Abstract
Salicylic acid (SA) is an endogenous growth regulator that participates in the regulation of physiologic processes in plants. It plays an important role in plant response to adverse environmental conditions. In this study, SA in three concentrations—0, 0.1 mM, and 1 m—was sprayed on the leaves and fruits of two grape cultivars (Bidane Ghermez and Bidane Sefid) at two growth stages: unripening and ripening. The SA 0.1-mM concentration effectively increased leaf chlorophyll a of the Bidane Sefid cultivar at both growth stages. Chlorophyll b and proline of the leaves and berry skin of both cultivars increased at both growth stages under SA treatment, especially with the 0.1-mM concentration. The SA 0.1-mM concentration increased carotenoids in leaves and berry skin of Bidane Ghermez cultivar at both growth stages and of Bidane Sefid cultivar in the unripening stage. The SA 0.1-mM concentration was also associated with increased soluble sugars of the leaves and berry skin of both cultivars at both growth stages. However, the effect of SA on insoluble sugars was different. At the unripening stage, the SA 1‑mM concentration significantly increased the total protein of the berry flesh and leaves of Bidane Sefid. The results show that in both growth stages, the SA treatment, especially the 1‑mM concentration, effectively increased the superoxide dismutase (SOD) enzyme activity of leaves, skin, and flesh of berries of both cultivars. The results indicate that SA treatment in grapes may stimulate the synthesis of some photosynthetic pigments, carbohydrates, proline, protein, and SOD enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most important sugars in fresh grapes are sucrose, glucose, and dextrose (Combe 1992; Zhang et al. 2021). Larronde et al. (1998) showed that high levels of sucrose and glucose are necessary to accelerate the anthocyanin biosynthesis, and insoluble sugars do not double these results. A high concentration of carbohydrates reduces the oxidative damage and protects the protein structure during water shortages (Fazelian and Asrar 2011; Li and Wang 2021).
Proline is the most abundant amino acid in grapes (Nassar and Kliewer 1966), and proline accumulation has been reported to activate the antioxidant mechanism (Ben Ahmed et al. 2009). In fact, proline increases the tolerance and resistance to stresses by several mechanisms, including inhibiting hydroxyl radicals, regulating osmosis, preventing denaturation of enzymes, and preserving and synthesizing proteins (Ashraf and Foolad 2007). Studies have shown that when plants are confronted with different stressors, they have osmotic adjustment mechanisms to maintain their water condition by the accumulation of compounds such as proline, soluble sugars, and some ions. Under stress conditions such as salinity stress, soluble sugars and proline can act as osmotic proteins (Bartles and Sunkar 2005).
Sugars and amino acids that accumulate in grapes during ripening are imported via the phloem, while many secondary metabolites are synthesized within the berry itself. Grapes import sucrose but accumulate hexoses. Conversion of sucrose to hexoses is most likely catalyzed by invertase. Expression of genes and an increase in invertase activity occur before veraison, so it seems unlikely that synthesis of this enzyme is a controlling factor for sugar accumulation during ripening. A number of proteins are newly synthesized in grapes during ripening, and several of these proteins have now been identified. Studies indicate that a dramatic change in gene expression occurs in grape berries at veraison, suggesting that ripening involves a coordinated increase in transcription of a number of different genes (Robinson and Davies 2000).
One of the effects of environmental stresses is oxidative damage and free radical production of reactive oxygen species (ROS) (Mittler et al. 2004). Antioxidant enzymes cooperate to scavenge free radicals. The most important antioxidant enzymes include ascorbate peroxidase (APZ), superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR). Nonenzymatic antioxidants such as glutathione (GSH), carotenoids, and tocopherols are antioxidant defense systems (Darvizheh et al. 2019). The enzyme SOD converts O2− into H2O2 and O2, then peroxidase (POD) and CAT, H2O2 to H2O (Wang et al. 2009).
Salicylic acid (SA) is an important plant hormone that influences various physiologic processes and biochemical properties of the plant. Studies show that when SA is applied at appropriate concentrations, this hormone increases the abilities of the antioxidant system of plant tissues by activating antioxidant enzymes and increasing some nonenzymatic substances such as anthocyanins, flavonoids, and carotenoids (Luo et al. 2012).
