Abstract
Human activities altering ecosystems structure and function worldwide strongly affect rivers. We studied aquatic macroinvertebrate communities (taxonomic and functional diversity) from rivers immersed in a forest matrix and rivers flowing through croplands. As rivers of the region experience a monsoon climate, high and low water seasons were also considered and their effect tested. We expected lower taxonomic and functional diversity in rivers flowing through croplands, and also during high water periods. We selected five Piedmont forest and three sugarcane crop rivers in Austral Yungas piedmont forests (Argentina), where marginal vegetation, land use, and hydromorphological variables were studied. Samplings were performed in these 8 sites during high and low water seasons of three consecutive years, totaling 32 samples. We analyzed differences between categories through nonparametric analyses of variance and SIMPER analysis. We studied taxonomic diversity through effective number of species and functional diversity using feeding groups with a factorial ANOVA. We calculated different biotic indices to test differences in water quality. We identified 11,034 specimens from 58 families of aquatic macroinvertebrates. Piedmont forest rivers showed higher richness (order 0) than crop rivers, but diversities of orders 1 and 2 showed the opposite pattern. Functional feeding groups were different between both situations. Season greatly influenced the assemblages, with reduced diversity and abundances during high water periods. Biotic indices showed good water quality, except during high water season for crop sites. A complex response of aquatic communities was found, but generally crop sites were more markedly affected during high water season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropic activities substantially modify natural landscapes, exerting powerful effects on the ecosystems (Foley et al. 2005). These modifications alter the physical environment, and consequently, the river quality and the biotic community together with its ecological functions (Príncipe et al. 2007). Factors such as habitat destruction and fragmentation, pollution, and climate change represent the main causes of biodiversity loss in rivers and streams (Carpenter et al. 2011). However, the effects depend not only on the intensity of the impact but also on the presence of riparian coverage (Naiman et al. 2006; García et al. 2017). The environment or matrix in which riparian forests are set may exert a strong influence on the dynamics of the communities inhabiting these forests. Agricultural landscapes, as well as natural areas, have a great potential for biodiversity because of the habitat heterogeneity they create. However, increasing agricultural activities have exposed natural landscapes to high amounts of fertilizers, herbicides, and pesticides, turning them into hostile areas for numerous species and promoting biodiversity loss (Grashof-Bokdam and van Langevelde 2004).
Argentinian ecoregions have also suffered this type of modification, especially in the Austral Yungas ecoregion at its lowest altitudinal level, the Piedmont forest (Brown et al. 2001; 400–700 m asl). The structural characteristics of this altitudinal level (e.g., low slope) have favored the establishment of large urban and agro-industrial cores, replacing original landscapes and altering riparian forests (von Ellenrieder 2007). Additionally, the monsoonal climate of the region originates two marked pluvial seasons: summer and autumn rains (November to April) and a drought during winter and spring (May to October; Brown et al. 2001). Flow regulation is to a large extent provided by the forest cover surrounding rivers (García et al. 2017).
Different works have demonstrated a high diversity of Yungas freshwater macroinvertebrates: von Ellenrieder (2007) mentioned 143 taxa, Molineri et al. (2009) reported 132 taxa, and Dos Santos et al. (2011) 171 taxa (as many groups are identified at the genus or family level in these works, the number of species is much higher). These assemblages are dominated by dipterans (Chironomidae), mayflies (Ephemeroptera), caddisflies (Trichoptera), water beetles (Elmidae), and other groups (Domínguez and Fernández 2009).
Taxonomic and functional structure of the macroinvertebrate assemblages are different approaches to understand aquatic ecosystems functioning (Dolédec et al. 1996). Taxonomy relies on systematic knowledge that is generally scarce in the region for some groups, and species diversity for the entire community is difficult to obtain. For example, some diverse groups as non-biting midges (Chironomidae) are generally identified to family level only. Also, taxonomic redundancy (many taxa with similar ecological traits) may cause bias in the comparison between naturally rich and poor ecosystems. On the other hand, functional structure approach relies on a limited number of biological traits, useful in different biogeographical regions, and making comparisons possible between ecosystems with dissimilar natural species-pool sizes (Basset et al. 2012; Menezes et al. 2010). We used Cummins et al. (2005) groupings of benthic organisms after their food habits and kind of ingested particles. This classification is known as functional feeding groups (FFGs), and the state of knowledge in regional lotic allows for the inclusion of this additional aspect in our analysis ecosystems (Cummins et al. 2005; Reynaga and Rueda Martin 2010; Reynaga and Dos Santos 2012). Functional analyses in lotic systems have been frequently used in Europe (e.g., Resh et al. 1994); USA (e.g. Bêche et al. 2006) and South America (e.g. Fossati et al. 2003; Reynaga and Dos Santos 2012).
