Abstract
Background
Polymerase chain reaction (PCR) can be used to confirm or deny infectious ocular inflammation such as uveitis. The purpose of this article is to review the current practical use of PCR examination in ophthalmology, especially multiplex and broad-range PCR, and a novel PCR, termed Strip PCR.
Summary of contents
At first, in the Introduction, we show the development of the PCR examination in ophthalmology. We next show the clinical applications of multiplex PCR and broad-range PCR. These advances in PCR continue to contribute greatly to the ophthalmology field. We also show how the sample for PCR is collected. Recently, we established a novel examination, a multiplex real-time PCR (Strip PCR) prototype for detecting 24 pathogens responsible for ocular infectious diseases. Moreover, we developed the Direct Strip PCR method, which skips the DNA extraction step in the procedure. This PCR is anticipated to ease etiologic evaluation, increasing pathogen detection in the intraocular fluids of uveitis patients even by general ophthalmologists. We also describe the following: (1) representative cases in which PCR is useful, (2) representative cases in which PCR can exclude a diagnosis, (3) the current status of PCR in the diagnosis of infectious uveitis and advanced medical service, and (4) the prospects for clinical PCR in ophthalmology.
Conclusion
We have established and developed the multiplex PCR, broad-range PCR, Strip PCR, and Direct Strip PCR methods and have reported the efficacy of such tests in multicenter studies. Our goal is “rapid and simple comprehensive PCR diagnosis anywhere and by anyone” for ocular infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogen detection is important in the diagnosis and treatment of ocular infections. However, pathogen detection is often difficult because of the small volume of specimens obtained from the ocular region. Therefore, a method to detect foreign antigens with high sensitivity and rapidity from a small number of specimens is required for the diagnosis of ocular infections in daily practice.
Polymerase chain reaction (PCR) is a method of amplifying DNA by using DNA polymerase. In PCR methods, the synthesis reaction at a specific DNA site is induced using 2 types of primers. Because DNA synthesis takes only a few minutes, the use of PCR is rapidly spreading. Recently, PCR diagnosis using local ocular specimens has become indispensable in the treatment of uveitis [1, 2]. PCR can be used to confirm or deny infectious uveitis, and PCR is often useful for viral keratitis, such as that caused by herpes simplex virus, and for infectious intraocular inflammation, such as that caused by bacteria or fungi. Recently, comprehensive PCR testing has become more common [1, 2].
Before the advent of PCR testing, which directly identifies the viral genome, patients were diagnosed and treated only by local antibody production (Goldmann–Witmer coefficient in the sample) or the ocular findings, or by both. For bacteria and fungi, culturing and microscopic examination (smear) are mainly used and remain the gold standard. However, culturing takes time in terms of obtaining the results and smears have a low detection rate. Moreover, the sensitivities of these methods vary depending on the facility where they are performed.
The PCR method was developed approximately 35 years ago, and about 20 years ago PCR examination became widely used in the ophthalmology field. Initially, PCR was a test in which amplified gene products were poured into a gel for determination, and the entire process took 4–5 h to complete (Fig. 1a). In addition, because of the problem of trace specimens unique to ophthalmology, only 1–2 tests could be performed, making it difficult to say that it was a comprehensive test. Ten years have passed since the advent of “ophthalmic multiplex PCR,” which can test multiple microorganisms simultaneously on microspecimens of ophthalmology [2,3,4]. These tests were introduced, and their use became widespread in many facilities because they permit the testing of multiple items quickly and simultaneously. In addition, we have entered the era of multiplex PCR in which positive and negative results are graphically reviewed on a personal computer (Fig. 1b), as opposed to visualization by gel electrophoresis. Five years have passed since the launch of a new comprehensive PCR test kit, termed Strip PCR [5]. In Strip PCR, the reagent is solidified in a small tube, which makes the test simpler and more rapid (Fig. 1c). In addition, we conducted a multicenter collaborative study using Strip PCR and were able to increase the efficacy with high sensitivity and specificity [6].
PCR results of cytomegalovirus (CMV)-associated uveitis. a Representative photographs of qualitative PCR results. Previously, PCR results were visualized using gel electrophoresis and considered to be positive if a band of the desired size was detected. In this representative image, the sample (vitreous humor) was CMV DNA-positive because a band of approximately 346 bp in size was identified, which is the same size as that for the positive control (PC); NC negative control. b Representative graph of multiplex PCR results. Multiplex PCR results are determined by graph analysis. The probe is set to indicate a positive result for CMV DNA at a peak of approximately 62 °C in the melting curve graph. The specimen in this case (aqueous humor) was negative for several other viruses. This method is faster and simpler than gel electrophoresis because it eliminates the time and effort needed for gel making. c Representative graph of Strip PCR results. Strip PCR results are analyzed in a similar manner as multiplex PCR. In the first sample (sample 1: aqueous humor), the curve is elevated at the fluorescence for CMV, indicating a positive result for CMV DNA. Although it is a qualitative PCR, the Cp value (number of cycles acquired) also allows for semiquantitative quantification (Cp value in this graph = 23.5). In another sample (sample 2: aqueous humor), the curve was flat, indicated a negative result for CMV DNA
The first part of this article focuses on the clinical applications of multiplex PCR and broad-range PCR in ophthalmology, specimen collection for PCR, and the establishment of Strip PCR. The second part reviews representative cases in which PCR testing was useful, representative cases in which diagnosis of exclusion was made using PCR, the current status of PCR coverage in the advanced medical service system in Japan, and the prospects for clinical PCR in ophthalmology. In this article, we would like to provide useful information related to PCR testing in the diagnosis and treatment of uveitis.
