Abstract
Helwingia, a shrub or small tree of four species distributed in East Asia, has been assigned to various families, mainly Cornaceae. However, molecular analyses show that the genus belongs to its own family Helwingiaceae which is sister to Phyllonomaceae (Phyllonoma only) in the order Aquifoliales. On the basis of H. japonica, we investigated the poorly understood floral and embryological characters of Helwingia, and compared their features with those of other Aquifoliales, particularly those of Phyllonomaceae. Results showed that perianth leaves of Helwingia represent sepals, because in plesiomorphic pentamerous flowers, they agreed in position with sepals (not with petals) in pentamerous flowers of Phyllonoma. Overall comparisons based on available information show that, while sharing with Phyllonoma the epiphyllous inflorescence, the inferior ovary, and an epigynous disc nectary as syapomorphies, Helwingia is characterized by loss of petals, obhaplostemony, large recurved stigmas, poorly developed disc nectary, tenuinucellate ovules with a mature female gametophyte filled with densely stained cytoplasm, and a thin mature seed coat. Morphological evidence, like molecular evidence, confirms that Helwingia is sufficiently distinct to be placed in its own family. Morphological and field observations suggest wind and insect pollination in H. japonica, which is the first example of ambophily in Aquifoliales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helwingia is a shrub or small tree of four species distributed from Himalaya to Japan (Hara and Kurosawa 1975; The Plant List 2013). The genus has been placed in various families such as Cornaceae (Cronquist 1981, 1988; Harms 1898; Hutchinson 1926; Lawrence 1951; Melchior 1964; Nakai 1909; Ohwi 1965; Rendle 1925; Wangerin 1910), Araliaceae (Bentham and Hooker 1862–1867; Eyde 1967; Hutchinson 1959, 1967, 1969, 1973), or its own family Helwingiaceae (Brummitt 1992; de Candolle 1868; Decaisne 1836; Takhtajan 1969, 1986, 1997, 2009; Thorne 1992). However, with the accumulation of molecular evidence (Morgan and Soltis 1993; Olmstead et al. 2000; Soltis and Soltis 1997), the Angiosperm Phylogeny Group (APG 1998; APGII 2003; APGIII 2009) has consistently accepted the monogeneric family Helwingiaceae in the asterid order Aquifoliales, while placing Cornaceae in Cornales and Araliaceae in Apiales (see also Reveal 2012; Stevens 2001 onwards). Within Aquifoliales, Helwingiaceae are sister to Phyllonomaceae (Phyllonoma only); a clade of Helwingiaceae and Phyllonomaceae is sister to Aquifoliaceae (Ilex only); and a clade consisting of the three families are sister to a clade of Cardiopteridaceae and Stemonuraceae (Soltis et al. 2011; Tank and Donoghue 2010).
Helwingia, like most of the other Aquifoliales, is poorly understood morphologically, particularly with respect to floral and embryological characters (Tobe 2012, 2013). As for floral characters, Horne (1914) described the general external morphology of flowers, with special reference to the number and positions of perianth leaves, stamens and stigmas in H. japonica (“H. ruscifolia”), and showed the vascular anatomy of several pistillate flowers. Eyde (1967) presented one micrograph of a transverse section of an ovary of H. japonica to show the vascular system. Tobe (2013) recently discussed on the basis of published data available that, although Helwingia shares an epiphyllous inflorescence and an inferior ovary with Phyllonoma, its (staminate and pistillate) flowers differ from those of Phyllonoma in having only a single whorl of perianth leaves. In Helwingia, perianth leaves always alternate with stamens in staminate flowers and with stigmas (or carpels) in pistillate flowers (Horne 1914). Given that perianth leaves are homologous in staminate and pistillate flowers, their positions correspond to those of petals in staminate flowers and to those of sepals in pistillate flowers. Bentham and Hooker (1862–1867); Harms (1898); Takhtajan (1997, 2009) described the calyx limb as obsolete (thus, perianth leaves representing the petals), whereas Horne (1914) and Tobe (2013) suggested that the petals, rather than the sepals, were lost. Which organs are missing in Helwingia flowers, sepals or petals? This question has long been reiterated without resolution.
Besides the aforementioned differences, a varying number of perianth leaves also distinguish Helwingia from Phyllonoma. In Phyllonoma, flowers usually have five or rarely four perianth leaves (Tobe 2013), whereas in Helwingia, flowers have three, four, or five perianth leaves (Bentham and Hooker 1862–1867; de Candolle 1868; Harms 1898; Horne 1914). Trimerous or tetramerous flowers are much more frequent than pentamerous ones in Helwingia, as stated later, so that the tetramerous (pistillate) flowers were investigated as representatives of the genus (e.g., Eyde 1967; Harms 1898; Horne 1914; Wangerin 1910). However, pentamerous flowers are rather common to all other Aquifoliales and therefore are plesiomorphic in Helwingia as well as in the entire order. Tobe (2012, 2013) showed that in both Cardiopteris (Cardiopteridaceae) and Phyllonoma, pentamerous flowers have one sepal in the abaxial position and one petal in the adaxial position. Such sepal and petal positions are generally unusual in asterids (Leins and Erbar 2005). What about in the pentamerous flowers of Helwingia? If we could determine that the perianth leaves in Helwingia agree in their positions with either the sepals or petals in Cardiopteris and Phyllonoma, then we may be able to determine the organs to which perianth leaves of Helwingia correspond? Analyses of pentamerous flowers of Helwingia will likely resolve this question concerning perianth leaves.
We must also determine whether the “disc” of staminate and pistillate flowers is nectariferous. The presence of the disc has been described inside the stamens in staminate flowers and outside of the stigmas in pistillate flowers (Bentham and Hooker 1862–1867; de Candolle 1868; Harms 1898; Wangerin 1910). Takhtajan (1997, 2009) described the disc as a nectary, but no evidence has been provided to indicate that it produces nectar. Tobe (2012, 2013) showed that both Cardiopteris and Phyllonoma have a disc nectary with nectarostomata scattered on its surface. We must examine disc nectary presence, and if present, how nectarostomata are distributed on its surface in Helwingia. The flowers of Helwingia are unisexual and have large, recurved stigmas. These floral features, in addition to the loss of one whorl of perianth leaves, suggest wind pollination in Helwingia (see Culley et al. 2002). Do flowers of Helwingia really have nectarostomata on the disc surface?