It seems that SA spray with increasing antioxidant capacity of chamomile, including carotenoids, reduces H2O2 and lipid peroxidation and also protects more cell membranes and photosynthetic and photosynthesis pigments, as well as prevents chlorophyll catabolism (Costa et al. 2005). Changes in the chlorophyll pigments under SA treatment are highly dependent on the species and the treated concentration. In many observations, there was a significant increase in chlorophyll pigments with treatment of low concentrations of SA (Pancheva et al. 1996). Other studies have shown that SA increased chlorophyll and carotenoid pigments in corn (Khodary 2004) and that it increased the photosynthetic pigments, total carbohydrates, proline, and protein in basil (Abdel and Gharib 2006; Haghshenas et al. 2020). Other studies have shown that SA increased soluble and insoluble sugars in tomato (Poor et al. 2011) and wheat (El Tayeb and Ahmed 2010). In apple fruit, SA significantly increased the fruit weight, chlorophylls, carotenoids, proline, protein, and SOD (Turkyilmaz Unal et al. 2015). It has also been shown that SA, having an effect on antioxidant enzymes, causes plants to be resistant to environmental stress. Plants produce some proteins in response to biotic and abiotic stresses, some of which are produced by phytohormones such as SA (Hussein et al. 2007). There are many reports of increasing and decreasing protein with SA stress. The study of corn showed that all amino acids were enhanced by SA; increasing the amino acids in tissue under stress was associated with protein portions (El-Tayeb 2005). In research on two beans and tomato under stress, the use of SA increased the activity of antioxidant enzymes, including SOD and APX (Senaratna et al. 2000). Some researchers have shown that SA (0.1-mM concentration) increased the protein and antioxidant enzyme activity compared with control (Chakraborty and Tongden 2005). Studies in parsley also showed that SA increased the activity of the chloroplast SOD bands and cytosol, but SA was more effective on SOD chloroplasts (Antonova Ananieva et al. 2004).
Therefore, this study was done to evaluate the effect of SA in different concentrations on photosynthetic pigments, soluble and insoluble sugars, proline, total protein, and SOD activity in leaf and different tissues of grapevine fruits of the Bidane Ghermez and Bidane Sefid cultivars in two stages, unripening and ripening. Variations in these compounds in different tissues of both cultivars were also compared in both growth stages.
Materials and Methods
In this study, two cultivars of grapevine, Bidane Sefid (BS; white berries) and Bidane Ghermez (BG; red berries), grown in the garden of the Malayer Grape Research Center affiliated with the Ministry of Jihad Agricultural (Iran) were used. The grapevines are 10 years old, and their breeding system was row cropping and drip irrigation. The index for determining the unripening stage of grapevine fruit varieties was the berry size and the acidity, and for the ripening stage it was juiciness and sweetness of the berries. The fruits and leaves of BG and BS cultivars were treated in two growth stages (unripening and ripening) with three concentrations of SA: 0 (control), 0.1 mM, and 1 mM.
Determination of Carotenoids and Chlorophyll a and b
Carotenoids and chlorophyll a and b were measured based on the Lichtenthaler (1987) method: 0.2 g of tissue was homogenized in 5 ml of 80% acetone and filtered through Whatman no. 2 filter paper. Then the volume of the extract obtained with acetone reached 10 ml. The intensity of the light absorption of the extract was read at 646.8 nm, 65.15 nm, and 470 nm using a spectrophotometer (Jenus model UV-12000, USA).
Determination of Sugars
The reducing sugar was estimated using the method of Nelson (1944). The sulfuric acid phenol method (Dubois et al. 1956) was used to measure insoluble sugars (oligosaccharides and polysaccharides).
Determination of Proline
Free proline was determined by the method of Bates et al. (1973). Approximately 0.5 g of fresh or frozen plant material was homogenized in 10 ml of 3% sulfosalicylic acid and filtered through Whatman no. 2 filter paper. Then 2 ml of the filtrate was mixed with 2 ml acid-ninhydrin and 2 ml of glacial acetic acid in a test tube. The mixture was placed in a water bath for 1 h at 100 °C. The reaction mixture was extracted with 4 ml toluene, and the chromophore containing toluene was aspirated and cooled to room temperature. The absorbance was measured at 520 nm with a spectrometer (Jenus model UV-12000).
Determination of Total Soluble Protein
For determination of total soluble protein and enzyme activity, 1 g of plant material was homogenized in a chilled (4 °C) mortar using a 50 mM Tris-HCl buffer (pH 7.0) containing 10 mM EDTA, 2 mM MgSO4, 20 mM cysteine, 10% (v/v) glycerol, and 2% (w/v) PVPP (Jasska 1996). The homogenate was centrifuged in a refrigerated centrifuge, and the supernatant obtained was used for protein determination and enzyme assay. The protein of the extracts was determined according to the method of Bradford (1976), using bovine serum albumin as the standard.
Determination of Superoxide Dismutase Enzyme Activity
Superoxide dismutase enzyme (SOD) activity was determined by measuring its ability to inhibit the photochemical reduction of thiazolyl blue (MTT) as described by Giannopolitis and Ries (1977). The reaction mixture consisted of 0–100 µl enzyme extract, 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 75 µM MTT, 0.1 mM EDTA, and 75 µM riboflavin.
