Abstract
For closed-channel microfluidic chips, a layer of the cover plate is usually bonded to the substrate layer to enclose the fabricated microstructures on the surface of the substrate. Various irreversible and reversible bonding approaches have been invented for glass, thermoplastic, and PDMS-based microfluidic devices in the recent decade, including anodic bonding, thermal fusion bonding, chemical solvent assisted bonding, sandwich adhesion layer, and oxygen plasma treatment. Currently, most of the reversible bonding methods have to make compromise on bonding strength to achieve the reversible bonding between the substrate and cover plate. In this study, a novel reversible bonding method is proposed with the help of a UV curable release tape, compared with previous methods on reversible bonding using a magnetic or adhesive layer, the proposed method offers a higher bonding strength and easily debond with a simple UV exposure process. The proposed reversible bonding method using UV release tape is inspired by the application of UV release tape in the wafer dicing process in MEMS and IC industry. For the demonstration of the proposed reversible bonding method for microfluidic devices, conventional and hybrid reversible bonding between thermoplastics and glass were achieved, the bonding strength was also measured with different UV radiation doses, the biocompatibility of the UV release tape was tested, finally, several microfluidic devices were fabricated with the proposed reversible bonding method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microfluidics has been widely used in the chemical, biological, medical, and environmental fields (Shang et al. 2017; Sackmann et al. 2014). Except open-channel microfluidic devices, e.g., paper-based microfluidics (Zhang et al. 2019), and 3D printed microfluidics (Au et al. 2016), most of the current fabrication approaches are conducted by fabricating the microchannel (microstructure) on the surface of the substrate material and then bonding another layer of material (cover plate) to enclose the channel. The substrate and cover plate could be the same material (conventional bonding) or different materials (hybrid bonding) according to the requirement of the applications (Pitingolo et al. 2019a). The achieved bonding between the substrate and cover plate could be permanent (irreversible) or reversible. With the wide applications of microfluidics in life science, there is a growing demand on reversible bonding (Dinh et al. 2015), the detachment of substrate and cover plate could facilitate the retrieval of tissue (Pitingolo et al. 2018) or cell (Moon et al. 2021) in the microchannel or reaction chamber after the experimental process.
Various approaches have been invented for irreversible bonding according to the types of material to bond. PDMS (polydimethylsiloxane) is the most commonly used elastomer in microfluidics, the PDMS-glass or PDMS-PDMS bonding is normally achieved by oxygen plasma treatment (Bodas and Khan-Malek 2007). Another commonly used type of material is thermoplastics, to achieve the bonding between the substrate and cover plate with the same thermoplastics (e.g., PMMA, PS, PC) (Mahmoodi et al. 2019; Kurihara et al. 2020), the most effective approach is a thermal fusion (Fan et al. 2014), two plates are compressed with a temperature slightly higher than the glass transition temperature, the molecule entanglement at the contacted surface could achieve a secured bonding, in addition, chemical solvent or UV exposure can also be used to lower the bonding temperature (Gu et al. 2011) with the same bonding strength. However, to achieve irreversible hybrid bonding between two very different materials, e.g., plastic-glass or PDMS-plastics bonding, adhesive material (Kojic et al. 2019), chemical solvent (Lynh and Pin-Chuan 2018; Sivakumar and Lee 2020) or UV curable material (Vulto et al. 2005) is usually sandwiched between the substrate and cover plate to achieve the bonding. Usually, the forced opening between the substrate and cover plate for irreversible bonding will cause serious damage to the chip structure.
