Abstract
The causal agent of apple mosaic disease has been previously thought to be solely caused by apple mosaic virus (ApMV). In this study, we report that a novel ilarvirus is also associated with apple mosaic disease. Next-generation sequencing analysis of an apple tree showing mosaic symptoms revealed that the tree was infected with three apple latent viruses (apple stem pitting virus, apple stem grooving virus, and apple chlorotic leaf spot virus) and a novel ilarvirus (given the name apple necrotic mosaic virus (ApNMV)) that is closely related to Prunus necrotic ringspot virus (PNRSV) and ApMV. The genome of ApNMV consists of RNA1 (3378 nt), RNA2 (2767 nt), and RNA3 (1956 nt). A phylogenetic analysis based on the coat protein amino acid sequences indicated that the novel virus belongs to the same subgroup 3 of the genus Ilarvirus as PNRSV and ApMV. The presence of mosaic leaves, which tend to be unevenly distributed in diseased apple trees, was correlated with the internal distribution of ApNMV. RT-PCR detection of mosaic-diseased apple trees in Japan indicated that ApNMV was detected in apple trees introduced from China, whereas ApMV was detected from cultivated apple trees in domestic orchards. Consistent with these findings, a survey of mosaic-diseased apple trees in major apple-producing provinces in China revealed that the majority of apple trees showing mosaic symptoms in China are infected with ApNMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple mosaic disease has been reported since the beginning of the nineteenth century in Europe (Bradford and Joley 1933; Fukushi and Tahama 1960; Fulton 1972; Petrzik and Lenz 2011) and was one of the first viral diseases proven to be transmittable by grafting (Bradford and Joley 1933). Leaves of apple trees infected with mosaic disease develop bright pale-yellow chlorotic spots and mosaic patterns. Chlorosis can develop along leaf veins, creating a reticulated appearance or, in other cases, can manifest as large, amorphous chlorotic areas between leaf veins (Fukushi and Tahama 1960; Fulton 1972; Petrzik and Lenz 2011; Posnette and Cropley 1956). Trees with symptomatic leaves throughout the tree lack vigour (Nemeth 1986); symptoms do not appear on branches or fruits.
Until now, the causal agent of apple mosaic disease was believed to be apple mosaic virus (ApMV). ApMV belongs to the genus Ilarvirus, family Bromoviridae, and is distributed throughout the world (Bujarski et al. 2012; Petrzik and Lenz 2011). In addition to apple trees, known natural hosts of ApMV include pear, plum, peach, apricot, cherry, almond, hazelnut, silver birch, chestnut, blackberry, raspberry, hop, European elder, mahaleb cherry, and several species of Trebouxia lichen (Fulton 1972; Grimová et al. 2013; Kanno et al. 1993; Petrzik et al. 2014).
The genus Ilarvirus comprises viruses having tripartite genomes and icosahedral particles (Bujarski et al. 2012). The genome consists of single-stranded positive-sense RNA molecules (RNA1, RNA2, and RNA3) and an encapsidated subgenomic RNA4. RNA1 and RNA2 each encode single replication-associated proteins. RNA3 encodes a movement protein (MP) and coat protein (CP), whereas RNA4 is a subgenomic RNA that functions as an mRNA for CP (Bujarski et al. 2012; Guo et al. 1995). The complete genome of ApMV has been sequenced (Sánchez-Navarro and Pallás 1994; Shiel et al. 1995; Shiel and Berger 2000), and ApMV was shown to belong to subgroup 3 in the genus Ilarvirus as does Prunus necrotic ringspot virus (Bujarski et al. 2012; Hammond and Crosslin 1998).
