Abstract
Grapes contain high contents of phenolics, which are known to possess health promoting properties. Exogenous application of phytoregulators, mainly methyl jasmonate and abscisic acid, to grapevines to enhance phenolic content has been reported (Portu et al. Sci Hortic 240: 378-386, 2018; Ranjbaran et al. J Faculty Agric Kyushu Univ 56: 263-267, 2011). However, these phytohormones possess some drawbacks that can be overcome by using other phytoregulators as an alternative. In this work the effect of an additional phytohormone, salicylic acid, to grapevines on the phenolics and antioxidant activity of grapes was investigated. To our knowledge, salicylic acid has been earlier applied to grapevines to affect grape ripening and quality (Lóay. Egyptian J Basic Applied Sci 4: 227-230, 2017). However, this is the first time it is applied to increase the total phenolic content. As a result of our study, total phenol content and the free radical scavenging activity increased with 100 mg l−1 of salicylic acid. In particular, the total phenol content increased from 768.3 to 1843.5 mg 100 g−1 and the IC50 values decreased from 45.2 to 13.2 mg ml−1. Also the contents of individual phenolics mostly increased significantly with 100 mg l−1 of salicylic acid, except anthocyanins. Higher concentrations of salicylic acid (ie, 500 mg l−1vs 100 mg l−1) did not result in higher contents of phenolics. Therefore, 100 mg l−1 was selected as the best salicylic acid concentration to be used in the treatment. The application of exogenous salicylic acid to grapevines is an interesting agronomic practice to obtain table grapes with improved health-promoting properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolics are important constituents in food plants because of their physiological role and their nutritional features, which are mostly related to their antioxidant activity. Plant phenolics not only possess a number of health promoting activities [1] but also influence sensorial characteristics such as astringency and bitterness [2]. Grapes are rich in phenolics including phenolic acids, anthocyanins and flavonols [3]. Phenolic acids and flavonols are usually present as free aglycones and bound in conjugated forms. The concentration of phenolics in grapes varies with a number of factors [4]. In general terms, the total grape phenolic concentration slowly increases during maturation until a maximum is reached one or two weeks before harvest. In particular, anthocyanins increase significantly during veraison, contributing to the total increase in phenolics during this time [5]. Phenolics can also be enhanced by agronomical practices such as the application of elicitors [6]. In fact, exogenously applied elicitors are capable of triggering the accumulation of phenolics by stimulating the biochemical pathways responsible for their bioformation [6]. In grapes, some elicitors have been already used; however, methyl jasmonate (MJ) is one of the most effective up to now. As a result of the application of MJ to grapes, increases in the resveratrol content and the total phenolic content (TPC) have been reported [7]. However, despite its effectiveness, MJ action is at times too high in such a way that the ripening process is considerably accelerated. This results in overripe berries which involves decrease in the quality of the fruit. This advantage makes necessary the search for additional phytoregulators that can be used as an alternative.
In this regard, the effect of abscisic acid (ABA) on the phenolic composition of plant foods and its relation with the antioxidant and anti-inflammatory activities has been in the last years studied in our research group [8]. As a result of these studies, ABA has proven to be equally successful in increasing fruit phenolic content; however, its economic cost limits its use. More recently the capacity of salicylic acid (SA) in modifying phenolic content in samples other than grapes have been reported to be equivalent to that of MJ [9]. Similarly, in our laboratory, SA has been recently demonstrated to be satisfactory in increasing the olive phenolic composition (data submitted for publication). Similar effects of MJ and SA on phenolic content have been reported in samples other than grapes. Results obtained from the application of SA to olives suggest its potential as an alternative to MJ to enhance phenolic content in fruits other than olives. The objective of the present work is the search for a phytoregulator that can be used as an alternative to MJ to increase phenolic content in grapes. To that end, the effect of the treatment of grapevine with SA on the phenolic profile and on the antioxidant activity of the grapes was evaluated. Bibliographic reports on the effect of SA on grapevines in the literature are mostly focused to improve the quality, affect the ripening process and extent of the storage life. However, no study on the influence of SA treatment of grapevines on the antioxidant composition of grapes has been to our knowledge accomplished up to date.
