Abstract
Bonding is the critical step in the fabrication of thermoplastic-based microfluidic devices to enclose the microchannel. Conventional bonding method for thermoplastic is usually irreversible with thermal fusion or chemical treatment approaches. The reversible bonding for microfluidic applications is sometimes desirable to retrieve the samples inside the microstructures. In this study, a low cost, rapid, and low-residue reversible bonding method was proposed with the freeze-release adhesive. The poly(butyl methacrylate)-based freeze-release adhesive can achieve a relatively high bonding strength between the thermoplastic plates after applying a gentle compression force with/without thermal treatment. The detachment of the thermoplastics plates can be achieved by either storing the bonded chip in the refrigerator for several minutes or using freeze spray (immediate detachment). With the help of freeze-release adhesive, bonding between PMMA plates and hybrid bonding between PMMA and PS/PC was achieved. Bonding strengths were studied in detail with various temperature settings in the bonding process. The detachment process was also studied with different low-temperature treatment durations. The biocompatibility of the freeze-release adhesive was also discussed in this study. Compared to the previous reversible bonding methods, the proposed reversible bonding approach is simple to handle, rapid on bonding/debonding, low residue, and harmless to biological samples inside the microfluidic chip.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bonding is the critical step in the fabrication of polymer-based microfluidics, in the conventional fabrication procedure of polymer-based microfluidics, the substrate with fabricated microstructures and cover plate (same or different materials) are bonded with various approaches to enclose the microchannel. Some of the most commonly used bonding methods include thermal fusion bonding (Tsao and DeVoe 2009), laser bonding (Li et al. 2022), solvent bonding (Sivakumar and Lee 2020), and ultrasonic bonding (Zhang et al. 2010). However, the aforementioned bonding methods are usually irreversible. Currently, microfluidics has been widely used in the biological field, in some application scenarios reversible bonding is inevitable, e.g., surface patterning of immunoglobins (Delamarche et al. 1998) and cell recovery (Funano et al. 2021; Pitingolo et al. 2019), the biosamples need to be retrieved by detaching the substrate and cover plate. Less commonly, reversible bonding is also required for microchannel surface treatment or reuse of the chip.
Various reversible bonding methods have been invented for PDMS (polydimethylsiloxane) or thermoplastic-based microfluidics. Gecko gasket-like structures were fabricated on PDMS for the reversible bonding of microfluidic devices (Wasay and Sameoto 2015). Uncured PDMS can also be used for the reversible bonding of PDMS-based microfluidic devices (Chu et al. 2017). For thermoplastic-based microfluidics, a releasable adhesive layer (intermediate layer) can be used for the bonding process, while the release process can be triggered with UV radiation (Yao et al. 2022a), thermal, or chemical approaches (Gong et al. 2010). In addition, magnetic force (Pitingolo et al. 2018) or mechanical clamping (Moon et al. 2020) can also be used for the reversible bonding of microfluidic devices with less selectivity on thermoplastic materials. Generally, the reversible bonding methods suffer from low bonding strength (which may cause leakage) and/or residues from adhesives after the detachment of the substrate and cover plate.
In this study, a novel reversible bonding method for thermoplastic-based microfluidic devices is proposed. The thermoplastic substrate and cover plate were bonded with a poly(butyl methacrylate)-based freeze-release adhesive film, after use, the substrate and cover plate can be easily detached with a low-temperature treatment either using a refrigerator or freeze spray. Compared to the previous reversible bonding methods, the proposed bonding method has the advantage of a simple and rapid bonding/debonding process, low residue on the contact surface, and relatively high bonding strength comparable to the irreversible bonding method. The low-temperature treatment process is also less harmful to the biosample to be retrieved inside the chip. The proposed reversible bonding method could have wide application potential in thermoplastic-based microfluidic devices for biological or chemical study.