In the past few years, many reversible bonding approaches have been reported (Chen et al. 2014; Vézy et al. 2011), the current reversible bonding method can fall into two categories: physical assisted bonding and chemical assisted bonding. For physical reversible bonding, aspiration (Berre et al. 2006), gecko-like gaskets (Wasay and Sameoto 2015), and magnetic attraction (Rasponi et al. 2011) are some of the commonly used approaches. For chemical-assisted reversible bonding, a layer of adhesive agent is sandwiched between the substrate and cover plate to achieve reversible bonding. The adhesive agent including wax, (Gong et al. 2010), hydrogel (Le and Lee 2020; Pitingolo et al. 2019b), and silicone (Chu et al. 2017). In addition, the deliberately weakened PDMS-PDMS or PDMS-glass bonding achieved by oxygen plasma treatment followed with ethanol (Bhattacharya et al. 2005) or acetone (Shiroma et al. 2016) rinse before attaching two plates is also considered to be reversible. However, an obvious drawback of the current reversible bonding methods is the compromise on bonding strength to achieve reversible bonding, besides, the residual (often sourced from adhesive agents) left on the surface of the substrate and cover plate after debonding may also contaminate the tissue or cells to be retrieved inside the chip.
Various adhesive films (e.g. acrylic film, dry adhesive film) or tapes have been previously used in the fabrication of microfluidic devices as a structural layer or intermediate layer to achieve the bonding (Serra et al. 2017; Nath et al. 2010). However, most of these methods are irreversible, and forced detachment between two bonded layers archived by the adhesive film will damage the chip or lead to a large number of the residual left inside the microchannel and on the detached surfaces.
In this study, reversible bonding between thermoplastic or glass was achieved with the help of UV curable release tape, which is originally used in the wafer dicing process in the semiconductor industry. Unlike other UV curable materials used in the irreversible bonding, in which the bonding was achieved by UV exposure of the photosensitive material sandwiched between the substrate and cover plate. In this study, the UV curable dicing tape could achieve a high bonding strength by sandwiching between the substrate and cover plate with a gentle press, and the bonding could be immediately detached after a UV exposure process without residuals left on the substrate and cover plate. The PMMA-PMMA, glass-glass, PMMA-glass bonding was achieved and tested with the UV curable tape. Compared with the previously reported reversible bonding method, the proposed method makes no compromise on bonding strength to achieve reversible bonding, and the bonding strength is comparable to irreversible bonding methods. Another significant advantage is the detachment between substrate and cover could easily be achieved by UV exposure without the forced detachment process, which is commonly used in other reversible bonding approaches by hand peeling or inset a blade, in this study, no residuals were observed on the surface of the substrate and cover plate after debonding.
2 Materials and methods
Materials and instruments The UV curable release film (HUV-D7125-30) is sourced from Nippon Pulse Motor Taiwan Co., Ltd. The structure of the film is illustrated in the inset in Fig. 1, the PET base film has a thickness of 125 μm, the UV curable adhesive layer (acrylic) on both sides of the PET base film has a thickness of 30 μm, the release liner (PET) for film protection on both side of the film has a thickness of 25 μm. The UV release film is originally used in the silicon wafer grinding and cutting process for the protection of the microstructures fabricated on the wafer in MEMS or IC industry and the UV release properties can help to ease the process to retrieve the loose dies.
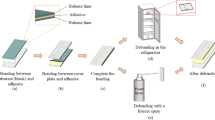
Chip fabrication, bonding, and debonding process for PMMA-based microfluidic chips. a Laser ablated microchannels and through holes on the substrate and cover plate, respectively; b adhesive bonding between the substrate and cover plate with sandwiched UV release tape, c UV exposure in a custom-made UV exposure box with a peak wavelength at 365 nm for debonding. d The detached substrate and cover plate
PMMA and glass were used as the bulk material used for the fabrication of microfluidic devices in this study, the casted PMMA plate with a thickness of 1.5 mm is sourced from TONDA Co., Ltd., China. The glass plate (microscope slide) is sourced from Ted Pella, Inc., USA. The Bacillus subtilis CMCC(B) 63,501 bacteria used in this study for the demonstration of the biocompatibility is sourced from China General Microbiological Culture Collection Center (CGMCC).