Materials and Methods
Chemicals
Both acetic acid and MeOH (HPLC grade) were obtained by VWR Inc. (Bridgeport, PA, USA). 1,1-diphenyl-2-picrylhydrazil (DPPH), SA and sodium carbonate standards were supplied by Sigma-Aldrich (Steinheim, Germany). Folin-Ciocalteu reagent was obtained from Merck (Darmstadt, Germany). Phenolic acids and flavonols standards were purchased from Sigma-Aldrich (Steinheim, Germany). Anthocyanins and resveratrol standards were provided by Extrasynthase (GenayCedex, France).
Pre-Harvest Salicylic Acid Treatments
The experiments were conducted over two consecutive years (2017–2018) in a 10-year-old vineyard of Vitis vinifera cv Syrah to Badajoz, western Spain. All the vineyards were grown under the same agronomical and environmental condition. Bunches were sprayed with aqueous solutions of SA at 100 mg l−1 (so-called SA100) and 500 mg l−1 (so-called SA500), respectively, using a back-pack spray unit (200 L ha−1). Concentrations of SA were selected on the basis of previous studies in our laboratory on the best conditions of the treatments with phytoregulators [10, 11]. For comparison, controls were also included in our study by spraying only water over the vineyards. The treatments were carried out when half the grapes were at the veraison, exactly from 8 to 9 weeks after full bloom. During the application of the treatment, at least one untreated grapevine row between test grapevine rows was used to avoid contamination. Besides, the spraying was only carried out on no windy days. At harvest, 8 weeks later, batches of 250 berries were picked from a block of 5 vines. Three blocks were used per treatment. The experimental blocks were set up on different vines each year. For analyses, table grapes were weighed and split into two batches. One of them was used for TPC, antioxidant activity and determination of anthocyanins, quercetin-3-glucoside and trans-resveratrol (batch 1). The other batch was used to analyze total quercetin and myricetin and total phenolic acids (free aglycones plus conjugated forms) (batch 2). Both batches were stored in the dark at −80 °C until analyses.
Sample Preparation
Table grapes were analyzed by using the whole fruit including pulp, seed and skin. Two different extraction methods were performed to determine the target phenolics. The batch 1 was extracted without hydrolysis to keep intact conjugated forms of phenolic acids and flavonols whereas the batch 2 was extracted with hydrolysis to determine the total content of phenolic acids and flavonols. Both extraction methods were accomplished as detailed below.
Extraction without Hydrolysis
Frozen grapes in the batch 1 were first freeze-dried by using a lyophilizer and a 5 g-weight of dry grapes was mashed with a mortar. Polyphenols were extracted by adding 60 mL-volume of 80:20 (v/v) methanol:water to the sample and using an Ultraturrax homogenizer (IKA, Sigma-Aldrich, Madrid, Spain). The mixture was then centrifuged at 1500 rpm for 10 min at room temperature and the supernatant was isolated and taken to dryness in vacuo. The resulting extract was re-extracted by adding an additional 60 mL of methanol:water. Finally, the extract obtained (so-called extract 1) was taken to a final concentration of 20 mg ml−1 by using methanol:water 80:20 (v/v) and split into aliquots for TPC, DPPH activity and HPLC analysis of anthocyanins, quercetin-3-glucoside and trans-resveratrol.
Extraction with Hydrolysis
First, frozen grapes in the batch 2 were freeze-dried by lyophilization. Then a 5 g-weight of dry grapes was homogenized with a blender. Acidified methanol (20 ml) containing 1% (v/v) HCl, 3 X 10−3 MTBHQ was added to the sample. Subsequently, HCl (1.2 M, 5 ml) was added to the mixture, which was stirred at 90 °C under reflux for 2 h. The resulting extract (so-called extract 2) was allowed to get cold and then centrifuged at approximately 22,000 g for 10 min. The upper layer was taken and then filtered through a 0.45 μm filter (Millipore) for HPLC analysis of total quercetin, myricetin and phenolic acids.