Clinical applications of multiplex PCR in ophthalmology
Establishment of the first multiplex PCR for viral diagnosis
Recent advances in virology and molecular biology have led to the discovery of the involvement of viruses in diseases of unknown cause in the field of ophthalmology. For example, a group found that acute retinal necrosis (also known as Kirisawa-type uveitis), whose cause was initially unknown, is caused by herpesviruses such as varicella-zoster virus (VZV) or by herpes simplex virus type 1 (HSV1) and HSV2 infection [7]. Subsequently, PCR testing made a major contribution to this diagnosis [3, 8,9,10,11]. As a result, acute retinal necrosis has been treated with antivirals and other therapies, which have made great progress, but it is still an intractable disease that cannot be completely avoided. One of the reasons for this is the difficulty of avoiding advanced retinal and optic nerve damage if diagnosis and the associated treatment are delayed. Many members have been identified in the Herpesviridae family, including cytomegalovirus (CMV), mainly found in opportunistic infections (eg, AIDS); Epstein–Barr virus (EBV), often found in uveitis; and human herpesvirus types 6–8 (HHV6–8), poorly associated with ocular inflammatory diseases.
Therefore, we developed a multiplex PCR test for use in clinical practice. This multiplex PCR test includes HSV1 (HHV1), HSV2 (HHV2), VZV (HHV3), EBV (HHV4), CMV (HHV5), HHV6, HHV7, and HHV8. In addition, we added BK virus (BKV, Polyomavirus family), JC virus (JCV, Polyomavirus family), and Parvovirus B19 (B19V, Parvovirus family), which have recently attracted attention as causative pathogens of chronic infections. Thus, our multiplex PCR test was used to simultaneously screen for the above 11 viruses by using a primer pool containing the virus-specific primers AccuPrime Taq (Invitrogen), and the LightCycler system (Roche). The 11 viruses were divided into 2 sets and tested simultaneously using 2 capillaries [3]. Hybridization probes and PCR products were then mixed and melting curve analysis was performed to detect the virus. An overview of the multiplex PCR test and its flow are shown in Fig. 2.
Use of multiplex PCR for the analysis of pathogenic genomic DNA in ocular fluid from patients with uveitis. DNA was extracted from each sample, and multiplex PCR was performed to screen for infectious agents using LightCycler capillaries. The capillaries include PCR reaction buffer and probe buffer. After centrifugation, we analyzed the PCR melting curve data. At 55 °C, a significant positive curve was seen, indicating the detection of HSV1 genomic DNA in the aqueous humor. This particular sample was negative for other herpesviruses, such as HSV2, VZV, EBV, CMV, and HHV6–8. When a positive result was observed, real-time PCR was subsequently performed to measure the viral load
Multiplex PCR results in patients with acute retinal necrosis
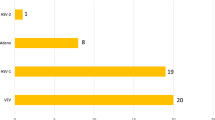
In our previous study, multiplex PCR examination identified either HSV or VZV in 19 intraocular fluid samples from 14 patients who were diagnosed with acute retinal necrosis on the basis of the clinical findings (Table 1). In particular, HSV1 (1/19, 5%), HSV2 (2/19, 11%), and VZV (16/19, 84%) were detected. For other herpesviruses, EBV coinfection was found in 9 of 19 (47%) samples. Coinfection with CMV was found in 1 of 19 (5%) samples. The genomes of the remaining herpesviruses tested, HHV6–8, were not detected in any of the samples.
The findings for all the patients were also negative for BKV, JCV, and B19V, which are often detected in transplant patients, immunocompromised patients, and patients with chronic infections. In contrast, in the control uveitis group, 1 patient with idiopathic uveitis had an aqueous humor sample that was VZV-positive (1/14, 7%) and 1 patient with sarcoidosis had a vitreous fluid sample that was EBV-positive (1/14, 7%) [3]. No other viruses were detected. In addition, DNA from these herpesviruses was not detected in the sera of either acute retinal necrosis or control uveitis patients [3].