Additionally, biological indicators are useful to assess the biological quality of a watercourse, its banks (riparian forest) and the surrounding matrix. Benthic communities are commonly used as indicators for several reasons: understanding the basins structure and dynamic, for the quick assessment of the ecosystems state, for basin restoration strategies, and for evaluating their resilience to strong impacts such as extraordinary spates (Heink and Kowarik 2010). Different biotic indices for estimating the quality of fluvial environments have been adapted and proposed for the region (Domínguez and Fernández 1998; von Ellenrieder 2007; Dos Santos et al. 2011).
The risk implied by biodiversity loss and landscape change in the Yungas Forest of northwestern Argentina is severe (Domínguez and Fernández 1998; von Ellenrieder 2007). Studies describing macroinvertebrate diversity or comparing different situations in the area are scarce (Molineri et al. 2009; García et al. 2017). Landscape transformation of Yungas forest to sugarcane croplands is expected to reduce taxonomic and functional diversity of macroinvertebrate. Additionally, larger amount of suspended solids and agrochemicals affect rivers during the rainy season, especially if riparian forests are lost or degraded (Naiman et al. 2006). Increased suspended solids are expected to affect strongly to filterer feeding groups, and agrochemicals are thought to affect more to predators due to trophic magnification and longer life cycles (Molina et al. 2010). We thus evaluate the taxonomic and functional structure of this community in eight sites from different matrices and seasons, expecting that sites immersed in crop matrix and/or high water season would be poorer than those located in better preserved basins and/or lower water period.
Material and methods
Study area
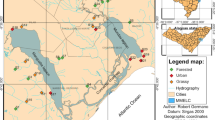
In northwestern Argentina, the distribution of the Yungas forest includes the provinces of Salta, Jujuy, Tucumán, and Catamarca (Fig. 1; Brown et al. 2001). River regimes in the Yungas are highly linked to rainfall: river flow increases between November and April, with floods in January through April, and then decreases between June and October, especially in August and September (Domínguez and Fernández 1998). Changes in the macroinvertebrate community during their natural annual cycle are so pronounced that sampling was adjusted in order to cover high and low waters limnologic periods (Fernández et al. 2016; García et al. 2017). We selected eight rivers associated with two different matrix states (Fig. 1): Piedmont forest (PF) and sugarcane crop (C). Rivers immersed in the crop matrix are Zora (ZR: 23° 46′ S, 64° 36′ W), Santa María I (SMIR: downstream, 23° 16′ S, 64° 19′ W), and Berro (BR: 23° 44′ S, 64° 40′ W), and those immersed in the piedmont forest matrix are Ledesma (LR: 23° 56′ S, 64° 57′ W), Sauzalito I (SIR: upstream, 23° 37′ S, 64° 35′ W), Sauzalito II (SIIR: downstream, 23° 39′ S, 64° 34′ W), Santa María II (SMIIR: upstream, 23° 15′ S, 64° 28′ W), and Aguas Negras (ANR: 23° 45′ S, 64° 51′ W). The classification of sites in relation to landscape matrix (crop vs forest) was done, in a first step, from the analysis of LandSAT 5TM images obtained from Instituto Nacional de Pesquisas Espaciais (INPE; http://www.dgi.inpe.br/CDSR), and a second step including direct field observations and measures (see vegetation transects below).
Riparian forests and macroinvertebrate sampling
Rivers were sampled during high waters (November–February) and low waters (July–August) between 2013 and 2015. At each site, a section of 500 m long was selected as the “sample site.” We measured variables related to riparian forests across 10 transects (of 50 m long, following the river channel, and 20 m wide, on each margin of the river): canopy cover (estimated using canopy pictures taken with a Nikon D3100), understory visibility, and diameter at breast height (DBH) of arboreal individuals (≥ 10 cm DBH). Physical variables related to the water body structure were also measured along each 500 m stream-reach: channel cover (canopy covering the river: absent = 0%; scarce = 1–25%; medium = 26–50%; present = > 50%, estimates by observations and photographs), granulometry (dominant substrate: fine sand, coarse sand, slime, clay, rocks), roughness (presence of emerged or submerged rocks), and stability (presence of attached or loose rocks). Dominant substrate was obtained from 4 transects (10 m long) with 25 points each (total 100 points) at each site; the size of the particle at each point was estimated by direct observation in the categories mentioned above.