With respect to embryological characters, Horne (1914) described the ovule structure of Helwingia japonica, and Sato (1976) documented the development of the female gametophyte in H. japonica. Embryology is concerned with the development of anthers, ovules, and seeds, and can provide more than 50 characters for a better understanding of the relationships within and between families [Tobe 1989; for more recent examples, see Tobe 2011 for Leitneria (Simaroubaceae); Tobe and Raven 2011 for Irvingiaceae (Malpighiales): Yamamoto et al. 2014 for Biebersteiniaceae (Sapindales)]. Previous studies mainly concerned with the development of female gametophytes (Horne 1914; Sato 1976) compared Helwingia with Cornus (Cornaceae) and/or Aucuba (placed previously in Cornaceae and now in Garryaceae). We need information on the development of the anthers and seeds in Helwingia, and if available, additional information on ovules. On the basis of these embryological features, we need to compare Helwingia with other Aquifoliales.
The purpose of the present paper is primarily to present an analysis of floral morphology and anatomy of Helwingia, with the aim of answering the questions regarding perianth leaves and disc, and providing additional information on embryological characters. This study provides most of the information lacking on the reproductive characters of Helwingiaceae along with a basis for critical comparisons with other Aquifoliales. On the basis of the overall information available, we will discuss how floral and embryological characters have evolved in Aquifoliales and how Helwingia can be characterized morphologically in the order.
Materials and methods
Staminate and pistillate (or carpellate) flower buds and fruits of Helwingia japonica (Thunb.) F. Dietr. in various stages of development were collected from along the Inugai River, Kyoto, Japan (vouchers: Tobe 1336, 1337 KYO) from early April to late August of 2011–2014, and some additional flower buds from cultivated plants at the Kyoto Prefectural Botanical Garden, Kyoto. They were fixed in FAA (five parts stock formalin, five parts glacial acetic acid, 90 parts 50 % ethanol). For anatomical observations, resin sections were used. Some staminate and pistillate flower buds, a few young fruits, and several mature seeds were dehydrated through an ethanol series and then embedded in Technovit 7100 (Kulzer, Wertheim, Germany) for sectioning on a microtome. Serial resin sections cut at a thickness of 5 µm were stained with Heidenhain’s hematoxylin and mounted in Entellan (Merck, Darmstadt, Germany). For observations of the number of cells in mature pollen grains, some pollen grains collected from liquid-preserved flowers were stained by 1 % acetocarmine (Tobe and Raven 1984). All microtome sections and aceto-carmine-stained pollen grains were observed with an Olympus BX-51 microscope (Olympus, Tokyo, Japan).
Further, observations of the external morphology were made using a stereoscope and scanning electron microscope (SEM). For SEM-observations of flowers, specimens dehydrated through an ethanol series were critical-point dried in CO2 and coated with platinum. Observations were made using a Hitachi Miniscope TM-1000.
Results
Morphology and anatomy of staminate inflorescences and flowers
Inflorescences are cymose, and each inflorescence is borne at the middle part on the midvein of the adaxial surface of a leaf (Fig. 1a). It consists of one to ten or more staminate flowers. Each flower has a short pedicel, and is small and approximately 3.0–4.0 mm in diameter at the time of anthesis. As stated in the Introduction, staminate flowers, like pistillate flowers, have a single whorl of perianth leaves which vary in number from three to five. Irrespective of their number, perianth leaves always alternate with stamens (Fig. 1b). Perianth leaves are triangular in shape, 1–1.3 mm wide, 1–1.8 mm long, and are recurved at the anthesis. Although we did not examine their respective frequencies, trimerous flowers consisting of three perianth leaves and three stamens (Fig. 1b, c) appeared most abundant. Tetramerous flowers consisting of four perianth leaves and four stamens (Fig. 1d) were also common, but fewer than trimerous flowers, and pentamerous flowers consisting five perianth leaves and five stamens were rare or absent (observations in 2014). We found no pentamerous flowers on the two relatively large trees cultivated at the Kyoto Prefectural Botanical Garden, as well as on the three trees growing along the Inuigai River, Kyoto.
Staminate inflorescences and flowers of Helwingia japonica. a Inflorescence epiphyllous. b Flower at anthesis, viewed from top. Note three perianth leaves alternating with three stamens. c Transverse section (TS) of trimerous flower. d TS of tetramerous flower. e–h TSs of inflorescence consisting of a terminal flower and three lateral flowers. Sections were obtained along with a subtending foliage leaf at the levels through a pedicel of the terminal flower (e), the basal part of the terminal flower (f), and anthers of the terminal flower (g), and at a slightly upper level than the section g (h). Note that flower is pentamerous with two of five stamens fused laterally. The median perianth leaf is positioned on the abaxial side and the median stamen on the adaxial side. i Floral diagram of pentamerous flower, obtained from h. j Scannning electron micrograph (SEM) of tetramerous flower, viewed from top, showing disc nectary in the center. k SEM of part of disc nectary enlarged, showing dispersed nectarostomata. Arrowheads in c, d, and h indicate that lateral margins of perianth leaves overlap (thus, perianth leaves imbricate). dc disc nectary, FL foliage leaf subtending inflorescence, lf lateral flower, pl perianth leaf, st stamen, tf terminal flower. Scale bars are 1 cm in a, 1 mm in b, e and j, 200 µm in c, d and h, and 40 µm in k. Scale bar in e is applied to f and g
However, one of three inflorescences that we examined anatomically using transverse sections had a pentamerous flower at the terminal. Figure 1e–h show four serial selected transverse sections of a four-flowered inflorescence, which consists of a terminal flower (bud) and three lateral flowers (buds). The four sections were obtained through a pedicel of the terminal flower (Fig. 1e), the basal level of the terminal flower (Fig. 1f), the lower level of the anthers (Fig. 1g), and the middle level of the anthers (Fig. 1h). All four sections are shown with the adaxial side above and abaxial side below. The terminal flower has five perianth leaves and five stamens, two of which are basally united to each other (Fig. 1h). It is noteworthy that there is one perianth leaf in a median plane and the abaxial position and one stamen in median plane and the adaxial position in relation to the inflorescence-bearing axis. Figure 1i shows a floral diagram of the pentamerous flower.
On the basis of the anatomical observations of a few lateral flowers, as well as observations of some terminal and lateral flowers under the stereoscope, floral diagrams were obtained for trimerous and tetramerous flowers (not shown in figures). Both trimerous and tetramerous flowers appeared dimorphic with respect to the positions of the three or four perianth leaves in relation to the axis. In trimerous flowers, the median of the three perianth leaves is present in either the adaxial or abaxial position. In tetramerous flowers, two of the four perianth leaves are present in the median position, and two others in the transversal position, or two each of the four perianth leaves are present in the latero-adaxial and latero-abaxial position, respectively.