Interestingly, in that study, EBV DNA was also detected by use of qualitative PCR in about half of the patients tested. However, a high copy number EBV DNA was detected by use of quantitative PCR (qPCR) in only 1 case [3]. That is, even if EBV DNA was positive on qPCR, the majority of EBV DNA was below the limit of detection by qPCR. The intraocular viral load of EBV differs from those of VZV and HSV1/2, which are thought to be the etiologic agents of acute retinal necrosis, and the involvement of EBV DNA in this disease as an etiologic agent was thought to be low. Ongkosuwito et al. observed a high EBV DNA load in local ocular specimens from patients with uveitis with HIV-negative immunosuppressed status but considered the likelihood of EBV as a direct causative virus of uveitis to be low [12]. We also detected EBV DNA in the intraocular fluid samples from patients with uveitis [13]. We believe that EBV-DNA is detected in the intraocular fluid as a secondary factor. The reasons are as follows: (1) The EBV-DNA copy number is lower than the main cause. (2) The intraocular infiltrating B cells may retain EBV-DNA during inflammation. (3) It is considered that the intraocular tissue such as RPE cells is destroyed during inflammation and that the EBV latent in the intraocular tissue may be released at that time.
Comparison of VZV levels using PCR analysis in vitreous and aqueous humor from patients with acute retinal necrosis
Eight vitreous humor and eight aqueous humor samples that were positive for VZV were grouped together and the viral load in the intraocular fluid was examined (Fig. 3a). The VZV viral load was significantly higher in the vitreous humor than in the aqueous humor, by > 106/mL (P = 0.0011). We also compared the viral loads of 5 patients who had both vitreous and aqueous humor samples available (Fig. 3b). In all the patients, the vitreous humor showed a higher viral load than that in the aqueous humor. These results suggest that viral infection occurs in the retina itself.

Reproduced from Sugita et al. [3] with permission
Comparison of viral load in vitreous and aqueous humor in patients with acute retinal necrosis. a The viral loads in VZV DNA-positive vitreous humor (n = 8) and aqueous humor (n = 8) samples were summarized and compared. Viral load represents the number of viral copies (mean ± standard deviation) in each sample detected by use of real-time PCR. *P < 0.005, Mann–Whitney test. b The amounts of VZV in vitreous and aqueous humor samples from the same patients (5 cases: cases 2, 7–9, and 11) were compared. The viral load represents the number of viral copies in each sample detected by use of real-time PCR. *P < 0.05, Mann–Whitney test.
Diagnosis of Toxoplasma gondii using multiplex PCR
Toxoplasma gondii DNA was identified by use of multiplex PCR testing of the intraocular fluids from 11 of 13 patients diagnosed with ocular toxoplasmosis on the basis of the clinical findings [14]. For the 2 remaining patients, they were in the inactive phase of the disease. Ocular toxoplasmosis with active uveitis could be diagnosed by means of PCR using either the aqueous or the vitreous humor, leading us to conclude that PCR examination is useful for this group of diseases. We recommend the collection of ocular fluids for the purposes of the diagnosis.
Clinical application of broad-range qPCR in ophthalmology
Establishment of broad-range qPCR for bacteria/fungi
Recently, a new method of PCR application, termed broad-range PCR, has been developed. Broad-range PCR targets ribosomal RNA (rRNA) genes that are conserved in different species, such as 16S rRNA in prokaryotes and 18S rRNA in eukaryotes. 16S rRNA (involved in protein synthesis of ribosomes in prokaryotes) and 18S or 28S rRNA (in the case of fungi) are among the most commonly used housekeeping genes (Fig. 4). Using broad-range qPCR, we were able to rapidly detect bacteria and fungi in samples such as the aqueous and vitreous humors. In addition, qPCR can be used to quantify the bacterium/fungus, and gene sequencing can be performed to identify the bacterium/fungus. For example, the 16S rRNA gene in bacteria contains conserved regions common to all bacteria and nonconserved regions (variable regions) that are species-specific (Fig. 4). Conserved regions are targets for PCR primers, and variable regions are sequenced to identify the bacterium. In fact, this method can cover about 60% to 80% of the more than 30,000 bacterial species in the world, and it could become a very important test in clinical settings. We have established and developed broad-range qPCR for detecting bacteria and fungi in infectious ocular diseases [15,16,17].
Schema of bacterial and fungal DNA used for broad-range quantitative PCR: 16S, 18S, and 28S rRNA regions. The ribosome of prokaryotes such as bacteria is composed of 3 rRNAs—50S, 16S, and 5S—and multiple proteins (53 types). On the other hand, ribosomes of eukaryotes such as fungi are composed of 4 rRNAs—28S, 18S, 5.8S, 5S—and multiple proteins (82 types). Recently, PCR amplification using primers of the 16S rRNA region to identify all bacterial species and amplification of primers using the 18S or 28S rRNA region to identify all fungal species have been performed. The lower figure is a schema of PCR of the 16S rRNA region of all bacterial species in broad-range PCR. Two primers and a probe for quantitative PCR are set in the conserved regions (yellow regions) in the bacterial gene, and theoretically all bacterial species can be identified
Method of broad-range PCR testing
At our institution, we perform broad-range qPCR with the following flow of testing: DNA extraction with the DNA Mini Kit (Qiagen) and qPCR using the ABI 7300 system (Thermo Fisher Scientific). The PCR is designed with bacterial 16S rRNA-specific primers and a TaqMan probe, and the PCR conditions are based on those of a previous report [18]. The detection sensitivity is set at 10 copies/mL and scored as follows: > 10 copies/mL, positive; 1–10 copies/mL, false positive; < 1 copy/mL, negative. During specimen collection and PCR, careful attention to bacterial and DNA contamination (particularly, lab contamination) is needed. For PCR amplification of the 16S rRNA gene, about 500 bp of the first half of the 16S rRNA is analyzed using 25F primers, while the above PCR-positive samples with high copy numbers are subjected to blast analysis (BLAST search) for bacterial identification. The amplified PCR product is directly sequenced using the ABI analyzer and evaluated for homology with reference sequences using NCBI BLAST. Bacterial sequences with 100% homology (98–99% also acceptable) were identified. We also conducted sequencing analyses of 18S rRNAs from Candida and Aspergillus species, which are considered to be highly ophthalmologically relevant among fungi, and broad-range PCR of the 28S rRNA gene region of fungi. We performed bacterial broad-range qPCR on intraocular fluid samples from patients with infectious endophthalmitis. The participants were 7 patients with a clinical diagnosis of infectious endophthalmitis after intraocular surgery or ocular trauma and who underwent bacterial broad-range qPCR using vitreous fluid or anterior humor samples. The same specimens were also cultured and subjected to smear tests.