Apple trees (tree no. P129, PK45, PK28, and P133) showing mosaic symptoms maintained at the National Agriculture and Food Research Organization (NARO)-Apple Research Station (Morioka City, Iwate Prefecture, Japan) had leaves with pale-yellow mosaic patterns and were believed to be infected with ApMV. However, enzyme-linked immunosorbent assay (ELISA) using an ApMV antibody yielded a positive reaction for a leaf extract of P133 only, but not for P129, PK45, and PK28 (Koganezawa and Besho 1987). Accordingly, the mosaic symptoms in P129, PK45, and PK28 were assumed to be caused by an agent other than ApMV. Although an attempt was made to isolate the causal agent from these trees via sap inoculation of herbaceous hosts, the agent was not transmitted to any test plants including the ApMV assay hosts Cyamopsis tetragonoloba and Cucumis sativus (Fulton 1972).
In this paper, we applied next-generation sequencing analysis to identify the causal agent in apple tree P129, which had “mosaic disease” symptoms but did not test positive for ApMV in the ELISA. We subsequently determined the genome sequence of a novel ilarvirus correlated with the mosaic symptoms.
Materials and methods
Plant material
Apple trees P129, PK28, PK45, and P133 maintained in the virus repository orchard at Apple Research Station, NARO were used in this study. Leaves were collected for next-generation sequence analysis and virus detection by RT-PCR. To clarify the relationship between the mosaic symptom and virus distribution, leaf samples (5 leaves per branch) were collected from branches with symptomatic leaves only, branches with a mixture of symptomatic and asymptomatic leaves, and branches with asymptomatic leaves only on a mosaic-diseased apple tree (PK28).
Deep sequence analysis
For next-generation sequencing, double-stranded (ds) RNAs were extracted from leaf tissues (20 g) of a mosaic-diseased apple tree (P129) using a previously described method (Morris and Dodds 1979; Yoshikawa and Converse 1990), then dissolved in 20 μL TAE buffer (40 mM Tris, 20 mM sodium acetate, 1 mM EDTA, pH 7.0). The dsRNAs were then subjected to library preparation according to the instructions for the NEBNext mRNA Library Prep Master Mix Set for Illumina (code No. E6110S, New England Biolabs, Tokyo, Japan) and subjected to reverse transcription to synthesize the first strand cDNA using a random primer according to the protocol for NEBNext Multiplex Oligos for Illumia Index primers 1–12 (code no. E7335, NEB). The cDNA was used as a template to synthesize a double-stranded cDNA, followed by end repair, addition of poly A, adapter ligation, and then PCR for library preparation. The prepared library was analysed using 6% polyacrylamide gel electrophoresis and the products (200 to 300 bp) were purified and subjected to paired-end sequencing (1 read = 101 bases) using an Illumina HiSeq 2000 (Illumina, San Diego, CA, USA). The resulting reads were assembled into contiguous sequences (contigs) by the Velvet software (European Bioinformatics Institute, https://www.ebi.ac.uk/~zerbino/velvet/), and the contigs were used to search for virus sequences using BLAST (blastn and blastx).
Complete virus genome sequencing
Total RNAs were extracted from apple leaves (P129) showing mosaic symptoms according to Gasic et al. (2004) and then dissolved in RNase-free water at 500 ng/μL. Unknown sequences of a novel ilarvirus (given the name Apple necrotic mosaic virus [ApNMV]) genome were amplified by RT-PCR and 3′- and 5′-RACE-PCR. RT-PCR was carried out using a TaKaRa RNA PCR Kit (AMV) ver.3.0 (TaKaRa, Siga, Japan) using primers (R1-node268+ as a sense primer and R1-node31—as an antisense primer) to amplify the sequence between NODE268 and NODE31 (NODE means next-generation sequencing-derived contigs) (Supplementary Table 1 and Fig. 1). For 3′-RACE-PCR, poly A tails were added to the 3′ ends of the viral RNAs using an A-PlusTM Poly(A) Polymerase Tailing Kit (Cellscript, Madison, WI, USA), and 3′-RACE-PCR was carried out using the TaKaRa RNA PCR Kit (AMV) ver.3.0 using primers (R1-node31+, R2-node305+, and R3-node76+ as sense primers and M13 Primer M4 as the antisense primer) to amplify the sequences between contigs and the 3′ end of each RNA (Supplementary Table 1 and Fig. 1). The 5′-RACE-PCR was carried out using the 5′-Full RACE Core Set (TaKaRa) as follows: reverse transcription was performed using the phosphorylated antisense primers 5′RACE-R1-RT, 5′RACE-R2-RT, and 5′RACE-R3-RT (Supplementary Table 1). The initial PCR was performed using 5′RACE-R1-S1, 5′RACE-R2-S1, and 5′RACE-R3-S1 as the sense primers and 5′RACE-R1-A1, 5′RACE-R2-A1, and 5′RACE-R3-A1 as the antisense primers. The second PCR was conducted using 5′RACE-R1-S2, 5′RACE-R2-S2, and 5′RACE-R3-S2 as the sense primers and 5′RACE-R1-A2, 5′RACE-R2-A2, and 5′RACE-R3-A2 as the antisense primers (Supplementary Table 1). The PCR products were purified using Mono Fas (GL Science, Tokyo, Japan), and then TA cloning was performed using the Mighty TA-cloning kit (TaKaRa). Plasmids were extracted from Escherichia coli using the Favor PrepTMPlasmid DNA Extraction Mini Kit (FAVORGEN, Tokyo, Japan) and used for sequence analysis.