Test tubes were shaken and placed 30 cm from three 30‑W fluorescent lamps. The reduction in MTT was measured by reading absorbance at 565 nm. Blanks and controls were run in the same manner but without illumination and enzyme, respectively. One unit of SOD activity was defined as the number of enzymes that produced a 50% inhibition of MTT reduction.
Results and Discussion
Effect of Salicylic Acid on Chlorophyll a
The results showed that chlorophyll a in leaves and berry skin of both cultivars at the unripening stage was greater than at the ripening stage. The use of SA in both concentrations did not have a significant effect on chlorophyll a of the leaves and berry skin of BG cultivar in either growth stage (Table 1). However, the SA in both concentrations had a significant effect on chlorophyll a of the leaves of BS cultivar at the unripening stage compared to control, with the 1‑mM concentrations having a significantly greater effect. At the ripening stage, SA in the 1‑mM concentration effectively increased the chlorophyll a of leaves compared to control. In addition, in the berry skin of BS cultivar, SA had an increasing effect in the 0.1-mM concentration at the unripening stage and a decreasing effect on chlorophyll a in both concentrations at the ripening stage. Studies have shown that SA increased chlorophyll a and b of strawberry fruit (Karlidag et al. 2009). In this study, the chlorophyll a of leaves and berry skin of BG cultivar at both growth stages was greater than the chlorophyll a of leaves and berry skin of BS cultivar. Meanwhile, except for the berry skin of BS cultivar in the ripening stage, in which the amount of chlorophyll a was higher than the berry skin of BG cultivars. In accordance with these results, chlorophyll a and b in red cultivars have been observed as greater than in white cultivars (Abbaspour et al. 2017). The chlorophyll a in both cultivars and at both growth stages was higher in the leaves than in berry skin. On the other hand, according to the results, chlorophyll a was higher in the control leaves of BG cultivar than in the control leaves of BS cultivar.
Effect of Salicylic Acid on Chlorophyll b
The results of comparing the two growth stages showed that chlorophyll b of leaves and berry skin of both cultivars at the unripening stage was greater than at the ripening stage. In the leaves and berry skin of BS cultivar at both growth stages and the leaves of BG cultivar at the unripening stage, the SA 0.1-mM concentration effectively increased chlorophyll b compared to control (Table 1). But in berry skin of BG cultivar, the SA 0.1-mM concentration at the unripening stage and the 1‑mM concentration at the ripening stage effectively increased chlorophyll b compared to control. In accordance with these results, SA has been shown to increase chlorophyll a and b SA the strawberry (Tohma and Esitken 2011). In this study, chlorophyll b was decreased at the ripening stage. Meanwhile, the use of SA could increase chlorophyll b. In addition, the chlorophyll b in leaves and berry skin of BS cultivar at both growth stages was greater than in leaves and berry skin of BG cultivar. Also, at the unripening stage for both cultivars, chlorophyll b in berry skin was higher than in leaves, but at the unripening stage, chlorophyll b in leaves was higher than in berry skin.
The effect of SA at the unripening stage was a little better than at the ripening stage, as its effect on the leaves and berry skin of BS cultivar was greater than for BG cultivar. The highest amount of chlorophyll b was in berry skin of BS cultivar treated with SA in the 0.1-mM concentration at the unripening stage. The study showed that the SA, depending on the concentration, time, and plant used, had dual effects, but in optimum concentrations contributed by reducing pigments and increasing antioxidant capacity.
Effect of Salicylic Acid on Carotenoids
The carotenoids of leaves and berry skin of both cultivars at the unripening stage were greater than at the ripening stage (Table 1). The use of SA 0.1-mM concentration in both cultivars at the unripening stage and in both concentrations at the ripening stage significantly increased the carotenoids of leaves and berry skin compared to control. But in leaves and berry skin of BS cultivar at the ripening stage, the SA in both concentrations significantly decreased the carotenoids. In accordance with these results, in strawberry fruits SA increased the carotenoids (Karlidag et al. 2009). Also, the results showed that at both growth stages, the carotenoids of leaves and berry skin were higher in the BG cultivar than in the BS cultivar. Except at the unripening stage, the leaf carotenoids of the BS cultivar were greater than in the BG cultivar. In accordance with these results, Abbaspour et al. (2017), in their studies of grape, showed that carotenoids of red cultivars were greater than in white cultivars. The carotenoids of both cultivars at the unripening stage and the BG cultivar at the ripening stage were greater in berry skin than in leaves.