2 Fabrication
2.1 Materials and instruments
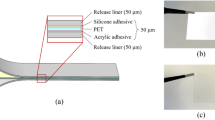
The freeze-release adhesive tape is sourced from Depin Packing Co., Ltd, China, the freeze-release tape consists of a 25 μm-thick poly butyl methacrylate (PBMA) adhesive layer sandwiched with two 50 μm-thick poly(ethylene terephthalate) release liner layers (shown in the inserts in Fig. 1a). PMMA (polymethyl methacrylate), PC (polycarbonate), and PS (polystyrene) plates were used as the bulk material for the fabrication of microfluidic devices in this study, all these thermoplastics plates have the same thickness of 2 mm and were sourced from Tonada Co., Ltd, China. The Bacillus subtilis CMCC(B) 63,501 bacteria used in the biocompatibility test of this study is sourced from China General Microbiological Culture Collection Center (CGMCC). The freezing spray used for the debonding process is Hiens PR14086, CRC Industries, USA.
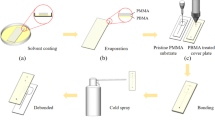
Chip bonding and detachment process. a Attachment of adhesive layer to the blank thermoplastic substrate; b Align and attach the cover plate to the adhesive layer; c Bonding with roll laminator; d Bonded chip was frozen in the refrigerator for 2–5 min to debond; e Bonded chip was treated with freezer spray for instant debond; f Detached chip
The SEM (scanning electron microscopy) used in this study (GeminiSEM 300) for the observation of the cross-section of the microchannel is sourced from ZEISS Group, Germany. A commercial CO2 laser ablation instrument (CMA1080, Guangdong Han's Yueming Laser Group Co., Ltd., China) was used for the direct laser ablation on the surface of PMMA for the formation of microchannels. The CO2 laser has a wavelength of 10.6 μm, the scan speed adjusts from 0 to 1000 mm/s, while the laser power is also adjustable from 0 to 100 W. A commercial hot/cold roll laminator (Golden Co., Ltd, China) was used in this study for the bonding and degassing of the thermoplastic substrate and cover plate with an intermediate freeze-release adhesive layer. The tensile bonding strength between the thermoplastic plates was measured with a universal testing machine (WDW-55, DahoMeter, China).
2.2 Bonding and detachment
The thermoplastics chip bonding process (the same process for PMMA-PMMA, PMMA-PS, and PMMA-PC bonding) was illustrated in Fig. 1. A 2 mm-thick PMMA plate was laser cut into a standard microscope slide’s size (25 mm by 75 mm). The liner on one side of the freeze-release tape was removed, freeze-release tape and blank substrate were then attached (Fig. 1a). After freeze-release tape was attached to the substrate plate, the liner on the other side of the tape was peeled off and the cover plate was attached (Fig. 1b). The final assembly has the freeze-release tape sandwiched between the PMMA cover plate and substrate.
As illustrated in Fig. 1b, the cover plate has the laser ablated microchannels with inlet/outlet through holes. Then, the whole bonding assembly was fed through a commercial roll laminator to remove the bubbles trapped between the tape and the PMMA plate, the bonding strength was also further enhanced in this process (illustrated in Fig. 1c). The bonding process with the roll laminator only takes around 10 s. Various temperature settings on the roll laminator were tested for the study of bonding strength (discussed in the following session).
The debonding (releasing) process was conducted with two different approaches for low-temperature treatment. The first method is to store the chip in a refrigerator at – 27 ℃, the substrate and cover plate debonded spontaneously after around 5 min (process illustrated in Fig. 1d). A detailed discussion on low-temperature treatment and bonding strength will be discussed in the following session. Another more convenient method was also used in this study for the debonding of the thermoplastic substrate and cover plate, a freeze cold spray was used for the instance temperature drop on the bonded thermoplastic chip to around – 51 ℃, causing the chip to detach within a few seconds (illustrated in Fig. 1e). Supplementary video footage was also provided for the demonstration of the debonding process with freeze spray. The low-temperature debonding is mainly due to the brittleness and stiffness of the PBMA under low temperature and the significant difference in the thermal expansion coefficient of PBMA and PMMA (Meyer et al. 1965).
The bonded PMMA-based microfluidic chip is shown in Fig. 2a, the PMMA substrate and cover plate were bonded with the help of freeze-release tape (bonding temperature was set at 50 ℃ on the roll laminator). After 5 min low-temperature treatment process in the refrigerator, the bonding failed spontaneously. As shown in Fig. 2b, almost no residues were left on the contacted surfaces. It should be noted that the released substrate (blank sheet before bonding) has some faint marks transferred from the patterns on the PMMA cover plate during the bonding process (same principle as the hot embossing process). The layout the chip is shown in Fig. 2c.