Genome organization of the Apple necrotic mosaic virus (ApNMV). Bars under each RNA indicate the position and length of contigs obtained from next-generation sequencing. The open reading frames (ORFs 1–4) of ApNMV-RNAs represent methyltransferase (MET), NTP-binding helicase (HEL), RNA polymerase (POL), movement (MP), and coat (CP) proteins. nt nucleotide
Homology comparison and phylogenetic analysis
The nucleotide and amino acid sequences of ORF1, ORF2, ORF3, and ORF4 of ApNMV were analysed using the software DANASIS (Hitachi, Tokyo, Japan). A homology search of nucleotide and amino acid sequences of CP and POL was performed using the software DANASIS (Hitachi) between ApNMV and the ilarviruses. Phylogenetic analysis was also performed using Clustal W software supplied by DDBJ based on the CP amino acid sequences of ApNMV and ilarviruses.
RT-PCR detection of ApNMV and ApMV
For the detection of ApNMV and ApMV, total RNAs were extracted from leaves of mosaic-diseased apple trees as described above, and RT-PCR was carried out using the TaKaRa RNA PCR Kit (AMV) ver.3.0 (TaKaRa) using primer pairs ApNMV-CP+ 1 and ApNMV-CP-1, or ApNMV-CP+ 2 and ApNMV-CP-2 for ApNMV detection (Table 1), and primer pairs ApMV-CP+ 1 and ApMV-CP-1, or ApMV-CP+ 2 and ApMV-CP-2 for the detection of ApMV (Table 1). In Japan, trees of five cultivars (Kaori, Redfield, Allington Pippin, Fuji-Iwafu 10, and Fuji-Yamafu 2) showing mosaic symptoms at the Apple Research Station, NARO were tested to survey the incidence of ApNMV and ApMV. In addition, 359 leaf samples (276 samples with mosaic and 83 without) were collected from major production provinces in China and tested by RT-PCR.
Results
Mosaic symptoms on apple leaves
Figure 2 shows the mosaic symptoms on leaves of four apple trees P129 (unknown cultivar), PK28 (cv. Zhong Guo Ping Guo from China), PK45 (cv. Qi Pan Tuo Ping Guo from China), and P133 (transcendent crabapple from the USA) maintained at the NARO Apple Research Station. Leaves from each tree had bright pale-yellow spots or mosaic symptoms. Mosaic symptoms ranged from small pale-yellow spots scattered across an entire leaf or part of a leaf, to large amorphous, contiguous, chlorotic spots covering an entire leaf. In some cases, chlorosis was observed along the leaf veins. Leaf symptoms were readily observed from spring to summer, but no symptoms appeared on the branches or fruits. Although no differences in symptoms were observed among P129, PK45, and PK28, a partial leaf necrosis was observed in these three trees when compared with P133 (Fig. 2). These symptoms were not distinguishable from symptoms of apple mosaic disease described before (Petrzik and Lenz 2011).