The CO2 laser ablation equipment (HTE-1206-W80, with a wavelength of 10.6 μm) for microchannel fabrication on the surface of PMMA substrate is sourced from Yue Ming Laser Group Co., Ltd, China, the power output of the laser is adjustable from 0 to 100 W, and the scan speed is also adjustable from 0 to 1 m/s. Optical images of the instruments and chips were taken by Nikon D3000 Digital SLR Camera (Nikon Corporation, Japan). The SEM (scanning electron microscope) used in this study for the observation of the cross-section of the microfluidic chip is model JSM-7160F, JEOL Ltd., Japan. The thermal roll laminator (GD-320) for bubble removal between the adhesive layer and substrate/cover plate is from Golden Ltd., China.
Fabrication of microfluidic chips and the bonding/debonding process The microfluidic chip fabrication and bonding/debonding procedure is shown in Fig. 1a. For the fabrication of PMMA-based microfluidic devices, a CO2 laser ablation procedure was used for the direct formation of microchannels on the surface of the PMMA substrate. The power of the laser was set at 15 W with a scanning speed of 40 mm/s, resulting in a microchannel with a Gaussian-like profile, the width of the microchannel is 265 μm with the depth of microchannel is around 316 μm. The profile of the microchannel is adjustable with various settings of laser power and scan speed. The minimum achievable channel width is around 80 μm with a depth of around 130 μm, the detailed fabrication procedure, limitation and system setup for CO2 laser ablation on thermoplastics were reported in our previous work (Liu et al. 2018).
The bonding procedure is shown in Fig. 1b, the PMMA substrate with laser-ablated microchannels was aligned with PMMA cover plate with laser-ablated through holes, then UV curable release tapes (with release liners removed on both sides) were sandwiched between the substrate and cover plate. The whole assembly was sent through a conventional office thermal laminator for the bonding process (with the heater off). The major reason to use the laminator is the roll-to-roll configuration of the laminator is helpful to remove the bubbles trapped between substrate/cover plate and adhesive layer. The glass-PMMA bonding procedure is identical to the PMMA-PMMA bonding procedure.
This study proposed a reversible bonding method, the debonding procedure is shown in Fig. 1c, the whole chip went through a UV exposure process for debonding, the applied dose is 4.25 mW/cm2 (with a duration of 180 s) for PMMA-PMMA debonding, the peak of the applied UV exposure is at 365 nm (same absorption peek of UV release tape). The UV exposure process was conducted in a custom-made UV exposure box (consisting of 8 UV lamps with the peak at 365 nm). It is worth mentioning that the exposure time significantly influenced the bonding strength, which will be discussed in the followed sessions. After detachment (Fig. 1d), hardly any residuals were observed on the surface of the substrate and cover plate.
Bonding test Two types of bonding strength tests (bust pressure test and tensile bonding strength test) were conducted for PMMA-PMMA and PMMA-Glass bonding with the help of UV release tape. The system setup for the burst pressure test is shown in Fig. 2, the compressed air from a compressor was supplied to a pressure regulator with a nylon pipe, then passing through a manual switch and used a T-shape air fitting to supply compressed air to bonded chip and pressure sensor (SDE1, Festo Corporation, Germany) simultaneously.
The PMMA/Glass-PMMA bonded chips have a cylindrical chamber (2 mm in diameter, 1.5 mm in height) connected to a nylon pipe for air supply (illustrated in Fig. 2). During the burst pressure test, the air pressure was supplied to the chip and gradually increase until the sudden detachment between glass/PMMA cover plate to PMMA substrate happens, and the whole process was monitored by the pressure sensor for a continuous measurement of air pressure till burst-open happen. The measured burst-open pressure for glass-PMMA bonding (average of three measurements) is 702 kPa, while the burst pressure for PMMA-PMMA bonding (average of three measurements) is 665 kPa.