In trimerous, tetramerous, and pentamerous flowers, perianth leaves are often imbricate, at least partially. Of the three, four, or five perianth leaves, all are imbricate (Fig. 1c) or, one, two, or more are imbricate, and the rest are valvate (Fig. 1c, d, h). Staminate flowers often have a pistillode (or a rudimentary pistil) (Fig. 2a). However, the pistillode has neither distinct stigmas nor locules that are comparable with constituent carpels; therefore, we could not establish positional relationships between the stamens and carpels.
Development of anthers and microspores in staminate flowers of Helwingia japonica. a Longitudinal section of flower bud (a flower with pistillode). b Transverse section (TS) of anther in flower bud. c TS of young anther wall. Note that the middle layer has a common histogenetic origin with endothecium. d TS of young anther wall showing binucleate tapetal cells. e TS of older anther showing simultaneous cytokinesis in meiosis of microspore mother cells. f TS of further older anther with young pollen grains. g TS of mature anther. Arrowheads indicate positions of longitudinal slit. h TS of mature anther wall enlarged. i Two-celled mature pollen grains. ent endothecium, ep epidermis, gc generative cell, ml middle layer, mmc pollen mother cell, p pollen, pl perianth leaf, ps pistillode, st stamen, t tapetum, v nucleus of vegetative cell. Scale bars are 200 µm in a, 100 µm in g, 50 µm in b, f and h, 20 µm in c, d, and e, 10 µm in i
Staminate flowers have a disc that is expanded in a horizontal direction and is approximately 1.1–1.2 mm in diameter (Fig. 1j). Nectarostomata (or stomata in the nectary epidermis) are scattered sparsely on its surface for the release of nectar (Fig. 1k). On the basis of the two flowers examined by SEM, the density of the nectarostomata was 7 mm−2.
Development of anthers and microspores
Three to five stamens develop around the pistillode in staminate flowers (Fig. 2a). The anther is tetrasporangiate (Fig. 2b; see also developed anthers in Fig. 1c, d, h). Prior to maturation, the wall is composed of four cell layers: an epidermis, an endothecium, one middle layer, and a tapetum (Fig. 2c, d). The middle layer has a common histogenetic origin with the endothecium (Fig. 2c). Thus, wall formation conforms to the “Dicotyledonous” type (Davis 1966, p. 10). The tapetum is glandular (Fig. 2e, f). Its cells are initially uninucleate and later become binucleate (Fig. 2c, d). During maturation, the middle layer degenerates, and cells of both the epidermis and endothecium become enlarged (Fig. 2d, f). By the time of anther wall dehiscence, the cells of the endothecium have developed fibrous thickenings (Fig. 2g, h). The cells of the epidermis are enlarged and persist as they were (Fig. 2h). Anther dehiscence takes place by longitudinal slits, with each slit common to two microsporangia of the theca (Fig. 2g).
Meiosis in a microspore mother cell is accompanied by simultaneous cytokinesis (Fig. 2e), and the resultant microspore tetrads are predominantly tetrahedral. Pollen grains are two-celled when they are shed (Fig. 2i).
Morphology and anatomy of pistillate inflorescences and flowers
Inflorescences are basically cymose, mostly consisting of a solitary flower and rarely two or three flowers. Each inflorescence is borne at the middle part on the midvein of the adaxial surface of a leaf. Like staminate flowers, pistillate flowers have a short pedicel, and are small, being approximately 3.0–4.0 mm in diameter at the time of anthesis. They have a single whorl of perianth leaves which vary in number from three to five. Irrespective of their number, perianth leaves always alternate with stigmas (Fig. 3a). A pistil consists of an inferior ovary and very short styles terminated with stigmas. The ovary is three-, four-, or five-loculed with a single ovule in each locule and has three, four, or five stigmas, one each above the locule. Thus, each locule or stigma represents one carpel. Tetramerous flowers, each consisting of four perianth leaves and four stigmas (locules or carpels), are most abundant. In contrast, trimerous and pentamerous flowers, each consisting of three or five perianth leaves and three or five stigmas, are fewer. Of the 66 pistillate flowers collected at the Inugai River in 2012, ten were pentamerous, 53 tetramerous, and three trimerous.
Pistillate flowers of Helwingia japonica. a Flower at anthesis, viewed from top, with the adaxial side above. Note five perianth leaves alternating with five stigmas. b Longitudinal section of pentamerous flower before anthesis, obtained by sectioning through line b in g. c–i Transverse sections of pentamerous flower at levels marked c–i in b, showing the median perianth leaf to be positioned on the abaxial side and median carpel to be positioned on the adaxial side. j Floral diagram of pentamerous flower, obtained from b–i. k Scannning electron micrograph (SEM) of tetramerous flower viewed from top, showing the disc nectary in the center. l SEM of part of disc nectary enlarged, showing dispersed nectarostomata. Arrowheads in i indicate that lateral margins of perianth leaves overlap. dc disc nectary, FL foliage leaf subtending inflorescence or flower, mv midvein, ov ovule, pc pedicel, pcv vascular bundle of pedicel, pl perianth leaf, sg stigma. Scale bars are 2 mm in a, 1 mm in b, g and k, 40 µm in l. Scale bar in g is applied to c–f, h and i
Figure 3b shows a median longitudinal section of the pentamerous flower, which was obtained through a flower-bearing foliage leaf near the midrib. Figures 3c–l show transverse sections of the pentamerous flower through different levels marked c–i in Fig. 3b. The sections are shown with the adaxial side above and abaxial side below. When traced upward, a vascular bundle diverges from a midvein of the subtending foliage leaf to a pedicel (Fig. 3c, d), and becomes cylindrical at the upper level of the pedicel (Fig. 3e). At the basal part of the flower, ten discrete vascular bundles diverge from the vascular cylinder in the pedicel to the periphery, leaving vascular tissue in the center (Fig. 3f). Five of the ten vascular bundles in the periphery supply the five perianth leaves, and the remaining five supply the five carpels as dorsal carpellary bundles. No vascular bundles correspond to a missing whorl of perianth leaves or stamens. The transverse section through the middle level of an inferior ovary shows that one locule (or carpel) is positioned in a median plane and at the adaxial position (Fig. 3g). Likewise, we can trace one dorsal carpellary bundle present in the adaxial position toward the upper level of an inferior ovary (Fig. 3h) and up to the stigma (Fig. 3i). We see that the pentamerous pistillate flower has the median perianth leaf in the abaxial position and the median carpel in the adaxial position (Fig. 3i). It is noteworthy that the perianth leaves are imbricate in part (see arrowheads in Fig. 3i). Figure 3j shows a floral diagram of the pentamerous flower. The second author examined 22 pentamerous flowers on the trees growing along in the Inugai River in 2014, and found that 20 had perianth leaves on the same positions as in the floral diagram. For two, the positions of perianth leaves were uncertain because their pedicels were too elongate for exact determination.