Deep sequence analysis of dsRNAs from an apple tree with leaf mosaic symptoms
cDNAs were synthesized using double-stranded RNA as a template that had been extracted from the leaves of apple tree P129 showing mosaic symptoms. After adapter ligation and polymerase chain reaction (PCR) amplification, paired-end sequencing (1 read = 101 bases) was performed by HiSeq2000 (Illumina Inc.). The resulting reads (92, 793, 386) were assembled into 843 contiguous sequences (contigs), ranging in length from 185 to 5359 nucleotides (nt). Based on the blastx analysis, 485 contigs had high sequence identity with known viral sequences (Supplementary Table 2). Of these, 298 contigs had high sequence identity with apple stem pitting virus (ASPV) and apricot latent virus (ALV) in the genus Foveavirus, while 65 and 5 contigs had high sequence identity with apple stem grooving virus (ASGV) in the genus Capillovirus and apple chlorotic leaf spot virus (ACLSV) in the genus Trichovirus, respectively (Supplementary Table 2). In addition, 11 contigs had 76.19–65.28% sequence identity with Prunus necrotic ringspot virus (PNRSV), and 1 contig had 57.34% sequence identity with ApMV, both in the genus Ilarvirus.
Among the contigs having sequence identity with PNRSV-RNA, NODE268 (1996 nt) and NODE31 (1290 nt) are thought to be part of RNA1 of a novel ilarvirus and NODE305 (2500 nt) as part of RNA2 of a novel ilarvirus (Fig. 1). NODE76 (566 nt) having high sequence identity with ApMV-RNA is thought to constitute RNA3 of ApNMV (Fig. 1).
Complete nucleotide sequence and genome organization of a novel ilarvirus from an apple tree with leaf mosaic
Based on the contigs yielded by next-generation sequencing (Fig. 1), the entire ApNMV genome from the tree P129 was amplified by reverse transcription (RT)-PCR and 3′- and 5′-rapid amplification of cDNA ends (RACE), then sequenced. As can be seen in Fig. 1, the ApNMV genome consists of a 3378-nt RNA1 (GenBank/EMBL/DDBJ accession LC108993), a 2767-nt RNA2 (accession LC108994), and a 1956-nt RNA3 (accession LC108995). On the positive-sense strand of RNA1, the sequence beginning with the ATG codon at position 51 and ending with a TAG at position 3219 encodes a 120-kDa protein (ORF1). Similarly, on the positive-sense strand of RNA2, the sequence beginning with the AUG codon at position 59 and ending with a UAG at position 2600 encodes a 97-kDa protein (ORF2). On the positive-sense strand of RNA3, the sequence beginning with the AUG codon at position 148 and ending with a UAG at position 988 encodes a 31-kDa protein (ORF3), while the sequence beginning with the AUG codon at position 1136 and ending with a UAG at position 1793 encodes a 25-kDa protein (ORF4).
Protein ORF1 (1056 aa) contained conserved methyltransferase and helicase domains, whereas ORF2 (847 aa) contained a conserved RNA polymerase domain. ORF3 (280 aa) is thought to be a movement protein MP and ORF4 (219 aa) to be a coat protein (CP) (Fig. 1).
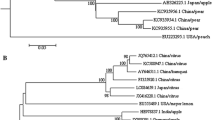
Comparison of the amino acid sequences for protein ORF4 (CP) and ORF2 (POL) between ApNMV and ilarviruses (Table 1) revealed 53 and 43% sequence identity of CP between ApNMV and PNRSV and between ApNMV and ApMV, respectively. Similarly, the highest amino acid sequence identity (64%) for POL was observed between ApNMV and PNRSV and between ApNMV and ApMV (Table 1). A phylogenetic analysis based on the CP amino acid sequence for ApNMV and other ilarviruses indicated that ApNMV is closely related to ApMV and PNRSV and belongs to genus Ilarvirus subgroup 3 (Fig. 3).