Analysis
Total Phenol Content (TPC)
The measurements were carried out by using a Beckman Coulter DU-800 spectrophotometer (Barcelona, Spain). The method used to determine TPC was that earlier described in the literature [12]. This method is based the use of the Folin-Ciocalteu reagent, which oxides the hydroxyl groups of phenols. In brief, 0.5 mL of Folin-Ciocalteu reagent and 10 mL of sodium carbonate solution (75 g/L) were added to a 0.1-mL volume of the extract. This mixture was made up to 25 mL with distilled water and was left for 1 h. Then the absorbance was measured at 750 nm and it was compared with that obtained from the blank (without Folin-Ciocalteu reagent). Calibration curves were prepared by using gallic acid. The results obtained were expressed as milligrams of gallic acid equivalents per 100 g of fruit. All analyses were accomplished in triplicate.
Antioxidant Activity
The 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH*) scavenging assay was performed by using Beckman Coulter DU-800 spectrophotometer (Barcelona, Spain). The assay was carried out according to the method elsewhere reported [13]. A Beckman Coulter DU-800 spectrophotometer (Barcelona, Spain) was used to measure the result of the DPPH assay. Each extract was diluted to final concentrations of 15.6, 62.5, 125, 250 and 500 μg/mL and then they were transferred to a 96-well microliter plate. A 150 μL-volume of a DPPH solution (400 μM) was added to 50 μL aliquot of the sample placed in each well. Absorbance was measured at 517 nm after 30 min at 37 °C. The value obtained was compared with that provided by the DPPH solution. Each extract without DPPH was used as a blank. The percentage inhibition of the DPPH by each dilution of samples was estimated by considering the percentage of the steady DPPH in solution after reaction. The experiments were performed in duplicate. A plot of percentage inhibition versus concentration was made and the IC50 values were calculated using linear regression analysis.
Phenolic Compounds
Both extracts 1 and 2 were analyzed by HPLC by using the same equipment (Alliance Separation Module 2695, Waters, Milford, USA) and the same method. The equipment was supplied with an automatic injector and a photodiode array detector 996 (DAD, Waters, Milford, USA). The separation of phenolics was accomplished on an ODS reverse phase (C18) column (250 mm × 4.6 mm i.d., particle size 5 μm, ACE, Madrid, Spain) and at a flow rate of 1 ml min−1. To protect the column, an Altima 5 μm C18 pre-column (Altech, Barcelona, Spain) was used. Both pre-column and column operated at 20 °C. The elution was performed using solvents A (water containing 5% formic acid) and B (acetonitrile). A linear gradient was applied from the initial eluent composition A/B 95:5 (v/v), which was maintained for 3 min, up to A/B 80:20 (v/v) from 3 to 15 min, and then to A/B 75:25 (v/v) within the next 5 min and then to A/B 70:30 (v/v) from 20 to 25 min. Subsequently, the composition was modified to A/B 65:35 (v/v) from 25 to 36 min and then to A/B 60:40 (v/v) within 4 min and to A/B 85:15 (v/v) from 40 to 45 min. Finally the composition was modified up to the initial composition A/B 95:5 (v/v) for within 2 min. Chromatograms were recorded from 250 to 600 nm and blanks between consecutive runs were carefully performed. Gallic acid was measured at 280 nm whereas caffeic, ferulic and chlorogenic acids were measured at 320 nm. Aglycones of quercetin and myricetin as well as quercertin-3-glucoside were all detected at 350 nm. Anthocyanins were all registered at 520 nm and resveratrol was recorded at 304 nm. Each HPLC run was performed three times. Stock solutions of the standard compounds were prepared in 70% (v/v) methanol to final concentration of 1 mg ml−1. In addition, calibration curves of the standards were established on six data points and each standard dilution was run in triplicate. Both grape extracts were also reconstituted in 700 ml l−1 methanol and injected in triplicate at a concentration of 20 mg ml−1 for quantification of the target phenolics. Analyses were performed in triplicate.