The tensile bonding strength test between glass/PMMA cover plate to PMMA substrate were measured with a universal tensile testing machine (HD-B615A-S, Haida Equipment Co., Ltd., China). The glass/PMMA cover plate was bonded to the PMMA substrate with a 10 mm by 10 mm overlap area, then use epoxy glue to firmly attach the cover plate and substrate to the upper and lower fixtures of the universal tensile testing machine (shown in Fig. 3c). During the tensile bonding test, the upper and lower fixtures gradually pull apart the substrate and cover plate, the applied force and displacement were continuously recorded till sudden detachment happens.
The tensile bonding test result for glass/PMMA-PMMA bonding with various UV exposure duration is shown in Fig. 3a, b. Figure 3a shows the measured tensile bonding test result with UV exposure duration from 0 to 180 s (at a dose of 4.25 mW/cm2). For the unexposed bonding between PMMA cover plate and PMMA substrate, the bonding failed with an applied force of 132 N (corresponds to 1.32 MPa with the 10 mm by 10 mm bonded area). However, the bonding strength dropped to 89 N (0.89 MPa) after 90 s’ UV exposure and 20 N (0.2 MPa) after 180 s’ UV exposure. Similar tensile bonding test results were also observed for the glass to PMMA bonding, the maximum force measured before bonding failure is 141 N (corresponds to 1.41 MPa with the 10 mm by 10 mm bonded area), the bonding force dropped to 122 N (1.22 MPa) after 90 s’ UV exposure and further decreased to 38 N (0.39 MPa) after 180 s’ UV exposure. In this study, the UV exposure duration needed for detachment was set at 180 s for PMMA-PMMA and glass-PMMA bonding, after 180 s’ UV exposure, the cover plate and substrate can be easily detached by hand and without any obvious residual left on the surface of the cover plate and substrate.
3 Result and discussion
Cross-section images of the PMMA-PMMA bonded microfluidic chip with microchannels are shown in Fig. 4. Figure 4a (SEM image) shows the PMMA substrate with laser-ablated microchannel bonded to a PMMA cover plate with the help of UV release tape. The cross-section of the laser-ablated microchannel on PMMA has a Gaussian-like profile, corresponds to the energy distribution of the CO2 laser-focused on the surface of the PMMA substrate. The microchannel in Fig. 4a was fabricated with a laser power of 15 W a scanning speed of 40 mm/s, the width of the microchannel is around 265 μm with the depth of the microchannel is around 316 μm. Figure 4b (optical microscope image) shows a near-rectangular microchannel fabricated by micro-milling on the surface of PMMA substrate, the width of the channel is around 610 μm with a depth of around 385 μm. The sandwiched UV release tape between the substrate and cover plate is visible in both of the cross-section images in Fig. 4.
Cross-section images of the microchannels fabricated with CO2 laser ablation and micro-milling on the surface of PMMA substrate and bonded with PMMA cover plate with the UV release tape. a SEM image of the cross-section of the CO2 laser-ablated microchannel on PMMA; b optical microscope image for the cross-section of the micro-milled microchannel on the PMMA substrate
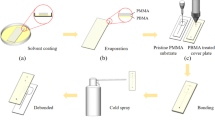
The adhesive layers on both sides of the UV release tape are composed of based polymer and fluidity oligomers before UV exposure (illustrated in Fig. 5), the fluidity oligomer has strong adhesion to the PMMA plates to achieve the bonding. However, after UV exposure, oligomers were cross-linked with the polymerization reaction. After UV exposure, the adhesive layers become rigid and thus lost the adhesive strength to the contacted surface.
The contact angle on the surface of PMMA substrate and UV curable release tape is shown in Fig. 6. The water contact angle on the surface of the PMMA substrate is 82.41°. Due to the similarity of the materials on the adhesive layer (acrylic oligomers with base polymer), the water contact angle on the adhesive layer of UV release tape is 85.75°, which is very close to the water contact angle of PMMA plate. The consistent water contact angle on the microchannel internal surface is helpful for fluid manipulation, and the consistent surface wettability on all the surface of the microchannel is especially helpful for the droplet microfluidics (Meng et al. 2016).