But in contrast, in the BS cultivar at the ripening stage, the carotenoids of leaves were greater than in berry skin. The SA effect at the unripening stage was greater than at the ripening stage. Also, at the unripening stage the effect of this treatment was the same for carotenoids of both cultivars. But at the ripening stage, the SA effect on carotenoids of leaves and berry skin in the BG cultivar was greater than in the BS cultivar. Therefore, the highest amount of carotenoid was observed in berry skin of the BG cultivar treated with the 0.1-mM concentration at the unripening stage.
Effect of Salicylic Acid on Soluble Sugar
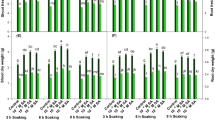
The soluble sugar of berry skin in both cultivars at the ripening stage was greater than at the unripening stage (Fig. 1). As observed, the soluble sugar of BS cultivar leaves in the ripening stage was greater than at the unripening stage, but in leaves of the BG cultivar, the soluble sugar at the unripening stage was more than at the ripening stage. The SA in both concentrations at the unripening stage had the effect of significantly increasing the soluble sugar of leaves and berry skin of both cultivars compared to control, and the 0.1-mM concentration was more effective.
Effect of salicylic acid (SA) in three concentrations—0 (control), 0.1 mM, and 1 mM—on soluble sugar (a at unripening stage and b at ripening stage) and insoluble sugar (c at unripening stage and d at ripening stage) of leaves and berry skin of grape Bidane Sefid (BS) and Bidane Ghermez (BG) cultivars. Different letters indicate significant differences at the level of p ≤ 0.05. DW dry weight
As at the ripening stage, the SA 0.1-mM concentration had an increasing effect on the soluble sugar of leaves and berry skin in both cultivars compared with control (Fig. 1). In accordance with these results, SA was observed to increase soluble sugar in apple (Mo et al. 2008). Also, the results of the comparison between the two growth stages show that the soluble sugar of berry skin of the BG cultivar was more than that of the BS cultivar at both stages. In addition, at the ripening stage the soluble sugar of leaves of the BS cultivar was greater than in the BG cultivar; in contrast, at the unripening stage it was greater in the BG cultivar. However, the soluble sugar in the studied tissues of both cultivars at the unripening stage was less than at the ripening stage. But the effect of SA in both concentrations on the soluble sugar of leaves and berry skin of both cultivars at the unripening stage was greater than at the ripening stage. The effect of SA in both concentrations on the soluble sugar of berry skin of the BS cultivar compared to the BG cultivar was quite significant. The highest amount of soluble sugar was seen at the ripening stage in berry skin (both cultivars) treated with SA in the 0.1-mM concentration.
Effect of Salicylic Acid on Insoluble Sugar
The insoluble sugar of leaves and berry skin of both cultivars at the ripening stage was greater than at the unripening stage (Fig. 1). The use of SA in both concentrations significantly increased the insoluble sugar of berry skin of both cultivars compared to control; at the unripening stage, the 0.1-mM concentration had more effect, and at the ripening stage the 1‑mM concentration had more effect. Also, in the leaves of both cultivars at the unripening stage, SA in both concentrations significantly increased the insoluble sugar of the leaves of both cultivars, with the 1‑mM concentration having a greater effect. But at the ripening stage, SA in both concentrations significantly decreased the insoluble sugar of the leaves in both cultivars compared to control (Fig. 1). In accordance with these results, SA was observed to increase insoluble sugar in the tomato (Wasti et al. 2012). In this study, the insoluble sugar at both stages in leaves and berry skin of the BG cultivar was greater than that of the BS cultivar. Also, at both stages and in both cultivars, the insoluble sugar of leaves was greater than that of berry skin (except for leaves of the BG cultivar, in which insoluble sugar was less than that of berry skin at the unripening stage,). The effect of SA on the insoluble sugar of the leaves of both cultivars at the unripening stage was greater than at the ripening stage, but in berry skin of both cultivars, the amount at the ripening stage was greater than at the unripening stage. The effect of SA on the insoluble sugar of berry skin of the BS cultivar at the ripening stage compared to another tissue was higher. In some plants such as pepper, SA (10−6 mM) increased the invertase enzyme of leaves and fruit and increased the insoluble sugar (Elwan and El-Hamahmy 2009). Also, the results indicate that the SA increased the sugar and antioxidant activity, so it helped to decrease environmental stresses (Elwan and El-Hamahmy 2009). Some studies have shown that SA increased the amount of photosynthetic pigment; decreased oxidative stress; protected chloroplast and cell membranes; protected macromolecules, such as proteins; increased the amount of sugar in plants; and also helped plants with regard to osmotic regulation (Khodary 2004). The highest amount of insoluble sugar was observed at the ripening stage in berry skin (both cultivars) treated with SA in the 0.1-mM concentration.