Statistical Analysis
The results are presented as the average of the all values obtained and standard deviation (mg kg−1dry weight ± SD). Data from grapes untreated and treated with 100 and 500 mg l−1 of SA were included in the statistical analysis. The data were analyzed using one-way analysis of variance (ANOVA) and differences were considered significant at p < 0.05. The effect of the phytoregulators was assessed by the Fisher test. Differences between data were compared by least significant differences (LSD).
Results and discussion
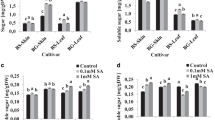
Figure Fig. 1 represents TPC, expressed as mg gallic acid 100 g−1 in dry matter, (a) and
Total phenol content (mg gallic acid 100 g−1) (a) and the free radical scavenging radical activity (expressed as IC50, extract that results in 50% reduction of DPPH) (b) of the control, SA100 treated and SA500 treated grapes. The values were estimated as means ± SD (n = 3). Different letters indicate significant (p < 0.05) differences between control and treated samples
the free radical scavenging activity in terms of IC50 (mg ml−1), (b) of the control, SA100 and SA500 treated grapes included in the extract 1. As seen in Fig.1a, TPC increased significantly (p < 0.05) from 768.3 in control grapes to 1843.5 and to 2131.8 mg gallic acid 100 g−1 in SA100 and SA500 treated samples, respectively. Concerning the IC50, values decreased (p < 0.05) from of 45.16 in controls to 13.2 and to 9.6 mg ml−1 after SA100 and SA500 treatments, respectively (see Fig. Fig. 1b). These TPC and IC50 data are within the range of values reported in the literature for grapes [14]. As seen, an improvement in both TPC and the free radical scavenging activity was observed in grapes after pre-harvest treatment of grapevines with SA. Surprisingly, the use of different concentrations of SA in the treatments (i.e., 100 and 500 mg l−1) did not affect significantly (p < 0.05) the measurements obtained either in terms of TPC or in terms of DPPH activity.
From these results, it is believed that SA is modifying the activity of enzymes responsible for the bioformation of grape phenolics. In this sense phenyl-alanine lyase, which is the first enzyme regulating the phenylpropanoid pathway [15], has been already reported to be affected by phytoregulators [16]. As a result, the effect of SA on phenylalanine lyase activity would eventually result in an alteration of the bioformation of phenolics. The reason why 500 mg l−1of SA did not lead to higher TPC and DPPH activity than 100 mg l−1 is probably the blocking of the entry into the active site of the phenylalanine lyase. No influence of SA concentration on phenolics in the elicitation of plants other than grapevines has also been already reported [17].
The possibility of a linear regression between TPC and the IC50 values was considered. As a result, the regression equation y = −0.0271x + 5.465 was obtained. In view of the correlation coefficient (r2 = 0.988) obtained, certain lineal relationship was established between TPC and the DPPH activity, which indicates that most phenolics present in the extract 1 were the major contributors to the free radical scavenging activity of the grapes.
Table 1 depicts the content of total phenolic acids (i.e ., sum of free aglycones and derivatives), expressed as mg g−1 dry matter, in grapes from the control, SA100 treated an SA500 treated grapevines used for the extract 2. As seen in the table, chlorogenic acid was the most abundant followed by caffeic acid. Trans-ferulic and gallic acids were however minor. Other researchers have reported distinct profile of phenolic acids from that of this study [18]. This is owing to differences in the experimental procedures used. As also observed in Table 1, the contents of all phenolic acids increased significantly (p < 0.05) as a result of the application of SA. Similarly to TPC and DPPH activity, phenolic acid content was not affected by higher level of SA. From the table, SA100 and SA500 treated grapes exhibited similar contents of all phenolic acids.