On the basis of additional anatomical and stereoscopic observations, floral diagrams were obtained for tetramerous and trimerous flowers. Like those of staminate flowers, both the trimerous and tetramerous pistillate flowers were dimorphic with respect to the positions of the three or four perianth leaves (not shown in figures). They have the three or four perianth leaves at the same positions as those in staminate flowers. In trimerous flowers, some had one perianth leaf in the median and adaxial position, and others had one in the median and abaxial position. In tetramerous flowers, two of the four perianth leaves were present in the median position, and two others in the transversal position, or two each of the four perianth leaves were present in the latero-adaxial and latero-abaxial position, respectively.
In trimerous, tetramerous, and pentamerous flowers, perianth leaves are rarely or partially imbricate. Pistillate flowers have an epigynous disc that expands in a horizontal direction and is approximately 1.4–1.5 mm in diameter (Fig. 3k). Nectarostomata are sparsely scattered on its surface (Fig. 3l). Based on two flowers examined by SEM, the density of the nectarostomata was 7 mm−2.
Development of ovules and female gametophyte
In the pistil consisting of three to five carpels, a single ovule is pendant from the axial placenta in each carpel (Fig. 3b, g). An ovule primordium first grows downward (Fig. 4a). As it develops, it turns its apex toward the horizontal direction and eventually upward (Fig. 4b). At maturity an ovule is anatropous and epitropous with the nucellar apex above and the raphe on the dorsal (or abaxial) side.
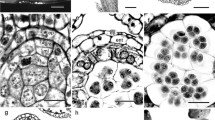
Development of ovules and female gametophytes in Helwingia japonica. a–f Longitudinal sections of pistils, presented with adaxial side of ovule on right. g and h Transverse sections of ovule, presented with adaxial side above. a Ovule primordium. b Ovule with megaspore mother cell. c Magnified view of ovule in b. d Ovule with two-nucleate female gametophyte. Note three degenerating megaspores (arrowheads) on micropylar side. e Ovule with eight-nucleate female gametophyte. An egg cell and two antipodals appear in adjacent sections. f Ovule with eight-nucleate female gametophyte at apical position of nucellus. g Ovule with immature female gametophyte. h Magnified view of a portion of g. ant antipodal cell, et endothelium, fg female gametophyte, it integument, mc megaspore mother cell, n nucleus in female gametophyte, nc nucellar tissue or nucellus, nep nucellar epidermis, po nucleus formed by fusion of two polar nuclei, rap raphe, sy synergid. Scale bars are 100 µm in b and g, 50 µm in a, f and h, 20 µm in c, d, and e
Early in development, the ovule has a one-celled archesporium differentiated beneath the apical dermal layer of the nucellus. The subsequent development of the archesporium follows the mode documented by Sato (1976). The archesporial cell differentiates directly into a megaspore mother cell (Fig. 4c). Thus, the ovule is tenuinucellate. Meiosis in the megaspore mother cell results in a linear tetrad of megaspores (Fig. 4d). While the three megaspores on the micropylar side degenerate, a megaspore on the chalazal side functions, successively developing into a two-nucleate (Fig. 4d), four-nucleate, and eventually eight-nucleate female gametophyte (Fig. 4e). Thus, the mode of female gametophyte development is of the monosporic Polygonum type. The mature female gametophyte is broadly rhomboidal to ellipsoidal, comprising an egg cell, two synergids, two polar nuclei, and three antipodal cells (Fig. 4e). It is always filled with a rich, densely stained cytoplasm (Fig. 4e, f). The three antipodal cells are ephemeral and disappear soon after fertilization.
Throughout the development of the female gametophyte, the apical dermal cells of the nucellus do not divide periclinally to form a nucellar cap (Fig. 4c, d). The apical dermal cells of the nucellus are destroyed by the developing female gametophyte and disappear, so that the upper half of the mature female gametophytes directly borders on the integument (Fig. 4e, f).
The nucellus is small early in development. At the megaspore mother cell stage, the nucellus has only a few cells below the megaspore mother cell (Fig. 4c). However, as the ovule develops, cell divisions rapidly proceed below the developing female gametophyte, forming a long, narrow cylindrical nucellar tissue on the chalazal side (Fig. 4f). No hypostase is formed (see chalazal region of a seed in Fig. 5a). The funicle is thick, but no obturator develops from the funicle.
Development of seeds and seed coats in Helwingia japonica. a–c, e and g Longitudinal sections of seeds. d and h Transverse sections of seeds. a Young seed with the primary endosperm nucleus. b Young seed with a zygote and a few endosperm cells. c Young seed with a two-celled proembryo and many endosperm cells. d Young seed with many endosperm cells. e Embryo in mature seed. f Mature fruit. Its transverse section is presented at lower right. g and h Mature seed coat. Mature seed viewed from lateral side is presented at lower left in h. ec endosperm cell, em embryo, en endosperm, ents endotesta, exts exotesta, nc nucellar tissue, pe proembryo, pt pollen tube, rap raphe, sc seed coat, se seed, z zygote. Scale bars are 1 cm in f, 2 mm in lower right of f, 700 µm in lower left of h, 200 µm in a, d and e, 100 µm in b, c, g and h
The ovule is unitegmic (Fig. 4b, c, g, h), as described by Horne (1914) and Sato (1976). The integument is multiplicative and is initially four cell layers thick. As the ovule develops, cells other than those of the outer epidermis further divide periclinally, so that the integument becomes more than eight cell layers thick in the nearly mature ovule (Fig. 4g, h). The thick integument forms a long micropyle above the female gametophyte. No vascular bundles differentiate in the integument (Fig. 4g, h). When the ovule reaches maturity, cells of the inner epidermis are more or less radially elongate and have cytoplasm stained relatively well, suggesting a weakly developed integumentary tapetum or endothelium (Fig. 4e, f).