Effect of Salicylic Acid on Proline
Proline in the skin and flesh of berries for both cultivars and in the leaves of the BG cultivar at the ripening stage was greater than at the unripening stage. But in leaves of the BS cultivar, proline was greater at the unripening stage than at the ripening stage (Fig. 2). The Drawert (1963) studies showed that proline increased during the maturity process in grape fruit. At the unripening stage, significant increases compared with control were observed for proline In the skin and flesh of berries of the BS cultivar treated with the SA 0.1-mM concentration and in BG cultivar with the 1‑mM concentration. At the ripening stage, SA in both concentrations significantly increased the proline in the skin and flesh of berries in both cultivars compared to control; the 1‑mM concentration was more effective. Also, the SA 0.1-mM concentration increased proline in the leaves of both cultivars at both growth stages compared to control.
Effect of salicylic acid (SA) in three concentrations—0 (control) 0.1 mM, and 1 mM—on proline content of leaves, skin, and flesh of berries of Bidane Sefid (BS) and Bidane Ghermez (BG) cultivars in unripening stage (a) and ripening stage (b). Different letters indicate significant differences at the level of p ≤ 0.05
In accordance with these results, in several fruits including tomato (Wasti et al. 2012), SA increased the proline. In this study, the proline of skin and flesh of berries at the unripening stage and the leaves of BG cultivar at the ripening stage were more than for the BS cultivar. Also, the proline of leaves at the unripening stage and of skin and flesh of berries at ripening stage were greater for the BS cultivar than the BG cultivar. Studies have shown that red cultivars such as Rasheh have more proline than white cultivars such as BS (Abbaspour et al. 2017). The comparison of the proline of different tissues at two growth stages also showed that the proline of leaves was more than for berry tissues in both cultivars at the unripening stage. But at the ripening stage, the proline of berry tissues was more than for leaves. The effect of the SA on the flesh and skin of berries at the ripening stage was greater than at the unripening stage. But in the leaves of both cultivars, the effect was great at the unripening stage. The studies showed that use of SA can neutralize the effect of stressors by increasing the concentration of organic solutions, including proline, to stabilize the essential proteins (Tayeb et al. 2006). Indeed, the highest amount of proline was observed at the ripening stage and in berry skin (both cultivars) treated with the SA 1‑mM concentration.
Effect of Salicylic Acid on Total Protein
The total protein differs in various tissues and stages. The total protein of the leaves and berry flesh of the BS cultivar at the ripening stage was greater than at the unripening stage and than in the BG cultivar contrast (Fig. 3). In accordance with the obtained results for BS cultivar in this research, in a study on grape the total protein increased during ripening (Giribaldi et al. 2007). At both growth stages, the total protein in leaves was more than in berry flesh (Fig. 3). At the unripening stage, total protein was significantly increased compared to control for the SA 1‑mM concentration in leaves and berry flesh of the BS cultivar, in berry flesh of the BS cultivar for the SA 1‑Mm concentration, and in berry flesh of the BG cultivar for the SA 0.1 mM concentration. However, SA had a decreasing effect on the leaves of the BG cultivar at this stage.
Effect of salicylic acid (SA) in three concentrations—0 (control), 0.1 mM, and 1 mM—on total protein content of leaves and berry flesh of Bidane Sefid (BS) and Bidane Ghermez (BG) grape cultivars in unripening stage (a) and ripening stage (b). Different letters indicate significant differences at the level of p ≤ 0.05
At the ripening stage, the SA 0.1-mM concentration significantly increased the total protein of leaves and berry flesh of both cultivars. In accordance with these results, SA has been shown to increase the total protein in other grape cultivars (Renault et al. 1996). In a study of the grape leaves of BS cultivar, SA in a 2‑mM concentration decreased the total protein, but concentrations of 0.5 mM and 1 mM did not have a significant effect on the total protein of leaves (Ershadi and Taheri 2013). The results in this investigation showed that the 0.1-mM concentration was effective in increasing the total protein of leaves of the BS and BG cultivars at the ripening stage. Studies have shown that SA played an effective role in regulating apoplastic proteins and antioxidant enzymes associated with stress resistance (Tasgin et al. 2003). Decrease in production, increase in protein degeneration, or both can be attributed to the increase and decrease of antioxidant activity (El-Tayeb 2005).
The total protein concentrations at both growth stages in the leaves of BG were greater than for the BS cultivar; in contrast, the total protein in berry flesh of BS was more than for BG cultivar (Fig. 3). The SA effect on the total protein for both cultivars at the ripening stage was greater than in the unripening stage, especially in the leaves and berry flesh of the BG cultivar, because in this cultivar the total protein of tissues decreased during ripening, but the use of the SA 0.1-mM concentration significantly increased the total protein in these tissues compared to control. Also, at both growth stages the total protein of leaves was (both cultivars) greater than that for berry flesh. The highest amount of total protein was at the ripening stage in leaves of BG and BS cultivars treated with SA in the 0.1-mM concentration.