The cross-section SEM image of the bonded chip is shown in Fig. 3, the freeze-release adhesive layer has a thickness of around 25 μm and is sandwiched between the PMMA substrate and cover plate. The cross-section profile of the microchannel is also shown in Fig. 3, the Gaussian-like profile of the laser ablated channel is sourced from the energy distribution of the CO2 laser beam. The microchannel shown in Fig. 3 was fabricated with a laser power of 17 W and a scan speed of 40 mm/s, the fabricated microchannel has a width of around 320 μm and a depth of around 445 μm. It is worth mentioning that, the profile of the microchannels can be adjusted with different laser power and laser scan speed (Liu et al. 2018). The SEM images of the surface of the substrate and cover plate before the bonding (bare plate without adhesive tape) and after the debonding process are shown in Fig. 4, the residue of the freeze-release tape is sparsely distributed on the surface of the substrate and cover plate. For the reuse of the chip, a cleaning process is required to remove the residues left on the PMMA plate.
3 Result and discussion
The water contact angle has a significant influence on the functionality of microfluidic devices, especially in fluid mixing or droplet generation. As illustrated in Fig. 5, with the proposed bonding method, one side of the enclosed microchannel will be covered with freeze-release tape while the other part of the channel is the pristine PMMA material. Thus, it is necessary to study the water contact angle on pristine PMMA and freeze-release tape. The measured water contact angles on the surface of the pristine PMMA plate and freeze-release adhesive tape are shown in Fig. 4, the water contact angle on the freeze-release adhesive (74.3°, average of three measurements) is slightly lower than the water contact angle on the PMMA plate (97.4°, average of three measurements).
The bonding strength was analyzed with the help of a universal testing machine. As discussed in the previous session, the freeze-release tape was sandwiched between the thermoplastic substrate and cover plate and processed with a roller laminator. The heated roller raises the temperature of the chip assembly while applying a compression force at the same time to enhance the bonding strength and remove bubble traps between the adhesive film and thermoplastic plates. We tested the bonding strength with various temperature settings on the roll laminator, the bonding strength measurement results are shown in Fig. 6 (each data point indicates an average of three measurements). The PMMA cover plate and the substrate have an overlap area of 7 mm by 7 mm, cover plate and substrate were fixed with the upper and lower grip of the universal testing machine (system setup shown in Fig. 7d), and the maximum tensile force (124.39 N) was obtained with a bonding temperature of 30 ℃ on the roll laminator, corresponding to a bonding strength of 2.54 MPa, which is comparable to the irreversible thermal fusion bonding strength and even higher than the conventional PDMS-glass bonding strength (Tsao and DeVoe 2009; Sivakumar and Lee 2020). Different from the thermal fusion bonding approach of thermoplastic-based microfluidics, a higher temperature doesn’t further enhance the bonding strength with the freeze-release tape.
Besides the tensile bonding test with a universal testing machine, a burst opening method was also used for the bonding strength test. The system setup is illustrated in Fig. 7, PMMA cover plate and substrate were bonded with freeze-release adhesive at 30 ℃, then compressed air was pumped into a chamber inside the chip with a size of 20 mm by 20 mm. The air pressure is gradually increased until the burst opens happen, and the measured maximum air pressure is around 0.8 MPa. Higher than the reported burst opening pressure of irreversible bonding between PDMS and glass (Tsai et al. 2011), and also higher than the reported ultrasonic bonding between thermoplastics (Zhang et al. 2009). Interestingly, the achieved bonding strength evaluated with the burst opening method is greater than the conventional bonding approaches with an intermediate layer (e.g., UV glue, double-sided tape, APTES) (Giri and Tsao 2022).