Aquatic macroinvertebrates were studied with a quantitative sample per site (using kick-net and hand-nets of 1 mm pore size). Quantitative samples involved all available aquatic habitats (in the 500 m stream reach) during a fixed time lapse of 1 h by 1 researcher (the same all the times): riffles, pools, submersed marginal vegetation, and adjacent ponds connected to the river. Samples were preserved in alcohol 96% and identified under magnification up to the family level. Regarding Ephemeroptera, Trichoptera, Elmidae, Megaloptera, and Plecoptera identification was up to the lowest possible level (species/morpho-species), to calculate biological indices at the species level. Particular keys were used for the identification of macroinvertebrates, mainly to genus and species (Domínguez et al. 2006; von Ellenrieder and Garrison 2007; Domínguez and Fernández 2009; Molineri 2010a; Isa Miranda and Rueda Martín 2014). All the material is deposited in the collection of Instituto de Biodiversidad Neotropical, Tucumán, Argentina.
Data analysis
Physical variables from riparian forest and rivers were compared to test differences between matrices (PF vs C) using Mann Whitney test (U’). An incidence matrix (site*taxa) was constructed, all sites and sampling dates (two seasons*three years) were included as rows, all taxa as columns. The obtained data were grouped according to the matrix type (PF or C) and season (high or low waters). We calculated the species richness of aquatic macroinvertebrates, and the total and relative abundance. We analyzed the diversity of C and PF matrix in terms of effective number of species (Jost 2006; Cultid-Medina and Escobar 2016). We used three “q values”: order zero (0 = species richness), 1 (exponential of Shannon’s entropy, typical diversity), and 2 (inverse Simpson concentration, number of dominant species). We analyzed high and low water seasons separately, and compared the two matrices (C vs. PF) adding up the abundances of each species in all C sites versus PF. Diversity in the assemblages of benthic macroinvertebrates was compared using dotplots and 95% CI. Non-overlapping CIs were considered to indicate statistically significant differences between the treatments (MacGregor-Fors and Payton 2013). In order to assess differences in assemblage composition and abundance between matrix and season, we performed an analysis of variance using distance matrices adjusted to a lineal model. This analysis is based on a dissimilarity measure that directly divides variation between individual MANOVA terms, measuring the simultaneous response of numerous non-independent variables (e.g., species abundances). This non-parametric analysis has a p value calculated from permutations (McArdle and Anderson 2001). Posteriorly, through a SIMPER analysis, we identified mean contributions of each taxa to intragroup similarity (Clarke 1993). For these analyses, we used the “adonis,” “simper,” and “betadisper” functions from the Vegan package (Oksanen et al. 2017), and the package “iNEXT” (Hsieh et al. 2016) in R software (R Development Core Team 2017). FFG is a categorization based on the feeding type and feeding particle size. We assigned each family to one of the different FFG based on data available for the Yungas ecoregion (Molineri et al. 2009; Reynaga and Rueda Martin 2010; Reynaga and Dos Santos 2012). We used a classification composed by nine categories, some of them are composite (more than one FFG co-dominate in a taxon): predators, predator-shedders, filterers, filterer-gatherer-predator, gatherers, scrapers, gatherer-filterers, gatherer-scrapers, shredders. Trophic preference data was used to calculate the percentage of each category found per matrix and season. FFG abundances were tested using factorial ANOVA (Quinn and Keough 2002), with nine levels for FFG, two levels for matrix and two for seasons.
Finally, different biotic indices were selected in order to evaluate site sensitivity to the surrounding matrix condition: BMWP (Biological Monitoring Working Party; Armitage et al. 1983, modified by Domínguez and Fernández 1998); EPT (number of species/morpho-species included in Ephemeroptera, Plecoptera, and Trichoptera; Klemm et al. 1990); ASPT (average score per taxon; Walley and Hawkes 1997); ElPT (number of species of Elmidae, Plecoptera, and Trichoptera; von Ellenrieder 2007), IBY-4 (Yungas biotic index based on the presence of 4 taxa [Elmidae, Plecoptera, Trichoptera and Megaloptera], Dos Santos et al. 2011) and EPT% (number of individuals EPT/total number of individuals in the sample). Cutoff values for each index reflected one of the two situations: crop/piedmont forest, as proposed by Dos Santos et al. (2011), who classified impacted those sites with values lower or equal to 2 (IBY-4), 66 (BMWP), 4 (ElPT), 8 (EPT), 6 (ASPT), and 14 (family richness). We evaluated these indices to detect significant differences between matrix and season categories through a permutational multivariate analysis of variance (PERMANOVA; Anderson and Walsh 2013). The relationship between biotic indices and environmental variables was explored through a canonic correlation analysis.