Development of endosperm and embryo
Fertilization is porogamous. The pollen-tube is observed entering from the micropyle above the nucellar apex (Fig. 5a). Endosperm formation is of ab initio Cellular type. Division of the primary endosperm nucleus is accompanied by cell wall formation. Figure 5b shows a longitudinal section of a seed representing the earliest stage of endosperm development. The seed appears to have a two-celled endosperm plus a zygote. At this stage of development, the nucellar tissue still remains in the lower half. However, as the endosperm cells divide further, the whole tissue of the nucellus is replaced by a developing cellular endosperm (Fig. 5c, d). The mature seed has copious endosperm composed of cells that appear to have accumulated abundant lipids and protein (Fig. 5g, h).
Embryogenesis proceeds very slowly compared with the development of endosperm (Fig. 5c). We did not examine embryogenesis in detail, but fragmentary data on early and late embryogenesis indicated that it proceeds normally to form globular and dicotyledonous embryos. The embryo in mature seeds is small, approximately 1/17–1/18 of the length of the endosperm. It has two slightly developed dicotyledons in addition to a short suspensor (Fig. 5e).
Development of seed and seed coat
When ripened, fruits become black, spherical drupes approximately 7–9 mm in diameter (Fig. 5f). A mature seed in each locule is enclosed by a hard endocarp composed of sclerotic cells (Fig. 5f, below). The seed coat is multiplicative during the early development, particularly on the antiraphal side. In the earliest stage of endosperm development, the seed coat is approximately 20 cell layers thick on the antiraphal side and 12 cell layers thick on the lateral sides (Fig. 5a, d).
Mature seeds are straight and exarillate. They are approximately 5.0–6.0 mm long and 1.2–1.5 mm wide (measured from side to side), and have a thin seed coat and a copious endosperm. During seed development, cells of the thick seed coat in immature seeds mostly degenerate and disappear, except for those of an outer epidermis (exotesta) and an inner epidermis (endotesta) [terminology of the unitegmic seeds of Helwingia followed Schmid (1986)]. At maturity, cells of both the exotesta and endotesta are irregularly collapsed and remain as permanent layers (Fig. 5g, h). There is no specific cell layer that develops as the mechanical layer.
Discussion
Summary of features of floral morphology and embryology
Prior to our analyses, information on floral morphology and embryology of Helwingia was fragmentary, although some characters were described by Eyde (1967), Horne (1914), and Sato (1976). Our work on H. japonica provides most of the missing information and updates current concepts for several characters, particularly concerning perianth leaves. The overall information on the features of the floral morphology and embryology of Helwingia can be summarized as follows.
Both staminate and pistillate inflorescences cymose (plants dioecious) and epiphyllous; each consisting of one to ten or more staminate flowers, or usually one, rarely two or three pistillate flowers; both staminate and pistillate flowers having a single whorl of perianth leaves that vary in number from three to five; perianth leaves always alternating with stamens in staminate flowers or carpels (and stigmas) in pistillate flowers; in pentamerous flowers (although rare in staminate inflorescences) one perianth leaf present in the abaxial position; trimerous and tetramerous flowers dimorphic with respect to the positions of perianth leaves. In both staminate and pistillate flowers, perianth leaves often partially imbricate, partially valvate. In staminate flowers a pistillode present which has neither stigmas nor locules; in pistillate flowers an ovary inferior with three to five locules corresponding to constituent carpels; both staminate and pistillate flowers having a disc nectary (epigynous in pistillate flowers); nectarostomata sparsely scattered on its surface (density 7 mm−2).
Anther tetrasporangiate; anther wall four cell layers thick, its formation of the Dicotyledonous type; anther epidermis persistent; its cells enlarged; endothecium fibrous; middle layer degenerating; and tapetum glandular with binucleate cells. Cytokinesis in the microspore mother cell simultaneous; microspore tetrads predominantly tetrahedral; pollen grains two-celled when shed.
Ovule pendant from axile placenta, anatropous and epitropous dorsal at maturity, and tenuinucellate; archesporium hypodermal and one-celled. An archesporial cell differentiating directly into a megaspore mother cell, which undergoes meiosis resulting in a linear tetrad of megaspores; chalazal megaspore functional, developing into an eight-nucleate Polygonum type female gametophyte; mature female gametophyte filled with rich, densely stained cytoplasm; antipodal cells ephemeral. Apical nucellar epidermal cells degenerating, and thus the upper half of mature female gametophytes directly bordering on the integument; hypostase not differentiating; obturator absent. Ovule unitegmic; integument multiplicative, thickening to more than 12 cell layers thick, having no vascular bundles; long micropyle formed by thick integument; cells of inner epidermis weakly differentiating into endothelium (integumentary tapetum).
Fertilization porogamous; endosperm formation of ab initio Cellular type; mature seeds having lipids in copious endosperm. Embryogenesis uncertain; embryo in mature seed small, approximately 1/17–1/18 in length of endosperm. Mature seed straight and exarillate. Early in development, seed coat multiplicative, thickening into as thick as 20 cells layers thick (antiraphal side), but cells eventually crushed except for those of exotesta and endotesta; cells of exotesta and endotesta collapsed at maturity to remain as permanent layers.
Which organs have been lost in Helwingia flowers, sepals or petals?
The interpretation that Helwingia flowers have lost sepals rather than petals has been based on staminate flowers, in which perianth leaves alternate with stamens, as do petals in other asterids. Our analyses based on pentamerous flowers (which represent a plesiomorphic state within Helwingia) showed that the five perianth leaves have an identical arrangement in both staminate and pistillate flowers, with the median perianth leaf in the abaxial position in relation to the axis. Although only one staminate flower was analyzed because of extreme paucity, this observation shows that perianth leaves are equivalent or homologous in both staminate and pistillate flowers. Floral diagrams of such staminate and pistillate pentamerous flowers are presented for comparison in Figs. 1i, 3j, respectively. When compared with flowers of Phyllonoma [which are perfect and usually pentamerous, rarely tetramerous (Tobe 2013)], we noted that the five perianth leaves of Helwingia agreed in their positions with sepals, not petals, of Phyllonoma (Fig. 6). Accordingly, perianth leaves of Helwingia are not petals, but sepals in both staminate and pistillate flowers.
As for aestivation, perianth leaves of Helwingia have been described as valvate (Bentham and Hooker 1862–1867; de Candolle 1868; Takhtajan 1997, 2009; Wangerin 1910). Because a valvate calyx is rare in pentamerous flowers (Ronse de Craene 2010) and Phyllonoma has imbricate sepals and valvate petals (Tobe 2013), one might consider the “valvate” perianth leaves of Helwingia as representing petals. However, our analyses showed that perianth leaves were variable in aestivation, and these are often (at least partially) imbricate in H. japonica. According to Ronse de Craene (2008), a valvate calyx is usually associated with even-numbered or trimerous whorls. In Helwingia, tetramerous and trimerous flowers are more common than pentamerous flowers. The variable aestivation of perianth leaves in Helwingia may reflect a tendency to oligomery, i.e., from pentamery to trimery.