Freeze-release tape relies on low temperatures to achieve the debonding process, in this study, the low-temperature environment was achieved either by a refrigerator or freeze spray. For further characterization of the debonding process, the bonding strength with various low-temperature treatment duration was tested for PMMA-PMMA, PMMA-PS, and PMMA-PC bonded chips (the bonded area is 7 mm by 7 mm, bonding temperature at 30 ℃). The bonded chips were stored in the refrigerator at – 27 ℃ for 2 or 5 min, then the bonding strengths of the untreated, 2 min stored and 5 min frozen chips were measured with the universal testing machine. The results are shown in Fig. 7, after 2 min’ low-temperature treatment, the bonding strength of the PMMA-PMMA bonded chips drop from 2.5 MPa to around 1.3 MPa, then drop further down to 0.35 MPa with 3 more minutes’ storage in the refrigerator (Fig. 8a). Similar trends were also found for PMMA-PC and PMMA-PS bonding with freeze-release tape. As shown in Fig. 8b and c, the untreated chipsets have a bonding strength of around 2 MPa, after 5 min’ low-temperature treatment in the refrigerator, the bonding strengths dropped to lower than 0.5 MPa. The bonding strength test system setup is shown in Fig. 8d.
Bonding strength measurements before and after low-temperature treatment. a PMMA-PMMA bonded chip before and after low-temperature treatment; b PMMA-PS bonded chip before and after low-temperature treatment; c PMMA-PC bonded chip before and after low-temperature treatment; d Universal tensile testing machine used for the bonding measurement
The experimental results shown in Fig. 8 indicate that the freeze-release tape is highly adaptable to various thermoplastics both in the bonding and debonding process. As shown in Fig. 8, the bonding strength is relatively stable between various thermoplastic materials. More importantly, the debonding process also reveals very low selectivity of the thermoplastic materials. All these results show freeze-release tape could be a very promising intermediate layer for the reversible bonding of thermoplastics.
A table was prepared (Table 1) for the comparison between the proposed study and the previous study on the reversible bonding of polymer-based microfluidics. The bonding methods were compared by means of bonding time, bonding strength applicable plastics, etc. The result indicates that the proposed bonding method with freeze-release adhesive has relatively higher bonding strength with a rapid bonding process and is also adaptable for various types of plastics.
For the demonstration of the proposed reversible bonding method, two kinds of microfluidic devices were fabricated. The fabricated T-shape droplet generator is shown in Fig. 9a, the continuous phase is liquid paraffin with 2 wt% of Span 80, and the disperse phase is DI water with food color. The continuous phase and dispersed phases were injected into the chip with syringe pumps at a rate of 0.2 mL/min and 0.1 mL/min. The fabricated gradient mixer is shown in Fig. 9b, the yellow and blue liquid phases are DI water with food dye both injected at the same rate of 0.2 mL/min. The droplet generator and gradient mixer functioned as expected, and no leakage was observed during the experimental process.
Biocompatity of the intermediate layer is always a big concern for the thermoplastics bonding approaches using an intermediate adhesive layer to achieve the bonding process. It is worth mentioning that biological compatibility is highly object-oriented, the intermediate layer could be very compatible with one biosample while highly toxic to another. In this study, with the constraints from the experimental condition, we performed a compatibility test by culturing the bacteria with a culture chamber in the PMMA-based microfluidic chip, the culture chamber was covered with freeze-release adhesives on the top and bottom sides.
The bacteria were loaded into the chip filled with culturing media (tryptone soy powder dissolved in DI water) and cultured at 37 ℃ for 24 h. The Gram staining of the Bacillus subtilis bacteria after 24 h’ culture is also shown in Fig. 10, the result doesn’t reveal any toxicity of the intermediate layer to the bacteria.