Results and discussion
Riparian forests and macroinvertebrates
Riparian forests immersed in the PF matrix were characterized by the presence of arboreal vegetation composed of species such as Erythrina falcata, Cedrela balansae, Salix humboldtiana, Tessaria integrifolia, Tipuana tipu, Anadenanthera colubrina, Parapiptadenia excelsa, Acacia aroma, and Enterolobium contortisiliquum, but some sectors are only composed of A. aroma or T. integrifolia. In these rivers canopy cover varied between 1 (scarce) and 50% (medium), dominant substrate formed by fine sand and slime, and high roughness and stability. On the other hand, riparian forests immersed in the C matrix were dominated by the exotic cane Arundo donax, alternating with monoespecific patches of T. integrifolia. Specimens of S. humboldtiana, Psidium guayava, and Coccoloba sp. also stand out together with monospecific forests of A. aroma. Canopy cover varied between 0 and 25% (absent to scarse), substrate formed by coarse sand and rocks, and low roughness and stability. Coverage was higher in PF matrix (Mann-Whitney: p ≤ 0.0001), DBH did not differ between matrices (Mann Whitney: p = 0.5), and understory visibility was higher in C matrix (Mann Whitney: p = 0.01; Supplementary Material 1). Riparian forests immersed in PF matrix, though characterized by unique elements, were similar in composition to Yungas piedmont forest. A marked stratification of these forests and the presence of well-developed undergrowth is evidenced by higher canopy cover and low understory visibility. Riparian forests included in C matrix were degraded, characterized by secondary vegetation, many pioneer elements, and poor developed understory, what is evidenced by higher visibility. This situation increases the sensibility of the river and its margins to natural and anthropic perturbations. Natural vegetation, at least in a marginal strip along the rivers reduces impacts and increases water quality (García et al. 2017).
During the period 2013–2015, we completed 32 samples collecting a total of 11,034 individuals belonging to 58 families, in addition to Oligochaeta, Hirudinea, and Acari (non-identified up to the family level). Arthropoda was the most abundant phylum, followed by Mollusca and Annelida, both of which were scarcely represented (Supplementary Material 2). Regarding Arthropoda, the most abundant group was represented by insects (98%) and, to a lesser extent by crustaceans and mites. In both sampled seasons, Baetidae (Ephemeroptera) showed the highest values of relative abundance, at almost every site inside the PF matrix, except for LR and ANR (high waters) dominated by Leptohyphidae (Ephemeroptera), and for LR (low waters) dominated by Helicopsychidae (Trichoptera). On the other hand, Baetidae was the family with higher relative abundance considering sites located in the C matrix, for both seasons (high and low waters). Diversity at order q = 0 was much higher in PF rivers than in C rivers (Fig. 2a). The other two diversities (q = 1 and q = 2) were higher in C (except at low waters for q = 1, Fig. 2b, c). Lower values of richness were expected in crop matrix sites, corresponding to poorer communities, typical of more stressed environments. On the other hand, we did not expect just the opposite pattern (C>PF) shown by diversities q = 1 (typical diversity) and q = 2 (number of dominant species). The higher richness in piedmont forest matrices is due to many rare species, but the community is dominated by fewer species showing a lower eveness if compared with crop matrices. Riparian forest with crop matrices show a larger set of dominant species (most of the abundance of the community is divided in more species). The closeness of all the sampling sites to a large preserved natural area (Calilegua National Park) probably guarantee a constant flux of new specimens and rare species to the studied communities. This may explain the relatively large richness values, even in impacted sites. More equitative communities found in crop matrices are due at least in part to new habitats generated by human activities, since in all these sites we observed side pools indirectly generated by gravel extraction.
The lineal model including all structural variables aimed to evaluate the differences in composition and abundance of the assemblages pointed the variable “season” as the only with significant differences (R2 = 0.18; F = 0.007, Fig. 3a). The remaining variables, including matrix type, did not differ. The strong effect of seasonality on the macroinvertebrate community is not surprising, since the region experience a monsoonal climate, with spates reseting the aquatic community much more frequently during four months of the year (January to April; Molineri 2010b). Taxa contributing significantly to this result (factor “season”) were: Baetidae (p = 0.004), Dixidae (p = 0.029), Polycentropodidae (p = 0.023), and Aeshnidae (p = 0.04). The dominance of the class Insecta is a common situation, in coincidence with other tropical and subtropical regions (von Ellenrieder 2007; Dudgeon 2011; Principe et al. 2015). Family richness is similar to that reported by von Ellenrieder (2007) in the studied area. Structural variables from the two matrices explain the differences in macroinvertebrate abundance (PF>C). Regarding the community composition, the dominance of Baetidae has been commonly reported due to its taxonomic and ecological diversity in South America (Domínguez et al. 2006); in our study, dominant species were from the genera Callibaetis and Americabaetis, associated to marginal vegetation and to slow or moderate water flow (Domínguez et al. 2006). On the other hand, Leptohyphidae was commonly found in sites immersed in the Piedmont forest matrix during the high water seasons, thus, reflecting the preference of the most abundant genus (Leptohyphes) for sandy substrates in areas with moderate to high water flux (Molineri 2010b). This group is also considered important as a bioindicator due to its sensitivity to anthropogenic impacts, as they are found exclusively in conditions with high dissolved oxygen concentration. The great abundance of dipterans is frequent in lotic environments, mostly of Chironomidae but also Simuliidae and Culicidae. This last family is of special medical importance in the study site since its species are vectors of human disease (Molineri et al. 2009). Anyway, a relative large pore size of the nets used in this study prevented the capture of most specimens of these groups.