Effect of Salicylic Acid on SOD Enzyme Activity
Salicylic acid has different effects on the SOD activity of different parts of the grape at both growth stages. In this study, the SOD activity in the leaves, skin, and flesh of berries for both BS and BG cultivars was higher at the ripening stage than at the unripening stage (Fig. 4).
Effect of salicylic acid (SA) in three concentrations—0 (control), 0.1 mM, and 1 mM—on superoxide dismutase enzyme (SOD) activity content of leaves, skin, and berry flesh of Bidane Sefid (BS) and Bidane Ghermez (BG) grape cultivars in unripening stage (a) and ripening stage (b). Different letters indicate significant differences at the level of p ≤ 0.05
At the unripening stage, the use of SA in both concentration significantly increased the SOD activity of the leaves, skin, and berry flesh in both cultivars compared to control; the effect of the 1‑mM concentration was greater than that of the 0.1-mM concentration. But at the ripening stage, the SA 0.1-mM concentration in the BG cultivar and the 1‑mM concentration in the BS cultivar significantly increased the SOD activity of leaves, skin, and flesh of berries (Fig. 4). In accordance with these results, the effect of SA on the antioxidant system of several grape cultivars (Darcherin, Ghezel Osum, and Sahebi) was such that when SA was applied at appropriate concentrations (2 mM), this hormone enhanced the antioxidant system of the plant’s tissues through the activation of SOD and CAT oxidant enzymes (Habibi 2012). Research on the tomato showed that SA increased the activity of antioxidant enzymes POD, SOD, and CAT; this activity may be due to the regulating role of SA on transcriptional or translational regulation (Hayat et al. 2005).
Salicylic acid directly and indirectly activates the antioxidant enzymes by increasing their activity due to ROS scavenging created by stress (Doulatabadian et al. 2008; Li and Wang 2021). In the present study, the activity of the SOD enzyme in berry flesh of both cultivars was higher than in the leaves and berry skin at the unripening stage, but this was higher in berry skin than in leaves and berry flesh in both cultivars at the ripening stage. The activity of the SOD enzyme of leaves, skin, and berry flesh of the BG cultivar was higher than for the BS cultivar (Fig. 4). On the other hand, at both growth stages, the effect of SA on the activity of the SOD enzyme of leaves, skin, and berry flesh of the BG cultivar was greater than for the BS cultivar. The highest activity of the SOD enzyme was in the unripening stage in the berry flesh treated with the 1‑mM concentration in both cultivars and at the ripening stage in leaves and berry flesh of the BG cultivar treated with the 0.1-mM concentrations. In accordance with these results, studies have shown that SA stimulates extraordinarily sensitive reactions during ripening of the grape, suggesting that there are active oxygen-scavenging systems such as SOD and CAT enzymes in grapes, which are stimulated at this stage (Kraeva et al. 1998; Zhou et al. 2019).
The results show a direct correlation between soluble and insoluble sugar and SOD activity. In fact, the amount of both of these compounds increased at the ripening stage. On the other hand, there was a positive correlation between chlorophyll a, carotenoids, insoluble sugar, and the activity of the SOD enzyme; that is, the content of all of these compounds in the tissues of the BG cultivar was greater than for the BS cultivar. Also, there was a direct correlation between proline and protein at the ripening stage, such that the proline and protein in the berry flesh of the BS cultivar were higher than in the BG cultivar, and in leaves, both of these compounds were higher in the BG cultivar.
In fact, the results show that the SA 0.1-mM concentration in most cases was more effective than the 1‑mM concentration. Even in some cases in which the compound content decreased at the ripening stage, SA increased the compounds studied. It was also observed that in some cases, although the amount of these compounds in the BG cultivar was higher than in BS, the effect of SA on the BS tissues was greater.
Conclusion
It has always been underestimated that due to modulating effect of SA in biotic and abiotic stresses on the direct role of this pseudohormone alone and in isolation from stress, which plays a very positive role in nutritional factors. In this study, comparing two growth stages of grape cultivars under SA treatment provided the most effective concentration of this treatment and the most effective stage of the treatment effect. In fact, the results showed that the effect of SA was different in different tissues and in two different stages of growth (unripening and ripening). However, in most tissues at lower concentrations, 0.1 mM was more effective. In fact, the results of this study show that chlorophylls a and b and carotenoids decreased in the fruit ripening stage (compared with the unripening stage). But SA in both concentrations of optimal concentrations (0.1 mM) in both BG and BS cultivars increased chlorophyll a and b and carotenoids. Therefore, although chlorophyll a and b and carotenoids decreased in the ripening stage, SA at this stage was able to slightly compensate for this reduction. The results also show that soluble and insoluble sugar, proline, protein, and SOD enzyme activity in most of the tissues of both BG and BS cultivars at the ripening stage were greater than at the unripening stage. On the other hand, the effect of SA on these compounds in the ripening stage was greater.