Phylogenetic tree based on the coat protein (CP) amino acid sequences of apple necrotic mosaic virus (P129) and viruses in the genus Ilarvirus spinach latent virus (SpLV) (accession U93194), asparagus virus 2 (AV-2) (accession X86352), CVV, elm mottle virus (EMoV) (accession U85399), citrus leaf rugose virus (CiLRV) (accession U17390), TAMV, lilac ring mottle virus (LRMV) (accession U17391), parietaria mottle virus (PMoV) (accession U35145), TSV, APLPV, ApMV, PNRSV, PDV, Fragaria chiloensis latent virus (FCILV) (accession AY707772), and Humulus japonicus latent virus (HJLV) (accession AY500238), viola white distortion associated virus (VWDaV) (accession GU168941), blackberry chlorotic ringspot virus (BCRSV) (accession GQ325716), tomato necrotic streak virus (ToNSV) (accession KT779206), strawberry necrotic shock virus (SNSV) (accession AY363229), privet ringspot virus (PrRSV) (accession KT290041), bacopa chlorosis virus (BaCV) (accession JQ015298), ageratum latent virus (AgLV) (accession no. JX463342), blueberry shock virus (BlShV) (accession KF031039), lilac leaf chlorosis virus (LLCV) (accession FN669169), and alfalfa mosaic virus (AMV) (accession K02703). The CP amino acid sequence of AMV was used as the outgroup. The tree was constructed using the neighbour-joining method with 1000 bootstraps. The number beside each node indicates bootstrap values
RT-PCR amplification of the CP region from four apple trees showing mosaic symptoms and their sequence analysis
Total RNA was extracted from the leaves of apple trees showing mosaic symptoms (P129, PK45, PK28, and P133), and the CP region was amplified by RT-PCR using ApNMV-specific and ApMV-specific primers (Fig. 4). For P129, PK45, and PK28, a DNA product of the expected size was amplified only in the case of RT-PCR using the ApNMV-specific primer. In contrast, in the case of RT-PCR using the ApMV-specific primer, amplified DNA was only observed for P133, and no amplified DNA was observed for P129, PK45, or PK28 (Fig. 4).
Detection of apple necrotic mosaic virus (ApNMV) and apple mosaic virus (ApMV) from apple trees showing mosaic symptoms by RT-PCR using primer pairs ApNMV-CP+1 and ApNMV-CP-1 for ApNMV and ApMV-CP+1 and ApMV-CP-1 for ApMV in Table 1. An arrow and arrowhead indicate the position of DNA products from ApNMV and ApMV amplification, respectively. M size makers, RT-PCR reverse transcription-polymerase chain reaction
Next, when the RT-PCR amplification products were sequenced and analysed, the amplified CP region for ApNMV from P129, PK45, and PK28, in all cases, consisted of 657 bases encoding 219 amino acids. The corresponding CP region of ApMV from P133 consisted of 669 bases encoding 223 amino acids. The nucleotide and amino acid sequences among the viruses infecting different trees showed strong sequence identity. Nucleotide identities between P129 and PK45, between P129 and PK28, and between PK45 and PK28 were found to be 94, 94, and 95%, respectively (Supplementary Table 3). The nucleotide sequence of the CP region amplified from P133 was found to show high sequence identity (88%) to ApMV (accession U15608). The nucleotide sequence identity between the CP extracted from P133 and that from P129, PK45, and PK28 was 56% (Supplementary Table 3). Similarly, a high degree of amino acid sequence identity was observed between P129 and PK45 (94%), between P129 and PK28 (95%), and between PK45 and PK28 (92%). The CP of the virus isolated from P133 showed 91% amino acid sequence identity with that of ApMV and 43–44% amino acid sequence identity with the CPs of viruses from the three other trees (P129, PK45, and PK28) (Supplementary Table 3).