The results are summarized in Table 2. High copy numbers of bacterial DNA (1.7 × 103–2.8 × 108 copies/mL) were detected in all 7 patients with infectious endophthalmitis. By contrast, the findings for 3 of the 7 patients were both culture- and smear-negative. One patient (case 4) was culture-negative but smear-positive for gram-positive cocci, whilst another patient (case 5) was culture-positive for Staphylococcus but smear-negative. (Table 2). In addition, 6 of the 7 patients with infectious endophthalmitis were treated with systemic and topical antimicrobials. These results suggest that broad-range qPCR targeting bacterial 16S rRNA genes may be useful for determining the causative pathogen in bacteria-related infectious endophthalmitis given that many intraocular fluid specimens are often culture- or smear-negative because the patients were treated with antibiotics early in life [19]. We have demonstrated the efficacy of this PCR method in cases of infectious bacterial intraocular inflammation [15] and to detect Candida and Aspergillus 18S rRNA genes [16] and general fungal 28S rRNA genes [17].
Sample collection for PCR
Sample collection is one of the most crucial procedures for successful pathogen detection by PCR. Ocular specimens can be collected from extraocular or intraocular specimens. Extraocular specimens involve discharge, tissue from corneal scraping, or tears. Discharge and tissue from corneal scraping can be obtained by standard methods. Tear specimens can be collected by rinsing the ocular surface with 500 µL of sterile saline 3 times and using approximately 200 µL for DNA extraction [20]. Because of possible pathogen contamination from the extraocular tissues, it is important to test tear samples from both eyes and to compare the results.
For intraocular specimens, aqueous samples can be obtained as an outpatient procedure with the patient under a surgical microscope after sterilization of the ocular surface with povidone iodine. Approximately 100 µL of aqueous sample can be taken via limbal paracentesis using a 30-gauge needle. A disposal pipette with a 30-gauge needle specially designed for such procedure is available from Inami [21] (Fig. 5). Regarding vitreous samples, either a nondiluted sample or vitreous residue in the drainage container can be obtained. The nondiluted sample is obtained at the beginning of the vitrectomy, before starting the intraocular infusion, and usually yields up to 1.5 mL. During dry vitrectomy, scleral indentation is necessary to compensate for the decreased intraocular pressure.
The total DNA yield from intraocular samples is typically quite small, making quantitative measurement of amplified DNA based on the amount of total DNA difficult. When performing PCR using aqueous humor or nondiluted vitreous samples, microorganisms are quantified by calculating the copy number of the amplicon on the basis of the amount of intraocular fluid, usually described as “copy number/mL of sample.”
Aqueous tap and vitrectomy are invasive procedures, and patient consent is necessary. Therefore, sample collection should be performed with the appropriate timing so that the target DNA can be detected in high quantities. The viral load in the aqueous humor correlates with the degree of intraocular inflammation [22,23,24]. Therefore, the aqueous tap should be performed when intraocular inflammation is prominent. However, in CMV anterior uveitis, the viral load is often as low as the detectable limit and does not necessarily correlate with intraocular inflammation [25]. Moreover, multiple aqueous taps may be necessary to detect CMV from patients with presumed Posner–Schlossman syndrome [26]. Therefore, when CMV is suspected as the cause of anterior uveitis, the sample collection should be conducted not only during prominent intraocular inflammation, but also when the intraocular pressure (IOP) is elevated, even if intraocular inflammation is weak or absent.
Establishment of Strip PCR
Our purpose was to develop a convenient real-time PCR kit that would be equivalent to qPCR in targeting the main pathogens of ocular infectious diseases and yield consistent results in different laboratories and with different users. Simplifying the PCR examination with general machines will improve the diagnostic fidelity, thereby providing a reliable option for diagnosis at general hospitals.