The contents of the main anthocyanins (expressed as mg g−1 dry matter) in grapes from the control, SA100 treated an SA500 treated grapevines are summarized in Table 2. Cyanidin-3-glucoside and delphinidin-3-glucoside constituted the smallest group in grapes whereas malvidin-3-glucoside represented the major anthocyanin. The predominance of malvidin derivatives over cyanidin and delphinidin derivatives in grapes here found is supported by the literature [19]. Regarding the treatment effect, significant (p < 0.05) changes in contents of anthocyanins after exposure of grapevines to SA were measured. However, in contrast to phenolic acids, contents of anthocyanins decreased significantly (p < 0.05) in treated grape fruits as compared with controls. In fact, malvidin-3-glucose dropped drastically from 2357.5 to 437.1 and 501.1 mg g−1after treatment with SA100 and SA500 respectively. Once more, the concentration of SA used in the treatments did not bring significant (p ˃ 0.05) variations.
It is believed that the effect of SA over the contents of phenolic acids and anthocyanins is related to the evolution of these compounds during the grape ripening. In this regard, variations in the concentrations of grape phenolics during the ripening period has been described to exhibit two stages, one around veraison and the second around maturity [20]. Hydroxycinnamates including phenolic acids show a peak prior to veraison and then a decline leading to a constant amount as the fruit ripened. On the contrary, anthocyanins start accumulating at veraison, then increase gradually during grape maturation and begin a decline by 60 days after veraison [25]. Since the treatments were applied at veraison, it is hypothesized that SA slows down the grape ripening. As a result, although untreated and treated grapes were both simultaneously collected, they were harvested at a slightly different maturation status because of SA effect. Particularly, SA treated grape fruits were harvested at a greener stage than the control, which were harvested at commercial maturity. These results are verified by those on TPC previously commented (see Table 1) since TPC has been described to decrease as ripening progresses [20]. Therefore, the increase in TPC content after SA application confirms the delay of the ripening process as a consequence of the treatment. That is most likely the reason why unripe grapes exhibit a maximum in TPC and phenolic acids but a minimum of anthocyanins. Variations in the grape phenolic composition after the application of SA have never been studied; however, delay in grape ripening by the action of SA treatment has previously been reported [21]. According to the literature [21], the retarding effect of SA on the grape maturation is due to its antagonism with abscisic acid. In this sense, whereas abscisic acid is the responsible for grape berry ripening, SA would be inhibiting the ABA effect.
Table 3 represents the contents of flavonols (expressed as μg g−1 dry matter) in grapes from the control, SA100 treated an SA500 treated grapevines. From the table, the effect of the treatments differed according to each individual compound. For total quercetin, which was extracted by the extraction 2, significant (p < 0.05) decreases with the application of SA were measured. Specifically, content of total quercetin declined from 215.8 in controls to 72.7 and 84.1 μg g−1 in SA100 treated and SA500 treated grapes, respectively. Similarly, quercetin-3-glucoside, which was isolated by extraction method 1, also resulted in a significant (p < 0.05) decrease from 172.4 in controls to 87.0 μg g−1 after treatment with SA100. It is interesting to point out that the use of higher concentration of SA (i.e., 500 mg l−1) did not alter the content of quercetin. By comparing Tables 2 and 3, the trend of total quercetin and quercetin-3-glucoside with the treatments was the same as that observed for anthocyanins (see Table 2). Based on our theory about the retarding effect of SA on grape maturation, it is thought that total quercetin increases gradually from veraison to maturity and then drops near overripe. This hypothesis could not be verified since no supporting information could be found in the literature. Actually contradictory conclusions on this topic have been reported by other authors. In general, flavonols have been described to vary considerably with cultivar and weather conditions [22]. In short, definite conclusions have not been to our knowledge published.