a Formplot of sites obtained with PCoA during low (red) and high waters (black) season according to the abundance of aquatic macroinvertebrates. b Box and whisker plot of the abundance of FFT in different matrices and seasons. Black circle = median. P = predators, P-Sh = predators-filterers, F = filterers, F-G-P = filterers-gatherers-predators, G = gatherers, Sc = scrapers, G-F = gatherers-filterers, G-Sc = gatherers-scrapers, Sh = shredders
Gatherers-scrapers were dominant, with 31.7% of total abundance (Supplementary Material 2). Predators and gatherers were also important with 24.1% and 18.8% of total abundance, respectively. During high water season, predators (39.32%) and gatherers-scrapers (25.68%) dominated in C matrices, but during low water season, only gatherers-scrapers (45.01%) were dominant. In PF matrices, during high water season, gatherers-filterers dominated (39.63%), and in low water season, gatherers-scrapers (35.19%) and predators (25.95%) were more important. Shredders and filterers abundances remained low in both seasons and matrices. Significant differences in the abundance of gatherers and gatherers-scrapers were found for “season” (low waters season>high waters season; F18,226 = 55.09, p < 2.2e−16; Fig. 3b), and “matrix” (PF>C; F18,226 = 27.36, p < 2.2e−16). The interaction of both factors (season*matrix) resulted significant only for gatherers (F = 33.43, p < 2.2e−16). The trophic analysis of macroinvertebrate communities is important to identify ecosystem atrributes contributing to services provision and conservation planning (Cummins et al. 2005). Gatherers and scrapers were both well represented at the study sites. Their higher contribution, reaching more than 50% of total abundance, coincide with other rivers of low altitudes (Molineri et al. 2009; Mesa 2014). Predators composed by odonates, plecopterans, megalopterans, hemipterans, and coleopterans, also resulted abundant, as was reported for mountain rivers in the area; filterers were rare, as Molineri et al. (2009) have shown for pedemountain rivers. The importance of gatherers and scrapers may be associated with the heterotrophic nature of the studied rivers, where leaves and other terrestrial material are abundant. The low abundance of shredders has been reported in other studies carried out in tropical and subtropical sites (Dudgeon 2000; Dobson et al. 2002; Mesa 2014), and has been mainly assigned to the processing of allochtonous material (e.g., leafs) by microbial activity, and to mechanical action of the current in fragmenting coarse material (Romero et al. 2010; Mesa 2014). Abundance of gatherers and gatherers-scrapers significatively responded to matrix and season, as expected since higher abundance of organic material in well-preserved forest and during low water season favor those FFG (Cummins et al. 2005; Romero et al. 2010; Mesa 2014).
Biotic indices
Regarding the biotic indices ASPT showed values lower than the established cutoff value across most of the sampled situations. ElPT and IBY4 showed this in only one site (Table 1). On the other side, the PERMANOVA did not show significant differences in the values of biotic indices when comparing between matrix type (PF and C, F1,13 = 0.07, p = 0.78) and seasons (high and low waters, F1,13 = 0.22, p = 0.65). The first two axes of the CCA explained 78% of total variability (Fig. 4). ASPT showed a positive relation with percent canopy cover, reaching high values in PF matrix, and a negative relation to zero-values of canopy cover. Riparian forests and their associated water bodies depend on the ecological integrity of basins (García et al. 2017). It is important to consider that changes in land and riparian forest use (total or partial removal, replacement with exotic species, etc.) affect the ecosystems ecological functioning (Romero et al. 2010). Success in the use of biotic indices is subjected to the accuracy of the sensitivity scores assigned to each group of taxa, and to the cutoff values distinguishing rivers with different impact condition (Dos Santos et al. 2011). The EPT index did not discriminate between matrix type or season. Dos Santos et al. (2011) suggested that relatively high diversity of macroinvertebrates of the studied region prevents some indices to find impacts, especially those strongly influenced by richness (i.e., EPT and BMWP). In this study, only the ASPT identified some sites as impacted. The ASPT has shown to be highly sensitive since it corrects for false results stemming from changes in taxa richness (regardless of its sensitivities; Romero et al. 2011). Indices were different between seasons but only inside the crop matrix, whereas sites inside the forest matrix showed no differences. Thus, under the same condition (e.g., high waters season), the effect on the benthic community is evidenced by the matrix type in which riparian forests are set. For instance, in the crop matrix, the higher runoff may be allowing the access of foreign material (sediments, nutrients, agrochemicals), thus affecting water quality and ultimately, the benthic community.