A thermal aging test was also conducted in this study for the examination of the thermal stability of the bonding achieved by UV release tape. As shown in Fig. 7, PMMA substrate and cover plate were first bonded with UV release tape and then exposed in a thermal chamber at 80 °C. The tensile bonding strength was measured after 0, 2, and 24 h, the result indicates the bonding strength decreased significantly after thermal treatment, dropped from 1.32 MPa to around 0.2 MPa after 24 h’ thermal aging. UV–Vis transmittance spectra of the PMMA-PMMA bonded and glass-PMMA bonded chips are shown in Fig. 8, results show relatively favorable transparency in the visible range, which is of great importance for the observation of the fluid propagation inside the chip.
For the demonstration of the proposed bonding techniques, a T-shape droplet generator and a fluid mixer were fabricated. As shown in Fig. 9a, the T-shape droplet generator has laser-ablated microchannel on PMMA substrate and then bonded with a PMMA cover plate. The laser-ablated microchannel on the surface of PMMA substrate was fabricated with a laser power of 15 W and a scan speed of 20 mm/s, result in a channel with a width of around 289 μm and a depth of around 386 μm. For the droplet generation, the continues phase is DI water at 100 μL/min, while the dispersed phase is dimethyl silicone oil (dye in red, with a viscosity of 10 mPa·s, Macklin Biochemical Co., Ltd., China) flowing at a rate of 200 μL/min, the generated droplet has a diameter around 220 μm with a generation rate around 230 Hz. The inset in Fig. 9a shows the enlarged image of the droplet formation process at the T-junction.
The microchannel on the fluid mixer (shown in Fig. 9b) was also fabricated with the laser power of 15 W and a scan speed of 20 mm/s on the surface of PMMA substrate and then bonded with a glass cover plate with the help UV release tape. The DI water dye in yellow and blue was pumped into the chip at the same rate at 200 μL/min. No leakage was observed for both of the microfluidic ships during the experimental process.
To study the biocompatibility of the UV release tape used in this study,we fabricated a PMMA-based culture dish with laser ablation and covered the whole bottom area with the UV released tape (shown in Fig. 10). The bacteria (Bacillus subtilis) was cultured in this device filled with culture medium (tryptone soy broth, sourced from Aoboxing Bio-Tech CO. Ltd, China) for 36 h, the result (Gram stain for direct visualization, inset in Fig. 10) indicates relatively good biocompatibility of the UV release tape.
This study proposed a reversible bonding method that can achieve relatively high bonding strength and even comparable to irreversible bonding. A comparison between the proposed work using UV release tape and previously reported reversible/irreversible bonding methods is listed in Table 1. Table 1 contains a brief description of the bonding method, tensile or burst-opening bonding strength measurement result and the required bonding time. The archived bonding strength (665 kPa for PMMA-PMMA bonding, 702 kPa for PMMA-glass bonding) in this study has obvious advantages on bonding strength and short processing time compared with other reversible bonding methods. The drawback of the proposed bonding method using UV release tape includes: firstly, we found it is not suitable for the bonding of PDMS, the adhesion force between PDMS and UV release film is very weak to maintain a secured bonding; secondly, the bonded chips need to avoid direct sunlight in the outdoor environment, we found the PMMA-PMMA bonding failed after 15 min’ exposure under the direct sunlight in summer.