Correlation between leaf mosaic and ApNMV detectability in apple trees with mosaic
In apple trees with leaf mosaic symptoms, symptom manifestation varied from branch to branch as described previously (Petrzik and Lenz 2011). On some branches, almost all leaves had pale-yellow spots or mosaic patterns, others had a mixture of symptomatic and asymptomatic leaves, and some had no symptomatic leaves at all. Thus, to clarify the relationship between mosaic symptoms and virus distribution, we collected 5 leaves per branch from branches with symptomatic leaves only, branches with a mixture of symptomatic and asymptomatic leaves, and branches with asymptomatic leaves from a mosaic-diseased apple tree (PK28), and the distribution of the virus was analysed using RT-PCR. As shown in Fig. 5, ApNMV was detected in all symptomatic leaves (branch nos. 1 and 2). In contrast, ApNMV was not detected or was only detected at extremely low levels in leaf samples from the branch with asymptomatic leaves only (branch nos. 5 and 6). In the case of the branch with both symptomatic and asymptomatic leaves, ApNMV was detected in all symptomatic leaves whereas it was detected in some asymptomatic leaves but not in others (Fig. 5). The above results demonstrate that the detectability of ApNMV in apple trees infected by mosaic disease varies from branch to branch and is correlated with the presence/absence of symptoms.
Distribution of ApNMV in leaves of different branches of an apple tree (P28) showing mosaic symptoms. Branch numbers are on left; on right, + mosaic present on all leaf samples from branch, – all leaf samples from branch were asymptomatic, +/– both symptomatic and asymptomatic leaves were sampled. White asterisks indicate asymptomatic leaf samples. Primer pair (ApNMV-CP+2 and ApNMV-CP-2) was used for RT-PCR. ApNMV Apple necrotic mosaic virus, M size makers, RT-PCR reverse transcription-polymerase chain reaction. H leaves from an uninfected tree, P control leaves with mosaic
Distribution of ApNMV in apple trees in Japan and China
ApMV was previously detected by ELISA in mosaic-diseased apple tree cvs. Fuji, Oorin, and Jonagold in several prefectures in Japan (Koganezawa and Bessho 1987; Nakatani, F., Iwate Plant Protection Association, personal communication; Ito, unpublished results). In this study, we also detected ApMV from all apple trees (cvs. Fuji, Kaori, Red Field, and Allington Pippin) showing mosaic symptoms in other fields. As mentioned above, ApNMV was detected in apple trees P129, PK28, and PK45 maintained in the virus repository orchard at the NARO Apple Research Station. As apple trees PK28 and PK45 were imported from China, we conducted a survey on the distribution of both viruses in mosaic-diseased apple trees (cvs. Fuji, Red Star, Gala, and Hua Niu) in major apple-growing areas (Shandong, Shaanxi, Shanxi, Gansu, and Beijing) of China using ApNMV-specific and ApMV-specific primers (Supplementary Table 1). As a result, ApNMV was detected at a high rate (228 positive trees of 276 trees tested, 82.6%) in trees showing mosaic symptoms, whereas ApMV was not detected from these trees at all. ApNMV was also detected in leaves without mosaic symptoms (37.35%); ApMV was again not detected.
Discussion
In this study, we investigated an apple tree with leaf mosaic (P129) that yielded a negative result on ELISA using an ApMV antibody, and performed next-generation sequencing analysis to comprehensively detect all viruses infecting the tree (Coetzee et al. 2010; Delwart 2007). The tree was infected with ASPV and ApLV (Foveavirus), ASGV (Capillovirus), and ACLSV (Trichovirus), as well as a novel virus (ApNMV) belonging to the genus Ilarvirus, which is closely related to PNRSV and ApMV. In Japan, ASPV, ASGV, and ACLSV are known to latently infect apple trees and are detected at high rates in apple trees that are asymptomatic (Magome et al. 1997; Menzel et al. 2002; Yanase 1974).