References
Abbaspour N, Babaei L, Farrokhzad AR (2017) Effect of salicylic acid treatment on some morphological and physiological characteristics of cultivars two grape (Vitis vinifera L.) under drought stress conditions. J Hortic Sci 31:269–280
Abdel F, Gharib L (2006) Effect of salicylic acid on the growth, metabolic activities and oil content of basil and marjoram. Int J Agri Biol 8(4):485–492
Ahmed BC, Rouina BB, Sensoy S, Boukhris M, Abdallah BF (2009) Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. J Environ Exp Bot 67:345–352
Antonova Ananieva E, Nikolov Christov K, Popova L (2004) Exogenous treatment with Salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to Paraquat. J Plant Physiol 161:319–328
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bartles D, Sunkar R (2005) Drought and salt tolerance in plants: a review. Plant Sci 24:23–58
Bates LS, Walderd RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantitiesof protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Chakraborty U, Tongden C (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. J Curr Sci 89:384–389
Combe BG (1992) Research on development and ripening of the grapeberry. Am J Enol Vitic 43:101–110
Costa M, Civell PM, Chaves AR, Martinez GA (2005) Effects of ethephon and 6‑benzylaminopurine on chlorophyll degrading enzymes and a peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 °C. Postharvest Biol Technol 35:191–199
Darvizheh H, Zahedi M, Abbaszadeh B, Razmjoo J (2019) Changes in some antioxidant enzymes and physiological indices of purple coneflower (Echinacea purpurea L.) in response to water deficit and foliar application of salicylic acid and spermine under field condition. Sci Hortic 247:390–399
Doulatabadian A, Modarres Sanavy SAM, Etemadi F (2008) Effect of pretreatment of salicylic acid on wheat (Triticum aestivum L.) seed germination under salt stress. J Plant Physiol Iran 4:692–702
Drawert F (1963) Biochemisch-physiologische untersuchungen an traubenbeeren. Das verhalten der aminosauren wahrend der reifung und der zucker nach einfrieren der beeren. Vitis 4:49–56
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith V (1956) Calorimetric method for determination of sugars and related substances. Am J Analyt Chem 28:350–356
El Tayeb MA, Ahmed NL (2010) Response of wheat cultivars to drought and salicylic acid. J Agron 3(1):1–7
El-Tayeb MA (2005) Response of barley gains to interactive effect of salinity and salicylic acid. J Plant Growth Regul 45:215–225
Elwan MWM, El-Hamahmy MAM (2009) Improved productivity and quality associated with salicylic acid application in greenhouse pepper. J Sci Hortic 122(4):521–526
Ershadi A, Taheri S (2013) Evaluation effect of salicylic acid on spring frost tolerance in grape (Vitis Vinifera) “Bidaneh Sefid” cultivar. J Crop Improv 15(2):135–146
Fazelian N, Asrar Z (2011) Arsenic and salicylic acid interaction on the growth and some other physiological parameters in Matricaria recutita. J Plant Biol 8:1–11
Giannopolitis CN, Ries SK (1977) Superoxide dismutases II. Purification and quantitative relationship with water-soluble protein in seedlings. J Plant Physiol 59:315–318
Giribaldi M, Perugini I, Sauvage FX, Schubert A (2007) Analysis of protein changes during grape berry ripening by 2‑DE and MALDI-TOF. J Proteomics 7:3154–3170
Habibi G (2012) Effect of salicylic acid on antioxidativ system some grape cultivar nuder cold stress. J Cell Mol Biol 9:101–105
Haghshenas M, Nazarideljou MJ, Shokoohian A (2020) Phytochemical and quality attributes of strawberry fruit under osmotic stress of nutrient solution and foliar application of putrescine and salicylic acid. Int J Hortic Sci Technol 7(3):263–278
Hayat S, Fariduddin Q, Ali B, Ahmad A (2005) Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron Hung 53:433–437
Hussein MM, Balbaa LK, Gaballah MS (2007) Salicylic acid and salinity effects on growth of maize plant. J Agric Biol Sci 3(4):321–328
Jasska V (1996) Isoenzyme diversity and phylogenetic affinities among the phaseolus beans (Fabaceae). Plant Syst Evol 200:233–252
Karlidag H, Yildirim E, Turan M (2009) Salicylic acid ameliorates the adverse effect of salt stress on strawberry. J Sci Food Agric 66(2):180–187
Khodary SEA (2004) Effect of salicylic acidon the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agric Biol 6:5–8