Oppositely to quercetin, the contents of total myricetin and trans-resveratrol increased significantly (p < 0.05) with the SA treatments. Therefore, their trend with SA application is in accordance with that of TPC, antioxidant activity and phenolic acids. This reflects that myricetin and trans-resveratrol also accumulate around veraison and then decrease as maturity progresses. It is necessary to emphasize that for these two phenolics higher concentration of SA (i.e., 500 mg l−1) did result in higher concentrations of myricetin and trans-resveratrol. This was striking since it was the only case in which higher SA concentration implied higher contents of the investigated phenolics.
Overall, the exogenous application of 100 mg l−1 to grapevines during the ripening process results in grapes enriched in total phenolic content and with higher free radical scavenging activity. In addition, a number of phenolic compounds with bioactive properties such as resveratrol, myricetin and phenolic acids also increased with the application of SA. The use of 500 mg l−1 of SA might be recommendable only when the objective is to increase the resveratrol content. However, considering the results on the rest of phenolics, total phenol content and antioxidant activity, the application of 100 mg l−1 appear to be advantageous. Studies on the content of some of the phenolics during the ripening process before and after SA treatment as well as other treatment conditions and types of grapes (i.e., wine grapes) are now scheduled to get a deeper insight of the action mechanism of SA over grape phenolics. In these studies, quality parameters and sensory characteristics will be included. The results obtained in the present study are interesting from a practical point of view to grape industry.
References
Ozcan T, Akpinar-Bayizit A, Yilmaz-Ersan L, Delikanli B (2014) Phenolics in human health. Intern J Chem Eng Appl 5:393–396. https://doi.org/10.7763/IJCEA.2014.V5.416
Cejudo-Bastante C, Durán-Guerrero E, García-Barroso C, Castro-Mejía R (2017) Volatile compounds, polyphenols and sensory quality in the production of tomato vinegar. J Food Nutr Res 5:391–398. https://doi.org/10.12691/jfnr-5-6-5
Padilha CV, Miskinis GA, de Souza ME, Pereira GE, de Oliveira D, Bordignon-Luiz MT, Lima MD (2017) Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem 228:106–115. https://doi.org/10.1016/j.foodchem.2017.01.137
Bavaresco L, Mattivi F, de Rosso M, Flamini R (2012) Effects of elicitors, viticultural factors, and enological practices on resveratrol and stilbenes in grapevine and wine. Mini-Rev Med Chem 12:1366–1381. https://doi.org/10.2174/13895575112091366
Downey MO, Dokoozlian NK, Krstic MP (2006) Cultural practices and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. Am J Enol Vitic 57:257–268
García-Ruiz Y, Gómez-Plaza E (2013) Elicitors: a tool for improving fruit phenolic content. Agriculture 3:33–52. https://doi.org/10.3390/agriculture3010033
Portu J, Santamaría P, López R, Garde-Cerdán T (2018) Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci Hortic 240:378–386. https://doi.org/10.1016/j.scienta.2018.06.019
Blanch GP, Flores G, Gomez MC, Ruiz del Castillo ML (2018) Abscisic acid sprayed on olive tree (Olea europaea L.) affects the phenolic composition of olive fruit cultivars. J Agric Sci 10:37–46. https://doi.org/10.5539/jas.v10n4p37
Muhammed A, Sevket M, Ahmet MM, Ebru B (2019) Effects of methyl jasmonate and salicylic acid on the production of camphor and phenolic compounds in cell suspension culture of endemic Turkish yarrow (Achillea gypsicola) species. Turkish J Agric and Forest 43:351–359. https://doi.org/10.3906/tar-1809-54
Flores G, Blanch G, Ruiz del Castillo ML (2018) Development of a new strategy based on the application of phytoregulator to induce phenolic acids in olive fruits. CyTA-J Food 16:692–697. https://doi.org/10.1080/19476337.2018.1465998