4 Conclusion
This study proposed a novel reversible bonding method for thermoplastic-based microfluidics using freeze-release tape. The bonding and debonding process were introduced in detail with the characterization of bonding strength under various bonding/debonding conditions, water contact angle, and biocompatibility. Compared to other reversible bonding methods for thermoplastic-based microfluidics, the proposed bonding and debonding processes were conducted in a simple, rapid, and low-cost manner, with very low residue left and high bonding strength. The proposed bonding method is also highly adaptive to various thermoplastic materials, conventional or hybrid bonding was all demonstrated in this study. Using freeze-release tape as an intermediate layer for the bonding of thermoplastic gives a new and effective alternative to the reversible bonding of thermoplastic-based microfluidics.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chu M, Nguyen T, Lee E, Morival J, Khine M (2017) Plasma free reversible and irreversible microfluidic bonding. Lab Chip 17(2):267–273
Delamarche E, Bernard A, Schmid H, Bietsch A, Michel B, Biebuyck H (1998) “Microfluidic networks for chemical patterning of substrates: design and application to bioassays.” J Am Chem Soc 120(3):500–508
Funano S-I, Ota N, Tanaka Y (2021) A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 21(11):2244–2254. https://doi.org/10.1039/D1LC00058F
Giri K, Tsao C-W (2022) Recent advances in thermoplastic microfluidic bonding. Micromachines 13(3):486
Gong X et al (2010) Wax-bonding 3D microfluidic chips. Lab Chip 10(19):2622–2627
Li L, Huang Y, Fan Y (2022) Low-cost irreversible blue diode laser transmission welding of poly (methyl methacrylate)-based microfluidics. Appl Phys A 128(12):1–8
Liu S, Fan Y, Gao K, Zhang Y (2018) Fabrication of Cyclo-olefin polymer-based microfluidic devices using CO2 laser ablation. Mater Res Express 5:095305
Meyer HH, Mangin P-MF, Ferry JD (1965) Dynamic mechanical properties of poly(n-butyl methacrylate) near its glass transition temperature. J Polym Sci Part a 3(5):1785–1792
Moon B-U, Morton K, Li K, Miville-Godin C, Veres T (2020) Reversible bonding of thermoplastic elastomers for cell patterning applications. Processes 9(1):54
Pitingolo G, Nizard P, Riaud A, Taly V (2018) Beyond the on/off chip trade-off: a reversibly sealed microfluidic platform for 3D tumor microtissue analysis. Sens Actuators B 274:393–401
Pitingolo G, Riaud A, Nastruzzi C, Taly V (2019) Tunable and reversible gelatin-based bonding for microfluidic cell culture. Adv Eng Mater 21(8):1900145
Sivakumar R, Lee NY (2020) Microfluidic device fabrication mediated by surface chemical bonding. Analyst 145(12):4096–4110
Song K-Y, Zhang H, Zhang W-J, Teixeira A (2018) Enhancement of the surface free energy of PDMS for reversible and leakage-free bonding of PDMS–PS microfluidic cell-culture systems. Microfluid Nanofluid 22:1–9
Tsai L-F, Dahlquist WC, Kim S, Nordin GP (2011) Bonding of polydimethylsiloxane microfluidics to silicon-based sensors. J Micro/nanolithogr MEMS MOEMS 10(4):043009
Tsao C-W, DeVoe DL (2009) Bonding of thermoplastic polymer microfluidics. Microfluid Nanofluid 6(1):1–16
Wasay A, Sameoto D (2015) Gecko gaskets for self-sealing and high-strength reversible bonding of microfluidics. Lab Chip 15(13):2749–2753. https://doi.org/10.1039/C5LC00342C
Yao Y, Li L, Jiang J, Zhang Y, Chen G, Fan Y (2022a) Reversible bonding for microfluidic devices with UV release tape. Microfluid Nanofluid 26(3):1–10
Yao Y, Li L, Jiang J, Zhang Y, Chen G, Fan Y (2022b) Reversible bonding for microfluidic devices with UV release tape. Microfluid Nanofluid 26(3):23
Zhang Z, Luo Y, Wang X, Zheng Y, Zhang Y, Wang L (2009) Thermal assisted ultrasonic bonding of multilayer polymer microfluidic devices. J Micromech Microeng 20(1):015036
Zhang Z, Wang X, Luo Y, He S, Wang L (2010) Thermal assisted ultrasonic bonding method for poly (methyl methacrylate)(PMMA) microfluidic devices. Talanta 81(4–5):1331–1338
Acknowledgements
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Author information
Authors and Affiliations
Contributions
Yusheng Li and Shuo Yang, experimental and data collection; Xiaoyang Wang, concept and performed the analysis; Jing Liu designed the microfluidic devices; Qi Zhang supervised the work; Yiqiang Fan drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 1960 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Wang, X., Yang, S. et al. Reversible bonding of thermoplastic-based microfluidics with freeze-release adhesive. Microfluid Nanofluid 27, 33 (2023). https://doi.org/10.1007/s10404-023-02643-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-023-02643-6