One might still wonder why perianth leaves of Helwingia, which we now understand to be the sepals, alternate with stamens in staminate flowers. This may merely show obhaplostemony. Horne (1914) discussed this subject about 100 years ago, stating that “If it were supposed that the flower was primitively ob- or diplostemonous, but has lost the inner or outer whorl of stamens, as the case may be, then perianth segments could be interpreted as sepals and the orientation of the whorls in both the male and female flower could be explained.” Euasterids (lamiids and campanulids) including Aquifoliales are characterized by haplostemony (Endress 2011), but obhaplostemony is widespread as a homoplasy in other eudicots (Ronse de Craene 2010; see also Ronse de Craene and Smets 1995). Ronse de Craene and Smets (1995) assert that obhaplostemony must necessarily be considered as derived from a diplostemonous ancestor. Among the four other aquifolialean families, Aquifoliaceae (sister to the clade of Helwingiaceae and Phyllonomaceae) have flowers with varying numbers of stamens (sepals, petals, and carpels) in one, two, or more whorls (Takhtajan 2009; Watson and Dallwitz 1992 onwards). It seems necessary to confirm the existence of diplostemonous flowers in Aquifoliaceae. Ronse de Craene and Smets (1995) further assert that the occurrence of obhaplostemony is often associated with loss of the petals (or the initiation of complex stamen–petal primordia). Helwingia has no petals in staminate and pistillate flowers. Although we did not investigate the early ontogeny of staminate flowers in Helwingia, their sepal primordia after initiation may leave no space for initiation of staminal primordia, except in the alternate position.
Character evolution in Helwingiaceae and other Aquifoliales
Aquifoliales are one of the 17 orders in asterids and are considered sister to the rest of the campanulids (Bremer et al. 2002; Kårehed 2001; Olmstead et al. 2000; Soltis et al. 2011). After pursuing a few key innovations of floral evolution in asterids in a phylogenetic context, Tobe (2013) discussed floral evolution in Aquifoliales based on analyses of available information (see also Tobe 2012). It can be summarized as follows: within Aquifoliales, Cardiopteridaceae and Stemonuraceae share a pseudomonomerous gynoecium composed of two carpels in median position (though the carpel position is uncertain in Stemonuraceae), and Phyllonomaceae and Helwingiaceae share an inferior ovary, an epiphyllous inflorescence, and an epigynous disc nectary. The disc nectary of Helwingia was previously confirmed by the presence of nectarostomata on the surface of the disc in pistillate flowers of H. japonica (Tobe 2013, unpublished data). We further confirmed that both staminate and pistillate flowers have nectarostomata on the disc surface. However, the density of nectarostomata in H. japonica is much lower than that in Phyllonoma. It is approximately 7 mm−2 in both staminate and pistillate flowers of H. japonica, but 46 mm−2 in P. tenuidens Pittier (Tobe, unpublished data). Such a low density of nectarostomata indicates that the disc nectary is less functional in H. japonica than in P. tenuidens. Tobe (2013) further reported that, while Phyllonomaceae have multicellular glandular trichomes on the lateral margins of the sepals and bicarpellate, unilocular gynoecium bearing many (six to seven) ovules per carpel on the parietal placenta as autapomorphies, Helwingiaceae show loss of petals (confirmed in the present study) as an autapomorphy. The present study of H. japonica has further shown that Helwingia is characterized by obhaplostemony.
The flowers of Helwingiaceae appear to be highly specialized as compared with those of Phyllonomaceae and other Aquifoliales. Loss of the petals, combined with dioecy, large recurved stigmas, less functional disc–nectary, and the number of staminate flowers higher than that of carpellate flowers per leaf appear to indicate an adaptation of the Helwingia flowers to wind pollination (for characters associated with wind pollination, see Culley et al. 2002). This observation contrasts with flowers of Phyllonoma, which are perfect and have simple stigmas and disc nectaries with more numerous macrostomata (Tobe 2013), and which are obviously pollinated by insects. Nonetheless, the second author has seen insects visiting staminate and pistillate flowers of H. japonica and bees visiting flowers of Ilex cornuta Lindl. et Paxt. (Aquifoliaceae) during field observations in 2014. In Helwingiaceae, ambophily (a combination of both wind and insect pollination) may have evolved as a transitional state from entomophily to anemophily, although this speculation must be confirmed by intensive studies in H. japonica and other species. Ambophily is rare in angiosperms and is known in species of certain families such as Arecaceae, Caryophyllaceae, Plantaginaceae, and Salicaceae (Clifford 1962; Culley et al. 2002). We expect that the present study of morphological characters will draw attention to pollination mechanisms in Helwingiaceae and other Aquifoliales.
As for embryological characters, we presented information on almost all characters for Helwingia. They are presented in Table S1 (supplementary Table) along with information available for four other aquifolialean families. Data were obtained from Krach (1976), Mauritzon (1933), Takhtajan (2000), and van Tieghem (1898) for Phyllonomaceae; from Brewbaker (1967), Copeland (1963), Corner (1976), Herr (1959, 1961), Schürhoff (1921), and van Tieghem (1898) for Aquifoliaceae; from Fagerlind (1945) and Mauritzon (1936) for Cardiopteridaceae; and from Fagerlind (1945), Mauritzon (1936), and Padmanabhan (1961) for Stemonuraceae. With respect to Cardiopteridaceae (comprising five genera), some data is published for Cardiopteris, Citronella (=Villarestia), Gonocaryum, and Leptaulus. Among these genera, data on Cardiopteris have been removed from Table S1. Kong et al. (2002) reported that C. platycarpa Gagnep. has orthotropous, ategmic ovules with a monosporic eight-nucleate female gametophyte, in which an egg apparatus is positioned on the chalazal side. They are very likely autapomorphic and restricted to the genus, and accordingly are not useful for extensive comparisons among the families.