First, we developed a multiplex real-time PCR test (Strip PCR) prototype for detecting 24 pathogens responsible for common ocular infectious diseases [5] as follows: HSV1/2, VZV, EBV, CMV, HHV6–8, human T-cell lymphotropic virus (HTLV)-1, adenovirus, Mycobacterium tuberculosis, Treponema pallidum, Propionibacterium acnes, bacterial 16S rRNA, Candida albicans, C glabrata, C krusei, Aspergillus, Fusarium, fungal 28S rRNA, Toxoplasma, Toxocara, Chlamydia trachomatis, and Acanthamoeba. Strip PCR comprises 12 wells, each precoated with primers and probes and targeting 1 to 3 different pathogens. The solid-phase technique relieves beginners of the technical challenge of quantitatively handling a small amount of liquid and provides stable results promptly with reproducibility and accuracy. The median examination time of Strip PCR (5 min 58 s for 24 pathogens) was shorter than that of qPCR (10 min 41 s for 1 pathogen). The intrainstitutional coefficient of variation (CV) of Strip PCR indicated low CV by PCR beginners (0.3–0.9%) as well as experts (0.1–0.4%). The interinstitutional CV was also low for both PCR beginners (4.3%) and experts (2.2%) [6].
However, the 24-pathogens Strip PCR prototype has some limitations. Minor contamination (with late Cq values) by bacteria, fungi, and P acnes was detected (1.6–88.7%, n = 124) using Strip PCR and qPCR on aqueous humor and vitreous humor nonuveitis samples. This finding may be explained by unavoidable contaminations from the ocular surface or the reagent, or both. Furthermore, the Strip PCR prototype is expensive and labor-intensive, as samples from different sites (eg, keratoconjunctivitis) and different samples (eg, cornea tissues) must be tested.
Therefore, we optimized the 24-pathogen Strip PCR prototype to detect 9 major pathogens of infectious uveitis (HSV1, HSV2, VZV, EBV, CMV, HHV6, HTLV-1, T gondii, and T pallidum; Fig. 6). We then evaluated the 9-pathogen Strip PCR test in a multicenter study using 772 intraocular samples from infectious uveitis patients [6]. The optimized Strip PCR successfully detected pathogen DNA at concentrations of 100–109 copies/mL in 252 of 255 qPCR-positive samples (Table 3). Strip PCR had high sensitivity (98.8%), specificity (98.5%), positive predictive value (98.8%), and negative predictive value (98.5%) against qPCR. Moreover, optimized Strip PCR showed a good relationship (r = 0.838, Fig. 7) with DNA copy numbers obtained by qPCR, which is the gold standard for accurately determining the number of pathogen copies in samples. Infectious uveitis is dependent on the pathogen load [22,23,24]; thus, quantitative Strip PCR will be useful for predicting disease condition. The high specificity of Strip PCR may be especially valuable in exclusive diagnosis before surgery or treatment with steroids, immunosuppressants, and tumor necrosis factor-alpha inhibitors.

Reproduced from Nakano et al. [6] with permission
Strip PCR setup. Strip PCR for infectious uveitis uses an 8-well multiplex PCR strip tube targeting 9 ocular infectious disease pathogens: HSV1, HSV2, VZV, EBV, CMV, HHV6, HTLV-1, T pallidum, and T gondii. The internal controls were glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (TBP). Each well targeted 1 to 2 types of pathogens and was coated with primers (forward and reverse) and multiple probes (6FAM and ROX) adapted for these target pathogens.

Reproduced from Nakano et al. [6] with permission
Correlation between Strip PCR and quantitative real-time PCR results. A high correlation value (r = 0.838, P < 0.001, Pearson correlation coefficient after Shapiro–Wilk normality test) was obtained between quantification cycle values (Strip PCR) and DNA copy numbers (copies/mL; quantitative real-time PCR).
Development of direct Strip PCR
DNA purification before qPCR or Strip PCR analysis is time-consuming but necessary. To avoid this step, we developed a new PCR amplification reagent cocktail and combined the reagent with Strip PCR, referred to as direct multiplex real-time PCR (Direct Strip PCR). We then confirmed in a multicenter study that Direct Strip PCR for the 9 causative pathogens of infectious uveitis demonstrated similar results to qPCR in terms of high accuracy, rapid detection, low cost, low sample volume required, and ease-of-use (manuscript under submission). Direct Strip PCR is anticipated to ease etiologic evaluation, increasing pathogen detection from intraocular fluids of uveitis patients even by general ophthalmologists. CMV was the most frequently detected pathogen in this multicenter study. Direct Strip PCR, which is comprehensive and quantitative, requires only 20 µL of sample; thus, it may be the most suitable method for CMV diagnosis. Direct Strip PCR is currently used in almost 35 institutions in Japan.
Representative cases in which PCR was useful
Good PCR targets in intraocular specimens are typically DNA of herpesviruses, such as HSV, VZV, and CMV, or T gondii. Even if corneal or dermal manifestations are absent, cases of sudden onset of unilateral anterior uveitis with mutton fat keratic precipitates (KPs) (Fig. 8a) and elevated IOP are highly suspected to be caused by HSV or VZV, and ophthalmologists should consider performing an aqueous tap. Additionally, zoster sine herpete may be diagnosed if a patient presents with segmental iris atrophy; however, PCR for aqueous humor is still useful to confirm the diagnosis.