Canonical correspondence analysis (CCA) of biotic indices. Stab= Stability; Rip_cov = riparian coverage; Rip_Cov = riparian coverage (null, low, high); Height = arboreal height; DBH = diameter at the breast height; Visibility = understory visibility; Chan_cov = channel coverage (absent, scarce, medium, present); HW = high waters season; LW = low waters season; ANR = Aguas Negras River; BR = Berro River; SIR= Sauzalito I River; SIIR = Sauzalito II River; SMIR = Santa Maria I River; SMIIR = Santa Maria II River; LR = Ledesma River; ZR = Zora River
Conclusion
Matrix type (PF or C) influenced structural variables of riparian forest. Piedmont Forest rivers and crop land rivers were not markedly different in macroinvertebrate composition, in spite of noticeable differences in landscape cover and riparian forest quality. PF rivers showed higher richness than crop rivers, but evenness showed the opposite pattern. Functional feeding groups were different between both situations. Biotic indices showed good water quality in all sites, except during high water season for crop matrices, what coincide with the loss of filtering function of degraded riparian forests in those stream reaches.
References
Anderson, M. J., & Walsh, D. C. I. (2013). PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecological Monographs, 83(4), 557–574.
Armitage, P. D., Moss, D., Wright, J. F., & Furse, M. T. (1983). The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running water sites. Water Research, 17(3), 333–347.
Basset, Y., Cizek, L., Cuénoud, P., Didham, R. K., Guilhaumon, F., Missa, O., Novotny, V., Ødegaard, F., Roslin, T., Schmidl, J., Tishechkin, A. K., Winchester, N. N., Roubik, D. W., Aberlenc, H. P., Bail, J., Barrios, H., Bridle, J. R., Castaño-Meneses, G., Corbara, B., Curletti, G., Duarte da Rocha, W., de Bakker, D., Delabie, J. H., Dejean, A., Fagan, L. L., Floren, A., Kitching, R. L., Medianero, E., Miller, S. E., Gama de Oliveira, E., Orivel, J., Pollet, M., Rapp, M., Ribeiro, S. P., Roisin, Y., Schmidt, J. B., Sørensen, L., & Leponce, M. (2012). Arthropod diversity in a tropical forest. Science, 338(6113), 1481–1483.
Bêche, L. A., Mcelravy, P. E., & Resh, V. H. (2006). Long-term variation in the biological traits of benthic-macroinvertebrates in two Mediterranean-climate streams in California, U. S. A. Freshwater Biology, 51, 56–75.
Brown, A. D., Grau, H. R., Malizia, L., & Grau, A. (2001). Argentina. In M. Kappelle & A. D. Brown (Eds.), Bosques nublados del Neotrópico (pp. 623–659). Santo Domingo de Heredia: Inbio.
Carpenter, S. R., Stanley, E. H., & Van Der Zanden, M. J. (2011). State of the world´s freshwater ecosystems: physical, chemical, and biological changes. Annual Review of Environment and Resources, 36, 75–79.
Clarke, K. R. (1993). Non – parametric multivariate analysis of changes in community structure. Australian Journal of Ecology, 18, 117–143.
Cultid-Medina, C. A., & Escobar, F. (2016). Assessing the ecological response of Dung Beetles in an agricultural landscape using number of individuals and biomass in diversity measures. Environmental Entomology, 45, 310–319.
Cummins, K. W., Merritt, R. W., & Andrade, P. C. N. (2005). The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Studies on Neotropical Fauna and Environment, 40(1), 69–89.
Dobson, M., Magana, A., Mathooko, J. M., & Ndegwa, F. K. (2002). Detritivores in Kenyan Highland streams: more evidence for the paucity of shredders in the tropics? Freshwater Biology, 47, 909–919.
Dolédec, S., Chessel, D., Ter Braak, C. J. F., & Champely, S. (1996). Matching species traits to environmental variables: a new three-table ordination method. Environmental and Ecological Statistics, 3, 143–166.