Except for Helwingiaceae, the other Aquifoliales (Aquifoliaceae, Cardiopteridaceae, Phyllonomaceae, and Stemonuraceae) are still poorly understood embryologically (see Table S1). Phyllonomaceae lack information on almost all embryological characters, except for mature seeds and seed coats; Cardiopteridaceae and Stemonuraceae have no data on seed and seed coat characters; and Aquifoliaceae and Cardiopteridaceae have no data on anther and microspore development. However, even at the present stage of knowledge, comparisons show that Helwingiaceae agree with the four other families in having anatropous, unitegmic ovules lacking nucellar tissue in the upper half of the ovule at maturity. These features can be found in many other families of other asterids. More data is needed from the four families for more critical comparisons with other asterids. Within Aquifoliales, Helwingiaceae appear to be distinct from other Aquifoliales in having tenuinucellate, instead of crassinucellate ovules (uncertain in Phyllonomaceae). Aquifoliaceae are currently described as having crassinucellate and tenuinucellate ovules (Takhtajan 2009; Watson and Dallwitz 1992 onwards). The occurrence of tenuinucellate ovules in addition to crassinucellate ovules is likely based on the observations of Herr (1959) who described ovule development in four species of Ilex. He reported that in all of the four Ilex species examined, i.e., I. opaca Ait., I. crenata Thunb., I. cornuta, and I. verticillata (L.) Gray, a hypodermal archesporial cell may differentiate directly into a megaspore mother cell (i.e., ovule tenuinucellate) or may divide to form several parietal (“sporogenous”) cells (i.e., ovule crassinucellate). To the best of our knowledge, no evidence has been provided, for instance, with micrographs to show that both the crassinucellate and tenuinucellate ovules occur in the same species of any other angiosperms and it may be necessary to reassess Herr’s observations. Here, we treated the ovules of Aquifoliaceae (Ilex) as crassinucellate, because Herr (1959) states that crasinucellate ovules are typical and because Copeland (1963) reported the ovules of I. aquifolium L. and I. cornuta to be crassinucellate.
Helwingiaceae may be characterized further by having the mature female gametophyte filled with a rich, densely stained cytoplasm and a thin mature seed coat composed of collapsed cell layers. Although its functional aspect is uncertain, the rich cytoplasm filling the mature female gametophyte is not known in other Aquifoliales (though uncertain in Phyllonomaceae). The thin seed coat is evidently associated with the development of the thick, hard endocarp in drupes of Helwingia. Ilex (Aquifoliaceae), sister to the clade of Helwingiaceae and Phyllonomaceae, also has drupes, but its seed coat is exotestal and composed of cuboidal or shortly longitudinally elongated cells with lignified walls (Corner 1976). Phyllonoma (Phyllonomaceae), sister to Helwingia, has berries, and its mature seed coat is exotestal and composed of hard radially elongated, thick-walled cells (Takhtajan 2000). Therefore, Helwingia is clearly different from Ilex and Phyllonoma in seed coat structure. In contrast, Cardiopteridaceae and Stemonuraceae (sister to each other) are likely characterized by having a single vascular bundle running in the integument; a characteristic unknown elsewhere in Aquifoliales (though uncertain in Phyllonomaceae).
Available information on floral and embryological characters thus corroborates molecular evidence (Morgan and Soltis 1993; Olmstead et al. 2000; Soltis et al. 2011; Soltis and Soltis 1997; Tank and Donoghue 2010), showing that Helwingia is sufficiently distinct to be placed in its own family. Overall, like floral characters, at least a few embryological characters appear to have evolved in Helwingiaceae and other Aquifoliales. However, additional studies are required for further critical comparisons.
References
APG (Angiosperm Phylogeny Group) (1998) An ordinal classification of the families of flowering plants. Ann Missouri Bot Gard 85:531–553
APGII (Angiosperm Phylogeny Group II) (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
APGIII (Angiosperm Phylogeny Group III) (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Bentham G, Hooker JD (1862–1867) Araliaceae. Genera plantarum. Reeve Co., London, pp 931–947
Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, AndErbarg AA, Källersjö M, Barkhordarian E (2002) Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Mol Phyl Evol 24:274–301
Brewbaker JL (1967) The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in angiosperms. Am J Bot 54:1069–1083
Brummitt RK (1992) Vascular plant families and genera. Royal Botanic Gardens, Kew
Clifford HT (1962) Insect pollination of Plantago lanceolata L. Nature 193:196
Copeland HF (1963) Structural notes on hollies (Ilex aquifolium and I. cornuta, family Aquifoliaceae). Phytomorphology 13:455–464
Corner EJH (1976) The seeds of dicotyledons. Cambridge University Press, Cambridge
Cronquist S (1981) An integrated system of classification of flowering plants. Columbia University Press, New York
Cronquist S (1988) The evolution and classification of flowering plants. The New York Botanical Garden, Bronx
Culley TM, Weller SG, Sakai AK (2002) The evolution of wind pollination in angiosperms. Trends Ecol Evol 17:361–369
Davis GL (1966) Systematic embryology of flowering plants. Wiley, New York
Decaisne J (1836) Remarques sur les affinités du genre Helwingia, et établissement de la famille des Helwingiacées. Ann Sci Nat Bot 6:65–76
de Candolle A (ed) (1868) Prodromus systematis naturalis regni vegetabilis. Sumptibus Sociorum Treuttel et Würtz, Paris
Endress PK (2011) Evolutionary diversification of the flowers in angiosperms. Am J Bot 98:370–396
Eyde HR (1967) The peculiar gynoecial vasculature of Cornaceae and its systematic significance. Phytomorphology 17:172–182
Fagerlind F (1945) Bau des Gynöceums, der Samenanlage und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. Svensk Bot Tidskr 1945:346–364
Hara H, Kurosawa S (1975) A revision of the genus Helwingia. Bull Univ Mus Univ Tokyo 8:393–413
Harms H (1898) Cornaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien. III-8:250–270. Wilhelm Engelmann, Leipzig
Herr JM Jr (1959) The development of the ovule and megagametophyte in the genus Ilex L. J Elisha Mitchell Sci Soc 75:107–128
Herr JM Jr (1961) Endosperm development and associated ovule modifications in the genus Ilex L. J Mitchell Soc 77:26–32