Representative case in which PCR is useful: VZV-associated anterior uveitis. The patient of this case was a 47-year-old man with anterior uveitis caused by varicella-zoster virus (VZV) in the left eye. a At the first visit, medium size mutton-fat keratic precipitates were observed in the lower part of the cornea by use of slit-lamp biomicroscopy. b Six months later, segmental iris atrophy had developed in the lower temporal area of the iris
The patient in case 1 was a 47-year-old man referred to our clinic because of sudden onset of foggy vision in the left eye. The IOP was 12 mmHg for the right eye and 26 mmHg for the left eye. In the anterior chamber of his left eye, medium size mutton-fat KPs (Fig. 8a) were present in the lower part of the cornea in a fan shape, and 3+ anterior chamber cells were observed. Aqueous tap was performed owing to the suspicion of herpesvirus infection, and the sample was analyzed using multiplex PCR. VZV was detected in high copy numbers (1.2 × 108 copies/mL), but other herpesviruses such as HSV or CMV were not detected. The patient received instillation of 1% betamethasone 4 times daily and oral valaciclovir. The IOP became normal and the intraocular inflammation was ameliorated, but segmental iris atrophy gradually developed (Fig. 8b). Steroid eye drops and acyclovir eye ointment were administered for more than 6 months.
Another pathogen frequently detected using PCR is CMV. Typically, patients with CMV anterior uveitis have a long history of sporadic episodes of elevated IOP accompanied by small KPs and moderate-to-faint anterior chamber cells. Patients with this condition usually respond well to topical steroid instillation. The diagnosis in such cases is often Posner–Schlossman syndrome, and the patients are observed until the next attack occurs. However, years later, steroid instillation may no longer suppress elevated IOP [27], and corneal endothelial cell density (CECD) is decreased when compared with the fellow eye [22]. The patient in case 2 was a 33-year-old woman who had a history of recurrent granulomatous anterior uveitis and elevated IOP (often > 30 mmHg) in the left eye for 2 years, which was treated with topical steroid instillation each time. She was then referred to our clinic for close examination. At the initial presentation, small, scattered white KPs (Fig. 9a) and small, clear KPs forming a coin-shaped lesion (Fig. 9b) were observed. Trace cells were observed in the AC. CECD was 2950/mm2 in the right eye and 1565/mm2 in the left eye. We suspected CMV infection and performed an aqueous tap. Multiplex PCR showed the presence of CMV DNA (5.1 × 104 copies/mL) but not HSV, VZV, or other pathogens. She received intravitreal ganciclovir, followed by 1% ganciclovir instillation 6 times a day. The IOP and ocular inflammation were well controlled thereafter.
The patient in case 3 was an 84-year-old woman who was referred to our clinic because of a history of decreased vision in the right eye for 1 week. Pigment KPs, 2+ AC cells, and blood clots in the iris and intraocular lens (Fig. 10a) were observed. The fundus could not be observed in detail owing to dense vitreous opacity, but a white lesion was observed in the upper part of the fundus (Fig. 10b). A systemic workup did not show remarkable findings except for anti-Toxoplasma IgG antibodies (Abs) (119 IU/mL). Anti-Toxoplasma IgM Abs were undetectable. We performed an aqueous tap and analyzed the samples by use of multiplex PCR, the findings of which were positive for T gondii (3.9 × 103 copies/mL) but negative for HHV, syphilis, and M tuberculosis. In our previous report, the copy number of toxoplasma DNA in the intraocular fluid of active uveitis patients who were finally diagnosed with ocular toxoplasmosis was 5.1 × 102 to 2.1 × 106 copies/mL [14].
Representative case in which PCR is useful for diagnosis: Toxoplasma infection. The patient of this case was an 84-year-old woman with ocular toxoplasma in the right eye. a Slit-lamp biomicroscopy showed a blood clot on the iris and intraocular lens at the first visit. b White lesions were seen in the upper part of the fundus. The fundus photo was taken 3 months after the first visit
On the basis of these findings, we diagnosed ocular toxoplasmosis and initiated oral acetylspiramycin and prednisolone therapy. PCR is thus useful for the diagnosis if typical ocular manifestations are not available because of cloudy media, as represented by this case.
Representative cases in which PCR could exclude a diagnosis
A 70-year-old woman with a history of primary central nervous system lymphoma was treated with radiation and had no recurrence throughout the year. She had blurred vision in the left eye and was treated with 0.1% betamethasone eye drops after HTLV-1-associated uveitis was diagnosed as a result of a blood test given by a local ophthalmologist. The ocular fluid was not tested. The vitreous opacity was unresponsive to therapy and worsened; thus, the patient was referred to Oita University Hospital after suspected acute retinal necrosis or central retinal artery occlusion with vision loss was diagnosed. The visual acuity of the left eye was 0.05 (n.c.). The ocular fundus is shown in Fig. 11. Direct Strip PCR was conducted using 20 µL of aqueous humor, and the results indicated an immediate exclusive diagnosis of HTLV-1 and HSV1, HSV2, CMV, EBV, VZV, HHV6, T pallidum, and T gondii within 1 h (Fig. 11a). Pars plana vitrectomy was performed (Fig. 11b); the vitreous sample was processed for repeat PCR assays, the findings for which were negative. Intraocular lymphoma was subsequently diagnosed as a result of immunohistochemistry, flow cytometry, cytokine evaluation, and molecular analyses and treated with intravitreal methotrexate (Fig. 11c). Direct Strip PCR was useful for exclusive diagnosis of the 9 main infectious ocular pathogens.