4 Conclusion
This study proposed a new reversible bonding method to achieve the PMMA-PMMA or glass-PMMA bonding with the help of UV release tape. Compared with other reversible bonding methods, the bonding process is easily accessible and has a higher bonding strength that is even comparable to irreversible bonding. In the conventional reversible bonding method, the substrate and cover plate were usually forced apart and usually left significant residuals on the bonded surface, however, in this study, the debond was achieved by UV exposure and no obvious residual was left on the bonded surface. Several microfluidic devices were also fabricated for demonstration and the bonding strength after various UV exposure duration were also discussed in this study. Biocompatibility of the UV release tape was also confirmed with a bacteria culture experiment.
The proposed reversiable bonding method is simple, straightforward, and without the requirement of highly sophisticated instruments, and could have wide application potentials to achieve reversible bonding between thermoplastics and the hybrid bonding between thermoplastic and glass plates.
References
Au AK, Huynh W, Horowitz LF, Folch A (2016) 3D-printed microfluidics. Angew Chem Int Ed 55(12):3862–3881
Bhattacharya S, Datta A, Berg JM, Gangopadhyay S (2005) Studies on surface wettability of poly (dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J Microelectromech Syst 14(3):590–597
Bodas D, Khan-Malek C (2007) Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—an SEM investigation. Sens Actuators B Chem 123(1):368–373
Chen Q, Li G, Nie Y, Yao S, Zhao J (2014) Investigation and improvement of reversible microfluidic devices based on glass–PDMS–glass sandwich configuration. Microfluid Nanofluid 16(1–2):83–90
Chu M, Nguyen T, Lee E, Morival J, Khine M (2017) Plasma free reversible and irreversible microfluidic bonding. Lab Chip 17(2):267–273
Dinh T et al (2015) Development of reversible bonding for microfluidic applications. Microfluidics Nanofluidics 19(3):751–756
Fan Y, Li H, Yi Y, Foulds IG (2014) PMMA to Polystyrene bonding for polymer based microfluidic systems. Microsyst Technol 20(1):59–64
Funano S-I, Ota N, Tanaka Y (2021) A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 21(11):2244–2254
Gong X et al (2010) Wax-bonding 3D microfluidic chips. Lab Chip 10(19):2622–2627
Gu P, Liu K, Chen H, Nishida T, Fan ZH (2011) Chemical-assisted bonding of thermoplastics/elastomer for fabricating microfluidic valves. Anal Chem 83(1):446–452
Kojic SP, Stojanovic GM, Radonic V (2019) Novel cost-effective microfluidic chip based on hybrid fabrication and its comprehensive characterization. Sens Actuators A 19(7):1719
Kurihara K, Hokari R, Satoh T, Sugiura S, Miyake K, Kanamori T (2020) Low-deformation precision thermal bonding of nanostructured microfluidic chips. Jpn J Appl Phys 59(1):8
Le Berre M, Crozatier C, Velve Casquillas G, Chen Y (2006) Reversible assembling of microfluidic devices by aspiration. Microelectron Eng 83(4):1284–1287. https://doi.org/10.1016/j.mee.2006.01.257
Le NXT, Lee NY (2020) Chitosan–polydopamine hydrogel complex: a novel green adhesion agent for reversibly bonding thermoplastic microdevice and its application for cell-friendly microfluidic 3D cell culture. Lab Chip 20(19):3524–3534
Liu S, Fan Y, Gao K, Zhang Y (2018) Fabrication of Cyclo-olefin polymer-based microfluidic devices using CO2 laser ablation. Mater Res Express 5(9):095305
Lynh HD, Pin-Chuan C (2018) Novel solvent bonding method for creation of a three-dimensional, non-planar, hybrid PLA/PMMA microfluidic chip. Sens Actuators A Phys 280:350–358