Kraeva E, Tesniere C, Terrier N, Romieu C, Sauvage FX, Bierne J, Deloire A (1998) Transcription of a R‑1,3‑glucanase gene in grape berries in a late developmental period, or earlier after wounding treatments. Vitis 27:107–111
Larronde F, Krisa S, Decendit A, Cheze C, Deffieux G, Merillon JM (1998) Regulation of polyphenol prpduction in vitis vinifera suspension cultures by sugars. Plant Cell Rep 17:946–950
Li B, Wang W (2021) Salicylic acid induces tolerance of Vitis riparia× V. labrusca to chilling stress by altered photosynthetic, antioxidant mechanisms and expression of cold stress responsive genes. Plant Signal Behav 16(11):1973711
Lichtenthaler H (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Luo Z, Wu X, Xie Y, Chen C (2012) Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. J Food Chem 3(131):456–461
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mo Y, Gong D, Liang G, Han R, Xie J, Li W (2008) Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J Agric Food Chem 88(15):2693–2699
Nassar AR, Kliewer WM (1966) Free amino acid in various parts of vitis vinifera at different stages of development. Sci Hortic 89(281):294
Nelson N (1944) A photometric adaptation of the somogyimethod for the determination of glucose. J Biol Chem 153(2):375–380
Pancheva TV, Popova LP, Uzunova AM (1996) Effect of salicylic acid on growth and photosynthesis in barley plants. J Plant Physiol 149:57–63
Poor P, Gemes K, Horvath F, Szepesi A, Simon ML, Tari I (2011) Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol 13(1):105–114
Renault AS, Deloire A, Bierne J (1996) Pathogenesis-related proteins in grapevines induced by salicylic acid and botrytis cinerea. Vitis 35(1):49–52
Robinson SP, Davies C (2000) Molecularbiology of grape berry ripening. J Grape Wine Res 2:175–188
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. J Plant Growth Regul 30:157–161
Tasgin E, Atici O, Nalbantoglu B (2003) Effect of salicylic acid and cold on freezing tolerance in winter wheat leaves. J Plant Growth Regul 41:231–236
Tayeb M, Enany A, Ahmed N (2006) Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). J Plant Growth Regul 50:190–199
Tohma O, Esitken A (2011) Response of salt stressed strawberry plants to foliar salicylic acid pre-treatments. J Plant Nutr 34(4):590–599
Turkyilmaz Unal B, Mentis O, Akyol E (2015) Effects of exogenous salicylic acid on antioxidant activity and proline accumulation in apple (Malus domestica L.). Hortic Environ Biotechnol 56(5):606–611
Wang W, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wasti S, Mimouni H, Smiti S, Zid E, Ben Ahmed H (2012) Enhanced salt tolerance of tomatoes by exogenous salicylic acid applied through rooting medium. Int J Integr Biol 16(4):1–8
Zhang D, Zhang Y, Lin K, Wang B, Shi X, Cheng W (2021) Comparison of sugars, organic acids and aroma components of five table grapes in Xinjiang. IOP Conf Ser Earth Environ Sci 792(1):12029
Zhou X, Tan J, Gou Y, Liao Y, Xu F, Li G, Chen Z (2019) The biocontrol of postharvest decay of table grape by the application of kombucha during cold storage. Sci Hortic 253:134–139
Acknowledgements
The authors thank the Grapes and Raisins Institute of Malayer University, as well as the Malayer Grape Research Station, which provided the opportunity to conduct this research.
Author information
Authors and Affiliations
Contributions
Fatemeh Nazari and Mousa Rasouli conceived of the presented idea. Fatemeh Nazari developed the theory and performed the computations. Fatemeh Nazari and Masoumeh Maleki verified the analytical methods. Masoumeh Maleki and Mousa Rasouli encouraged Fatemeh Nazari to investigate (a specific aspect) and supervised the findings of this work.
Corresponding author
Ethics declarations
Conflict of interest
F. Nazari, M. Maleki, and M. Rasouli declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nazari, F., Maleki, M. & Rasouli, M. Effect of Salicylic Acid on Changes in Superoxide Dismutase Enzyme Activity, Protein, Proline, and Some Photosynthetic Pigments in Grape (Vitis vinifera L.) Bidane Ghermez and Bidane Sefid Cultivars at Two Growth Stages. Erwerbs-Obstbau 64 (Suppl 1), 37–45 (2022). https://doi.org/10.1007/s10341-022-00683-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00683-w