Representative case in which PCR was used for exclusive diagnosis: intraocular lymphoma. a Direct Strip PCR was performed using 20 µL of aqueous humor, which made an immediate exclusive diagnosis of HTLV-1 and HSV1, HSV2, CMV, EBV, VZV, HHV6, T pallidum, and T gondii within 1 h. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (TBP) were the internal controls. b Pars plana vitrectomy was performed; the vitreous sample was processed for repeat PCR assays, the findings for which were negative. c After diagnosis of intraocular lymphoma, the patient was treated with intravitreal methotrexate
Current status of PCR in the diagnosis of infectious uveitis and the advanced medical service in Japan
According to a nationwide survey about the use of PCR for the diagnosis of uveitis in Japan, 101 of the 131 (77%) facilities that responded to the survey, including university hospitals and core training hospitals, performed PCR for 1616 patients from January 1, 2018 through June 30, 2018, and comprehensive PCR was performed for 674 (42%) of these patients. Thus, PCR using intraocular fluids to diagnose infectious uveitis is common and has important value in Japan [28]. However, the costs of diagnostic PCR for uveitis are not covered by the national health insurance system in Japan, which can prevent the use of PCR in appropriate cases.
Diagnostic PCR for uveitis has been approved for coverage by the advanced medical service since the end of 2013. From 2013 to 2017, PCR was performed for 409 herpesvirus cases and 112 bacterial/fungal cases, and the numbers of PCR cases and facilities are increasing annually (Fig. 12). PCR for herpes viruses or bacteria/fungi were useful to diagnose or diagnose by exclusion in all the cases, and no adverse effect was reported. Currently, 19 facilities have been involved in this system. It is expected that comprehensive diagnostic PCR will be approved for coverage by national health insurance in Japan in the near future.
Changes in the number of PCRs performed and participating facilities under the advanced medical service in Japan (cited and modified from Ref. [27] with permission). The upper panel shows changes in viral PCRs, and the lower panel, changes in bacterial/fungal PCRs. The solid lines represent the number of PCRs performed under the advanced medical service system in Japan; the white boxes represent the number of participating facilities
Prospects of clinical PCR in ophthalmology
As mentioned above, we have established and developed multiplex PCR, broad-range qPCR, and Strip PCR and have reported the efficacy of such tests in multicenter studies [2, 6]. We have developed Direct Strip PCR for keratoconjunctivitis and infectious endophthalmitis, and evaluations of these tests in multicenter studies are currently underway. The keratoconjunctivitis Direct Strip PCR kit covers herpesviruses, Chlamydia, Acanthamoeba, adenovirus, and Neisseria gonorrhoeae, whilst the endophthalmitis Direct Strip PCR kit provides a comprehensive identification of 14 bacterial species commonly found in infectious endophthalmitis.
Surprisingly, because the Direct Strip PCR infectious uveitis kit can be completed within 60 min, it is now used for intraoperative diagnosis of acute retinal necrosis and CMV retinitis during vitrectomy (manuscript under submission). The ability to identify a virus during vitrectomy allows intraoperative procedures to be performed according to PCR results.
In Table 4 we have summarized the sensitivity, specificity, positive predictive value, and negative predictive value of our PCR examinations such as multiplex PCR, broad-range PCR, Strip PCR, and Direct Strip PCR based on our previous results. In any of the PCR examinations, higher diagnostic parameters were exhibited (Table 4). As a result, we obtained high diagnostic parameters in a multicenter study. Our goal is “rapid and simple comprehensive PCR diagnosis anywhere and by anyone” for ocular infections, which we anticipate will occur in the near future.
References
Mochizuki M, Sugita S, Kamoi K, Takase H. A new era of uveitis: impact of polymerase chain reaction in intraocular inflammatory diseases. Jpn J Ophthalmol. 2017;61:1–20.
Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120:1761–8.
Sugita S, Iwanaga Y, Kawaguchi T, Futagami Y, Horie S, Usui T, et al. Detection of herpesvirus genome by multiplex polymerase chain reaction (PCR) and real-time PCR in ocular fluids of patients with acute retinal necrosis. J Jpn Ophthalmol Soc. 2008;112:30–8 (in Japanese).
Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92:928–32.
Nakano S, Sugita S, Tomaru Y, Hono A, Nakamuro T, Kubota T, et al. Establishment of multiplex solid-phase strip PCR test for detection of 24 ocular infectious disease pathogens. Invest Ophthalmol Vis Sci. 2017;58:1553–9.