Next, the entire genomic structure of ApNMV was analysed using RT-PCR and 5′- and 3′-RACE based on contigs yielded by next-generation sequencing analysis. It was thought that the ApNMV genome may consist of three strands of RNA, of which RNA1 encodes a protein containing conserved methyltransferase and helicase domains, RNA2 encodes a protein containing a conserved RNA polymerase domain, and RNA3 encodes a MP and a CP (Fig. 2). This arrangement of the genome is consistent with viruses in the genus Ilarvirus (Bujarski et al. 2012; Sánchez-Navarro and Pallás 1994; Scott et al. 1998; Shiel et al. 1995; Shiel and Berger 2000). When the amino acid sequence for CP and POL of ApNMV were compared with those of other ilarviruses, the CP amino acid sequence identity between ApNMV and PNRSV was 43%, while between ApNMV and ApMV was 53%. For POL, the amino acid sequence identity between ApNMV and PNRSV and between ApNMV and ApMV was 64%. According to the International Committee on Taxonomy of Viruses (ICTV) (Bujarski et al. 2012), species demarcation criteria in the genus Ilarvirus in terms of sequence similarity have not yet been defined. However, phylogenetic analysis based on CP amino acid sequence clearly places ApNMV in the same subgroup 3 of the genus Ilarvirus as ApMV and PNRSV (Fig. 3). We therefore concluded that ApNMV is a novel virus that is distinct from those viruses.
Conclusive determination that ApNMV is a causal agent of apple mosaic disease will require confirmation of pathogenicity by back inoculation based on Koch’s postulates. However, given the strong correlation between the ApNMV distribution within a given tree and the presence/absence of mosaic symptoms on leaves (Fig. 5), the likelihood is high that ApNMV causes the mosaic symptoms therein. As a next step, we are planning to synthesize full-length cDNA for the entire ApNMV genome using RT-PCR and to transcribe ApNMV-RNA via in vitro transcription using this full-length cDNA as a template. Apple seedlings will then be inoculated with this transcribed RNA to determine the pathogenicity of ApNMV.
Until now, the causal agent of apple mosaic disease was believed to be ApMV (Petrzik and Lenz 2011). In fact, ApMV has been detected from apple trees in the fields of several prefectures in Japan. On the basis of the results of this study, however, not only ApMV, but also ApMNV induces mosaic disease in apple. Because the mosaic symptoms induced by both viruses were indistinguishable, it is reasonable to think that both viruses are causal agents of apple mosaic disease.
Apple mosaic disease has been reported throughout the world (Petrzik and Lenz 2011), so it will be very interesting to determine whether the causal agent in these various cases is ApMV or ApNMV, or potentially another virus(es). In recent deep sequencing and RT-PCR of mosaic-diseased apples in China, ApMV was not detected in any of 111 samples with mosaic symptoms and 29 samples with no visible symptoms (Liang et al. 2015). In our survey of apple trees with mosaic symptoms in major apple-growing areas of China based on RT-PCR using ApNMV- and ApMV-specific primers, ApNMV was detected in the vast majority (82.6%) of trees, whereas ApMV was not detected in any of the trees. As noted already, trees PK45 and P28 were imported from China. In contrast, ApMV has so far only been detected in apple trees cultivated in ordinary Japanese orchards with mosaic symptoms. It is possible therefore that the causal agent of apple mosaic disease differs by country and region. It will thus be necessary to investigate which virus is responsible for the apple mosaic disease that occurs in different apple-growing areas of the world.