Blanch G, Gómez-Jiménez MC, Ruiz del Castillo ML (2019) Accumulation of the phenolic content in olive fruits by salicylic acid treatment of olive tree (Olea europaea L.). Food Res Int (Submitted for publication)
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Smith RC, Reeves JC, Dage RC, Schnettler RA (1987) Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem Pharmacol 36:1457–1460. https://doi.org/10.1016/0006-2952(87)90110-9
Komutiban O, Katkaew A, Jaisamut J (2018) Comparison of total phenolic content, antioxidant activity and trans-resveratrol content of fresh red grapes and raisin ethanolic extracts. J Food Health & Bioenv Sci 11:79–96. https://doi.org/10.1016/j.scp.2018.03.001
Flores G, de la Peña MF, Blanch GP, Ruiz del Castillo ML (2014) Phenylalanine ammonia-lyase, flavanone 3β-hydroxylase and flavonol synthase enzyme activity by a new in vitro assay method in berry fruits. Food Chem 153:130–133. https://doi.org/10.1016/j.foodchem.2013.12.034
Ketabchi S, Majzoob S, Charegani HA (2013) Effect of salicylic acid and methyl jasmonate on phenylalanine ammonia-lyase activity and total phenol in wheat infected by Pratylenchus thornei. Arch Phytop Plant Protect 48:10–17. https://doi.org/10.1080/03235408.2014.882104
Mendoza D, Cuaspud O, Arias JP, Ruiz O, Arias M (2018) Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol Rep 19:e00273. https://doi.org/10.1016/j.btre.2018.e00273
Shrikanta A, Kumar A, Govindaswamy V (2015) Resveratrol content and antioxidant properties of underutilized fruits. J Food Sci Technol 52:383–390. https://doi.org/10.1007/s13197-013-0993-z
Budic-Leto I, Mucalo A, Ljubenkov I, Zdunic G (2018) Anthocyanin profile of wild grape Vitis vinifera in the eastern Adriatic. Sci Hortic 238:32–37. https://doi.org/10.1016/j.scienta.2018.04.036
Garrido I, Uriarte D, Hernández M, Llerena JL, Valdés ME, Espinosa F (2016) The evolution of total phenolic compounds and antioxidant activities during ripening of grapes (Vitis vinifera L., cv. Tempranillo) grown in semiarid region: effects of cluster thinning and water deficit. Sci Hortic 238:32–37 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5133919
Ranjbaran E, Sarikhani H, Wakana A, Bakhshi D (2011) Effect of salicylic acid on storage life and postharvest quality of grape (Vitis vinifera L. cv. ‘Bidaneh Sefid’). J Fac Agr Kyushu U 56:263–269
Zheng J, Yang B, Ruusunen V, Laaksonen O, Tahvonen R, Hellsten J, Kallio H (2012) Compositional differences of phenolic compounds between black currant (Ribes nigrum L.) cultivars and their response to latitude and weather conditions. J Agric Food Chem 60:6581–6593. https://doi.org/10.1021/jf3012739
Ranjbaran E, Sarikhan H, Wakana A, Bakhshi D (2011) Effect of salicylic acid on storage life and postharvest quality of grape (Vitis vinifera L. cv. Bidaneh Sefid). J Faculty Agric Kyushu Univ 56:263–267
Lóay AA (2017) Preharvest salicylic acid and delay ripening of ‘superior seedless’ grapes. Egypt J Basic Appl Sci 4:227–230. https://doi.org/10.1016/j.ejbas.2017.04.006
Acknowledgments
This work was supported by the Comunidad of Madrid and European funding from FSE and FEDER programs (project S2018/BAA-4393, AVANSECAL-II-CM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
I would like to declare that I have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(ODT 71 kb)
Rights and permissions
About this article
Cite this article
Blanch, G.P., Gómez-Jiménez, M.C. & del Castillo, M.L.R. Exogenous Salicylic Acid Improves Phenolic Content and Antioxidant Activity in Table Grapes. Plant Foods Hum Nutr 75, 177–183 (2020). https://doi.org/10.1007/s11130-019-00793-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-019-00793-z