Nakano S, Tomaru Y, Kubota T, Takase H, Mochizuki M, Shimizu N, et al. Evaluation of a multiplex Strip PCR test for infectious uveitis: a prospective multi-center study. Am J Ophthalmol. 2020;213:252–9.
Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35:327–43.
Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–62.
Itoh N, Matsumura N, Ogi A, Nishide T, Imai Y, Kanai H, et al. High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol. 2000;129:404–5.
Tran TH, Rozenberg F, Cassoux N, Rao NA, LeHoang P, Bodaghi B. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87:79–83.
Gargiulo F, De Francesco MA, Nascimbeni G, Turano R, Perandin F, Gandolfo E, et al. Polymerase chain reaction as a rapid diagnostic tool for therapy of acute retinal necrosis syndrome. J Med Virol. 2003;69:397–400.
Ongkosuwito JV, Van der Lelij A, Bruinenberg M, Wienesen-van Doorn M, Feron EJ, Hoyng CB, et al. Increased presence of Epstein-Barr virus DNA in ocular fluid samples from HIV negative immunocompromised patients with uveitis. Br J Ophthalmol. 1998;82:245–51.
Yamamoto S, Sugita S, Sugamoto Y, Shimizu N, Morio T, Mochizuki M. Quantitative PCR for the detection of genomic DNA of Epstein-Barr virus in ocular fluids of patients with uveitis. Jpn J Ophthalmol. 2008;52:463–7.
Sugita S, Ogawa M, Inoue S, Shimizu N, Mochizuki M. Diagnosis of ocular toxoplasmosis by two polymerase chain reaction (PCR) examinations: qualitative multiplex and quantitative real-time. Jpn J Ophthalmol. 2011;55:495–501.
Sugita S, Shimizu N, Watanabe K, Katayama M, Horie S, Ogawa M, et al. Diagnosis of bacterial endophthalmitis by broad-range quantitative PCR. Br J Ophthalmol. 2011;95:345–9.
Sugita S, Kamoi K, Ogawa M, Watanabe K, Shimizu N, Mochizuki M. Detection of Candida and Aspergillus species DNA using broad-range real-time PCR for fungal endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:391–8.
Ogawa M, Sugita S, Watanabe K, Shimizu N, Mochizuki M. Novel diagnosis of fungal endophthalmitis by broad-range real-time PCR detection of fungal 28S ribosomal DNA. Graefes Arch Clin Exp Ophthalmol. 2012;250:1877–83.
Takai K, Horikoshi K. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol. 2000;66:5066–72.
Chiquet C, Cornut PL, Benito Y, Thuret G, Maurin M, Lafontaine PO, et al. Eubacterial PCR for bacterial detection and identification in 100 acute postcataract surgery endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49:1971–8.
Kakimaru-Hasegawa A, Kuo CH, Komatsu N, Komatsu K, Miyazaki D, Inoue Y. Clinical application of real-time polymerase chain reaction for diagnosis of herpetic diseases of the anterior segment of the eye. Jpn J Ophthalmol. 2008;52:24–31.
Kitazawa K, Sotozono C, Koizumi N, Nagata K, Inatomi T, Sasaki H, et al. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. Br J Ophthalmol. 2017;101:548–50.
Miyanaga M, Sugita S, Shimizu N, Morio T, Miyata K, Maruyama K, et al. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol. 2010;94:336–40.
Kandori M, Miyazaki D, Yakura K, Komatsu N, Touge C, Ishikura R, et al. Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Jpn J Ophthalmol. 2013;57:497–502.
Kido S, Sugita S, Horie S, Miyanaga M, Miyata K, Shimizu N, et al. Association of varicella zoster virus load in the aqueous humor with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br J Ophthalmol. 2008;92:505–8.
Takase H, Kubono R, Terada Y, Imai A, Fukuda S, Tomita M, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol. 2014;58:473–82.
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–40.
Koizumi N, Yamasaki K, Kawasaki S, Sotozono C, Inatomi T, Mochida C, et al. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol. 2006;141:564–5.
Takase H, Nakano S, Sugita S, Sotozono C, Goto H, Mochizuki M. Use of polymerase chain reaction analysis of intraocular fluids in diagnosis of infectious uveitis: survey on the status in Japan. J Jpn Ophthalmol Soc. 2019;123:764–70 (in Japanese).
Acknowledgements
This work was supported by a scientific research grant (B, 18H02959) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S.S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
S. Sugita, Lecture fee (Sumitomo Dainippon, Vision Care, Bayer, Santen); H. Takase, Lecture fee (Santen, Novartis, AbbVie, Senju, Eisai, Otsuka); S. Nakano, Grant (Novartis).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Sunao Sugita
Organizer: Annabelle A. Okada, MD
About this article
Cite this article
Sugita, S., Takase, H. & Nakano, S. Practical use of multiplex and broad-range PCR in ophthalmology. Jpn J Ophthalmol 65, 155–168 (2021). https://doi.org/10.1007/s10384-020-00794-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00794-5