación Centro de Estudios Interdisciplinarios Básicos y Aplicados (Interdisciplinary Foundation of Basic and Applied Studies). The work was also supported by the Instituto Amazónico de Investigaciones IMANI (Amazonian Research Institute) of the Universidad Nacional de Colombia (National University of Colombia), the Schiermeier Olentangy River Wetland Research Park, School of Environment and Natural Resources, The Ohio State University; and The Office of International Affairs, The Ohio State University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AMTB, GC, WG, CC, and SMPS. Writing was led by AMTB, GC, CC, and SMPS, with all authors contributing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no financial interests. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Consent to participate
All authors whose names appear on the submission: 1) made substantial contributions to the conception or design of the work, and the analysis and interpretation of data, 2) drafted the manuscript or revised it critically for important intellectual content, 3) approved the version to be published, and 4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Code availability
'Not applicable' for that section.
Consent for publication
We, the authors, declare to agree with the content of this manuscript, and we all gave explicit assent to send to the journal Aquatic Ecology.
Ethics approval
The material contained in this manuscript constitutes original research and has not been previously published in any journal. No data, text, or theories by others are presented as if they were the authors’ own (‘plagiarism’). In addition, the results showed have not been altered or manipulated. The authors have adhered to the specific rules of statistics information treatment for acquiring, selecting, and processing data. This research was conducted under study permits for the collection of specimens of wild species of biological diversity for non-commercial scientific research purposes, granted by the Autoridad Nacional de Licencias Ambientales ANLA (National Environmental Licensing Authority) to the Universidad Nacional de Colombia (National University of Colombia) issued by resolution No. 0255 of March 14 2014 (Article 3) and under IACUC Protocol 2009A0215 from The Ohio State University.
Data availability
All the authors declare transparency of the information in this manuscript. The data used in the study are available in Figshare for Access. https://doi.org/10.6084/m9.figshare.13483488.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Sébastien Villeger.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Torres-Bejarano, A.M., Sulliván, S.M.P., González-Daza, W. et al. Riparian vegetation structure and seasonality influence functional diversity more than taxonomic diversity of stream fish assemblages in the Colombian Amazon. Aquat Ecol 56, 153–172 (2022). https://doi.org/10.1007/s10452-021-09904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09904-y