Domínguez, E., & Fernández, H. R. (1998). Calidad de los ríos de la cuenca del Salí (Tucumán, Argentina) medida por un índice biótico (12th ed.). Tucumán: Talleres Gráficos de Fundación Miguel Lillo, Serie Conservación de la Naturaleza.
Domínguez, E., Molineri, C., Pescador, M.L., Hubbard, M.D. and, Nieto, C. (2006). Ephemeroptera de América del sur. In: Adis, J., Arias, J.R., Rueda-Delgado, G. and Wantzen, K.M. (Eds.), Aquatic Biodiversity in Latin America (ABLA). 2. Moscow: Pensoft Publishers.
Domínguez, E., & Fernández, H. R. (2009). Macroinvertebrados bentónicos sudamericanos. Sistemática y biología. Tucumán: Fundación Miguel Lillo.
Dos Santos, D. A., Molineri, C., Reynaga, M. C., & Basualdo, C. (2011). Which index is the best to assess stream health? Ecological Indicators, 11, 582–589.
Dudgeon, D. (2000). The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annual Review of Ecology, Evolution, and Systematics, 31, 239–263.
Dudgeon, D. (2011). Tropical stream ecology. London: Academic Press. Elsevier Science.
Fernández, R. D., Ceballos, S. J., González Achem, A. L., Hidalgo, M. D. V., & Fernández, H. R. (2016). Quality and conservation of riparian forest in a mountain subtropical basin of Argentina. International Journal of Ecology. https://doi.org/10.1155/2016/4842165.
Foley, J. A., Defries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, I. C., Ramankutty, N., & Snyder, P. K. (2005). Global consequences of land use. Science, 309(5734), 570–574.
Fossati, O., Dumas, P., Archaimbault, V., Rocabado, G., Fernández, H., Wasson, J. G., et al. (2003). Deriving life traits from habitat characteristics: an initial application for Neotropical invertebrates. Journal de Recherche Oceanographique, 28, 158–162.
García, A. K., Fernández, H. R., Rolandi, M. L., Gultemirian, L., Sánchez, N., Pla, L., et al. (2017). Effect of diffuse Pollution on Water Quality in Mountain Forest Streams. Forestry Research and Engineering: International Journal, 1(1), 1–7.
Grashof-Bokdam, C. J., & van Langevelde, F. (2004). Green veining: landscape determinants of biodiversity in European agricultural landscapes. Landscape Ecology, 20, 417–439.
Heink, U., & Kowarik, I. (2010). What criteria should be used to select biodiversity indicators? Biodiversity and Conservation, 19, 3769–3797.
Hsieh, T.C., Ma, K.H., and Chao, A. (2016). iNext: interpolation and extrapolation for species diversity. R package version 2.0.12.
Isa Miranda, A. V., & Rueda Martín, P. A. (2014). El Orden Trichoptera en Tucumán, Argentina: nuevo registro de Leucotrichia lerma (Angrisano and Burgos, 2002) (Trichoptera: Hydroptilidae), descripción de sus estados inmaduros, lista de especies y claves de identificación ilustradas. Acta Zoológica Lilloana, 58, 194–223.
Jost, L. (2006). Entropy and diversity. Oikos, 113(2), 363–375.
Klemm, D.J., Lewis, P.A., Fulk, F., and Lazorchak, J.M. (1990). Macroinvertebrate field and laboratory methods for evaluating the biological integrity of surface waters. EPA/600/4-90/030. U. S. Environmental Protection Agency. Environmental Monitoring. Systems Laboratory, Cincinnati, Ohio, Washington D. C.
Macgregor-Fors, I., & Payton, M. E. (2013). Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS One, 8, e56794. https://doi.org/10.1371/journal.pone0056794.
Mcardle, B. H., & Anderson, M. J. (2001). Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology, 82, 290–297.
Menezes, S., Baird, D. J., & Soares, A. M. (2010). Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. Journal of Applied Ecology, 47(4), 711–719.
Mesa, L. M. (2014). Influence of riparian quality on macroinvertebrate assemblages in subtropical mountain streams. Journal of Natural History, 48, 1153–1167.
Molina, C. I., Gibon, F. M., Duprey, J. L., Domínguez, E., Guimarães, J. R. D., & Roulet, M. (2010). Transfer of mercury and methylmercury along macroinvertebrate food chains in a floodplain lake of the Beni River, Bolivian Amazonia. Science of the Total Environment, 408, 3382–3391.