Horne AS (1914) A contribution to the study of the evolution of the flower, with special reference to the Hamamelidaceae, Caprifoliaceae and Cornaceae. Linn Soc Trans, 2nd ser. Bot 8:239–309
Hutchinson J (1926) The families of flowering plants. I. Dicotyledons. Macmillan and Co., London
Hutchinson J (1959) The families of flowering plants. Vol. I. Dicotyledons, vol I. Clarendon Press, Oxford
Hutchinson J (ed) (1967) The genera of flowering plants (Angiospermae). Dicotyledons. Vol II. Clarendon Press, Oxford
Hutchinson J (ed) (1969) Evolution and phylogeny of flowering plants. Dicotyledons: facts and theory. Academic Press, London
Hutchinson J (1973) The families of flowering plants : arranged according to a new system based on their probable phylogeny, 3rd edn. Clarendon Press, Oxford
Kårehed J (2001) Multiple origin of the tropical forest tree family Icacinaceae. Am J Bot 88:2259–2274
Kong DR, Peng H, Liang HX (2002) A new type of embryo sac in Cardiopteris and its systematic implication. Acta Bot Sinica 44:496–498
Krach JE (1976) Samenanatomie der Rosifloren. I. Die Samen der Saxifragaceen. Bot Jahrb Sys 97:1–60
Lawrence GHM (1951) Taxonomy of vascular plants. Macmillan Publishing Co., New York
Leins P, Erbar C (2005) Floral morphological studies in the South African Cyphia stenopetala Diels (Cyphiaceae). Int J Plant Sci 166:207–217
Mauritzon J (1933) Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. Håkan Ohlssons, Lund
Mauritzon J (1936) Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. Svensk Bot Tidskr 30:541–550
Melchior H (1964) Umbelliferae. In: Melchior H (ed) A Engler’s Syllabus der Pflanzenfamilien. Gebrüder Borntraeger, Berlin, pp 367–379
Morgan DR, Soltis DE (1993) Phylogenetic relationships among members of the Saxifragaceae sensu lato based on rbcL sequence data. Ann Missouri Bot Gard 80:631–660
Nakai T (1909) Cornaceae in Japan. Bot Mag (Tokyo) 23:35–45
Ohwi J (1965) Flora of Japan. Smithsonian Inst, Washington
Olmstead RG, Kim KJ, Jansen RK, Wagstaff SJ (2000) The phylogeny of the Asteridae sensu lato based on chloroplast ndhF gene sequences. Mol Phyl Evol 16:96–112
Padmanabhan D (1961) A contribution to the embryology of Gomphandra polymorpha. Proc Natl Inst Sci India B27:389–398
Rendle AB (1925) The classification of flowering plants. Vol II. Dicotyledons. Cambridge University Press, Cambridge
Reveal JL (2012) An outline of a classification scheme for extant flowering plants. Phytoneuron 37:1–221
Ronse De Craene LP (2008) Homology and evolution of petals in the core eudicots. Syst Bot 33:301–325
Ronse De Craene LP (2010) Floral diagrams. An aid to understanding flower morphology and evolution. Cambridge University Press, Cambridge
Ronse De Craene LP, Smets E (1995) The distribution and systematic relevance of the androecial character oligomery. Bot J Linn Soc 118:193–247
Sato Y (1976) Embryological studies of some cornaceous plants. Sci Rep Tohoku Univ Ser IV 37:117–130
Schmid R (1986) On cornerian and other terminology of angiospermous and gymnospermous seed coats: historical perspective and terminological recommendations. Taxon 35:476–491
Schürhoff PN (1921) Die Entwicklungsgeschichte von Ilex aquifolium. Ber Deutsch Bot Ges 39:377–379
Soltis DE, Soltis PS (1997) Phylogenetic relationships in Saxifragaceae sensu lato: A comparison of topologies based on 18S rDNA and rbcL sequences. Am J Bot 84:504–522
Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Matvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu YL, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot 98:704–730
Stevens PF (2001 onwards) Angiosperm phylogeny Website. Version 12, July 2012. http://www.mobot.org/MOBOT/research/APweb/ [accessed June 20, 2014]
Takhtajan A (ed) (1969) Flowering plants, origin and dispersal (Translation from the Russian by C Jeffrey). Oliver and Boyd, Edinburgh
Takhtajan A (1986) Floristic regions of the world. (Translation from the Russian by TJ Crovello). University of California Press, Berkeley
Takhtajan A (1997) Diversity and classification of flowering plants. Columbia University Press, New York
Takhtajan A (2000) Family Dulongiaceae. In: Anatomia seminum comparative. Tomus 6, Dicotyledones. Rosidae II. Nauka, Leningrad, pp 270–274
Takhtajan A (ed) (2009) Flowering plants, 2nd edn. Springer, New York
Tank DC, Donoghue MJ (2010) Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Syst Bot 35:425–441
The Plant List (2013) Version 1.1. Published on the Internet; http://www.theplantlist.org/. Accessed 1st January)
Thorne RF (1992) Classification and geography of the flowering plants. Bot Rev (Crawfordsville) 58:225–348
Tobe H (1989) The embryology of angiosperms: its broad application to the systematic and evolutionary study. Bot Mag (Tokyo) 102:351–367
Tobe H (2011) Embryological evidence supports the transfer of Leitneria floridana to the family Simaroubaceae. Ann Missouri Bot Gard 98:277–293
Tobe H (2012) Floral structure of Cardiopteris (Cardiopteridaceae) with special emphasis on the gynoecium: systematic and evolutionary implications. J Plant Res 125:361–369
Tobe H (2013) Floral morphology and structure of Phyllonoma (Phyllonomaceae): systematic and evolutionary implications. J Plant Res 126:709–718
Tobe H, Raven PH (1984) The number of cells in the pollen of Melastomataceae (Myrtales). Bot Mag (Tokyo) 97:131–136
Tobe H, Raven PH (2011) Embryology of the Irvingiaceae, a family with uncertain relationships among the Malpighiales. J Plant Res 124:577–591
van Tieghem P (1898) Structure de quelques ovules et patti qu’on en pent tirer am liorer la classification. J Bot (Morot) 12:197–220
Wangerin W (1910) Cornaceae. In: Engler A (ed) Das Pflanzenreich. Wilhelm Engelmann, Leipzig
Watson L, Dallwitz MJ (1992 onwards) The families of flowering plants: descriptions, illustrations, identification, and information retrieval. Ver 22nd April 2014. http://delta-intkey.com
Yamamoto T, Vassiliades DD, Tobe H (2014) Embryology of Biebersteinia (Biebersteiniaceae, Sapindales): characteristics and comparisons with related families. J Plant Res 127:599–615
Acknowledgments
We are grateful to staff members of Kyoto Prefectural Botanical Garden for their assistance in collecting materials used in this study and to Tomoki Kadokawa for his assistance in preparing some figures used in the present article. The study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25440208).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ao, C., Tobe, H. Floral morphology and embryology of Helwingia (Helwingiaceae, Aquifoliales): systematic and evolutionary implications. J Plant Res 128, 161–175 (2015). https://doi.org/10.1007/s10265-014-0672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-014-0672-9