Mahmoodi S, Sun P-K, Mayer M, Besser R (2019) Gas-assisted thermal bonding of thermoplastics for the fabrication of microfluidic devices. Microsyst Technol 25(10):3923–3932
Meng Q, Zhang Y, Li J, Lammertink RG, Chen H, Tsai PA (2016) Altering emulsion stability with heterogeneous surface wettability. Sci Rep 6(1):1–8
Moon B-U, Morton K, Li K, Miville-Godin C, Veres T (2021) Reversible Bonding of Thermoplastic Elastomers for Cell Patterning Applications. Processes 9(1):54
Nath P, Fung D, Kunde YA, Zeytun A, Branch B, Goddard G (2010) Rapid prototyping of robust and versatile microfluidic components using adhesive transfer tapes. Lab Chip 10(17):2286–2291
Pitingolo G, Nizard P, Riaud A, Taly V (2018) Beyond the on/off chip trade-off: A reversibly sealed microfluidic platform for 3D tumor microtissue analysis. Sens Actuators B Chem 274:393–401
Pitingolo G, Riaud A, Nastruzzi C, Taly V (2019a) Gelatin-coated microfluidic channels for 3d microtissue formation: On-chip production and characterization. Micromachines 10(4):265
Pitingolo G, Riaud A, Nastruzzi C, Taly V (2019b) Tunable and reversible gelatin-based bonding for microfluidic cell culture. Adv Eng Mater 21(8):1900145
Rasponi M, Piraino F, Sadr N, Lagana M, Redaelli A, Moretti M (2011) Reliable magnetic reversible assembly of complex microfluidic devices: fabrication, characterization, and biological validation. Microfluidics Nanofluidics 10(5):1097–1107
Ren Y, Ray S, Liu Y (2019) Reconfigurable acrylic-tape hybrid microfluidics. Sci Rep 9(1):1–10
Sackmann EK, Fulton AL, Beebe DJ (2014) The present and future role of microfluidics in biomedical research. Nature 507(7491):181–189
Serra M, Pereiro I, Yamada A, Viovy J-L, Descroix S, Ferraro D (2017) A simple and low-cost chip bonding solution for high pressure, high temperature and biological applications. Lab Chip 17(4):629–634
Shang L, Cheng Y, Zhao Y (2017) Emerging droplet microfluidics. Chem Rev 117(12):7964–8040
Shiroma LS et al (2016) Self-regenerating and hybrid irreversible/reversible PDMS microfluidic devices. Sci Rep 6(1):1–12
Sivakumar R, Lee NY (2020) Heat and pressure-resistant room temperature irreversible sealing of hybrid PDMS–thermoplastic microfluidic devices via carbon–nitrogen covalent bonding and its application in a continuous-flow polymerase chain reaction. RSC Adv 10(28):16502–16509
Song K-Y, Zhang H, Zhang W-J, Teixeira A (2018) Enhancement of the surface free energy of PDMS for reversible and leakage-free bonding of PDMS–PS microfluidic cell-culture systems. Microfluid Nanofluid 22(11):1–9
Tran HH, Wu W, Lee NY (2013) Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens Actuators B Chem 181:955–962
Tsao C-W, Syu W-C (2020) Bonding of thermoplastic microfluidics by using dry adhesive tape. RSC Adv 10(51):30289–30296
Vézy C, Haddour N, Dempsey N, Dumas-Bouchiat F, Frénéa-Robin M (2011) Simple method for reversible bonding of a polydimethylsiloxane microchannel to a variety of substrates. Micro Nano Lett 6(10):871–873
Vulto P et al (2005) Microfluidic channel fabrication in dry film resist for production and prototyping of hybrid chips. Lab Chip 5(2):158–162
Wasay A, Sameoto D (2015) Gecko gaskets for self-sealing and high-strength reversible bonding of microfluidics. Lab Chip 15(13):2749–2753
Zhang Y, Liu J, Wang H, Fan Y (2019) Laser-induced selective wax reflow for paper-based microfluidics. RSC Adv 9(20):11460–11464
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51804014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, Y., Li, L., Jiang, J. et al. Reversible bonding for microfluidic devices with UV release tape. Microfluid Nanofluid 26, 23 (2022). https://doi.org/10.1007/s10404-022-02532-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-022-02532-4