Molineri, C., Romero, F., & Fernández, H. R. (2009). Diversidad y Conservación de invertebrados acuáticos. In A. D. Brown, P. G. Blendinger, T. Lomáscolo, & P. García Bes (Eds.), Selva Pedemontana de la Yungas. Historia natural, ecología y manejo de un ecosistema en peligro (pp. 121–148). Ediciones del Subtrópico: Tucumán, Argentina.
Molineri, C. (2010a). Las especies de Leptohyphidae (Ephemeroptera) de las Yungas de Argentina y Bolivia: diagnosis, distribución y claves. Revista de la Sociedad Entomológica Argentina, 69(3-4), 233–252.
Molineri, C. (2010b). The influence of floods on the life history of dominant mayflies (Ephemeroptera) in a subtropical mountain stream. Studies on Neotropical Fauna and Environment, 45(3), 149–157.
Naiman, R. J., Decamps, H., & Mcclain, M. E. (2006). Riparia: ecology, conservation, and management of streamside communities. Bioscience, 56(4), 353–354.
Oksanen, J., Guillaume Blanchet, F., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., et al. (2017). Community Ecology Package. Package “Vegan”. Version, 2, 4–1.
Príncipe, R. E., Raffaini, G. B., Gualdoni, C. M., Oberto, A. M., & Corigliano, M. C. (2007). Do hydraulic units define macroinvertebrate assemblages in mountain streams of central Argentina? Limnologica, 37, 323–336.
Príncipe R. E., Márquez J. A., Martina L. C., Jobbágy E. G., Albariño R. J. 2015. Pine afforestation changes more strongly community structure than ecosystem functioning in grassland mountain streams. Ecol Indic 57, 366–375.
Quinn, G., and Keough, M. (2002). Experimental design and data analysis for biologists. Cambridge University Press.
Development Core Team, R. (2017). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical computing.
Resh, V. H., Hildrew, G. A., Statzner, B., & Towsend, C. R. (1994). Theoretical habitat templets, species traits, and species richness: a synthesis of long-term research on the Upper Rhône River in the context of concurrently developed ecological theory. Freshwater Biology, 31, 539–554.
Reynaga, M. C., & Dos Santos, D. A. (2012). Rasgos biológicos de macroinvertebrados de ríos subtropicales: patrones de variación a lo largo de gradientes ambientales espacio-temporales. Ecología Austral, 22, 112–120.
Reynaga, M. C., & Rueda Martin, P. A. (2010). Trophic analysis of two species of Atopsyche (Trichoptera: Hydrobiosidae). Limnologica, 40, 61–66.
Romero, F., Fernández, H. R., Molineri, C., & Domínguez, E. (2010). Ecología de ríos y arroyos de la Sierra de San Javier. In R. Grau (Ed.), Ecología regional de una interfase natural – urbana. La Sierra de San Javier y el Gran San Miguel de Tucumán (pp. 77–92). Tucumán: Editorial de la Universidad Nacional de Tucumán.
Romero, F., Manzo, V., Fernández, H. R., Domínguez, E., Molineri, C., Nieto, C., et al. (2011). Estudio integral de la cuenca del río Lules: aspectos biológicos. In H. Barber & H. R. Fernández (Eds.), La cuenca del río Lules (pp. 111–136). Tucumán: EDUNT.
von Ellenrieder, N. (2007). Composition and structure of aquatic insect assemblages of Yungas mountain cloud forest streams in NW Argentina. Revista de la Sociedad Entomológica Argentina, 66(3-4), 57–76.
von Ellenrieder, N., and Garrison, R.W. (2007). Dragonflies and Damselflies (Insecta: Odonata) of the Argentine Yungas: species, composition and identification. Scientific Reports no. 7. Italy: Societa Zoologica ‘La Torbiera’.
Walley, W. J., & Hawkes, H. A. (1997). A computer-based reappraisal of the Biological Monitoring Working Party scores using data from the 1990 River Quality Survey of England and Wales. Water Research, 31(2), 201–210.
Acknowledgments
We are grateful to our colleagues that helped with field and laboratory work at INECOA (L. Rivera, R. Ruggera, A. Benavídez, P. Puechagut, M. Morales, Y. Tejerina, and E. Ruiz de Los Llanos) and IBN (J. Rodríguez, G. Hankel, C. Cultid-Medina, D. Dos Santos, V. Manzo, C. Reynaga, C. Nieto, and F. Romero). Funding from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) is greatly thanked (PUE099 and PIP845).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Daniela, G., Carlos, M. Crop landscapes reduced taxonomic and functional richness but increased evenness of aquatic macroinvertebrates in subtropical rivers. Environ Monit Assess 191, 702 (2019). https://doi.org/10.1007/s10661-019-7864-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7864-7