References
Bradford FC, Joley L (1933) Infectious variegation in the apple. J Agric Res 46:901–908
Bujarski J, Figlerowicz M, Gallitelli D, Roossinck MJ, Scott SW (2012) Family Bromoviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. Academic Press, London, pp 965–976
Coetzee B, Freeborough MJ, Maree HJ, Celton JM, Rees DJ, Burger JT (2010) Deep sequencing analysis of viruses infecting grapevines: virome of a vineyard. Virology 400:157–163
Delwart EL (2007) Viral metagenomics. Rev Med Virol 17:115–131
Fukushi T, Tahama Y (1960) On apple mosaic. Mem Fac Agric Hokkaido Univ 3:116–123
Fulton RW (1972) Apple mosaic virus. CMI/AAB Description Plant Viruses No. 83. Association of Applied Biologists, Wellesbourne, UK
Gasic K, Hernandez A, Korban SS (2004) RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Rep 22:437–438
Grimová L, Winkowska L, Ryšánek P, Svoboda P, Petrzik K (2013) Reflects the coat protein variability of apple mosaic virus host preference? Virus Genes 47:119–125
Guo D, Maiss E, Adam G, Casper R (1995) Prunus necrotic ringspot ilarvirus: nucleotide sequence of RNA3 and the relationship to other ilarviruses based on coat protein comparison. J Gen Virol 76:1073–1079
Hammond RW, Crosslin JM (1998) Virulence and molecular polymorphism of Prunus necrotic ringspot virus isolates. J Gen Virol 79:1815–1823
Kanno Y, Yoshikawa N, Takahashi T (1993) Some properties of hop latent and apple mosaic viruses isolated from hop plants and their distributions in Japan. Ann Phytopathol Soc Jpn 59:651–658
Koganezawa H, Bessho H (1987) Detection of Apple mosaic virus by ELISA (in Japanese). Ann Phytopathol Soc Jpn 53:93
Liang P, Zhang Z, Liu F, Lu M, Li S, Wang H (2015) Problems of identification of pathogens associated with apple mosaic symptom and the exploration of its potential pathogens. Internat J Fruit Sci. doi:10.13925/j.cnki.gsxb.20150094
Magome H, Yoshikawa N, Takahashi T, Ito T, Miyakawa T (1997) Molecular variability of the genomes of capilloviruses from apple, Japanese pear, European pear, and citrus trees. Phytopathology 87:389–396
Menzel W, Jelkmann W, Maiss E (2002) Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J Virol Methods 99:81–92
Morris TJ, Dodds JA (1979) Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 69:854–858
Nemeth M (1986) Virus, mycoplasma and rickettsia diseases of fruit trees. Akademiai Kiado, Budapest
Petrzik K, Lenz O (2011) Apple mosaic virus in pome fruits. In: Hadidi A, Barba M, Candresse T, Jelkmann W(eds) Virus and virus-like diseases of pome and stone fruits. APS Press, St. Paul, MN, pp 25–28
Petrzik K, Vondrák J, Barták M, Peksa O, Kubešová O (2014) Lichens—a new source or yet unknown host of herbaceous plant viruses? Eur J Plant Pathol 138:549–559
Posnette AF, Cropley R (1956) Apple mosaic viruses. Host reactions and strain interference. J Hortic Sci 31:119–133
Sánchez-Navarro JA, Pallás V (1994) Nucleotide sequence of apple mosaic ilarvirus RNA 4. J Gen Virol 75:1441–1445
Scott SW, Zimmerman MT, Ge X (1998) The sequence of RNA 1 and RNA 2 of tobacco streak virus: additional evidence for the inclusion of alfalfa mosaic virus in the genus Ilarvirus. Arch Virol 143:1187–1198
Shiel PJ, Berger PH (2000) The complete nucleotide sequence of apple mosaic virus (ApMV) RNA 1 and RNA 2: ApMV is more closely related to alfalfa mosaic virus than to other ilarviruses. J Gen Virol 81:273–278
Shiel PJ, Alrefai RH, Domier LL, Korban SS, Berger PH (1995) The complete nucleotide sequence of apple mosaic virus RNA-3. Arch Virol 140:1247–1256
Yanase H (1974) Studies on apple latent viruses in Japan. Bull Fruit Tree Res Stn Jpn Ser C1:47–109
Yoshikawa N, Converse RH (1990) Strawberry pallidosis disease: distinctive dsRNA species associated with latent infectious in indicators and in diseased strawberry cultivars. Phytopathology 80:543–548
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 24380027.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noda, H., Yamagishi, N., Yaegashi, H. et al. Apple necrotic mosaic virus, a novel ilarvirus from mosaic-diseased apple trees in Japan and China. J Gen Plant Pathol 83, 83–90 (2017). https://doi.org/10.1007/s10327-017-0695-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-017-0695-x