Abstract
Senegalia was recently described as non-monophyletic; however, its sections exhibit robust monophyletic support, suggesting a potential reclassification into separate genera—Senegalia sect. Monocanthea p.p. is the largest section. It contains 164 species of pantropical distribution and includes all of the current 99 neotropical species of Senegalia; however, no morphological characteristics are available to differentiate this section. To characterize this section, we examined floral developmental traits in four species of Senegalia sect. Monocanthea p.p. These traits were previously considered as potentially distinguishing features within Acacia s.l. and include the onset patterns of the androecium, the timing of calyx union, the origin of the staminal disc, and the presence of stomata on the petals. Furthermore, we analyzed previously unexplored traits, such as corolla union types, inflorescence development, and micromorphological features related to the indumentum, as well as the presence and location of stomata. The characteristics proposed as potential synapomorphies of the group include the postgenital fusion of the corolla and the presence of a staminal disc formed at the base of the filaments. The other analyzed floral characteristics were not informative for the characterization of the group. Future studies of floral ontogeny will help to establish more precise patterns, mainly whether corolla union and staminal tube formation occur similarly in African and Asian sections of Senegalia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acacia s.l. is a genus long known as non-monophyletic within the mimosoid clade (Leguminosae: Caesalpinioideae). It comprises more than 1450 species, among which a polyandrous androecium with > 30 free stamens is one of the most striking floral features (Koenen et al. 2020; Lewis 2005; Luckow et al. 2003). Currently, it is segregated into seven genera: Acacia Mill., Acaciella Britton & Rose, Mariosousa Seigler & Ebinger, Parasenegalia Seigler & Ebinger, Pseudosenegalia Seigler & Ebinger, Senegalia Raf. and Vachellia Wight & Arn. (Maslin et al. 2003; Miller and Seigler 2012; Seigler et al. 2006, 2017). The position of these segregate genera varies within the mimosoid clade. All of them, except Vachellia, are in the ingoid clade, part of the central mimosoid clade (Koenen et al. 2020). Senegalia is one of the most diverse and widespread genera of mimosoid clade, with 219 species having a pantropical distribution (Terra et al. 2017).
Recent analyses have confirmed that Senegalia is paraphyletic and that its monophyletic, well-supported sections will probably be treated as separate genera in the future (Koenen et al. 2020; Ringelberg et al. 2022; Terra et al. 2022). Within Senegalia, the clade “sect. Monacanthea p.p.” comprises around 164 species with a pantropical distribution, and all neotropical species (99 species) are positioned within this clade. The morphological features considered critical to distinguishing sections within the genus, like patterns of prickles and the inflorescence morphology, are no longer key for most species (Terra et al. 2022). Since floral ontogeny can reveal characters not present in fully developed structures, studying it can be valuable for characterizing the sect. Monacanthea p.p. (Maslin and Stirton 1997; Tucker 1992a).
Although floral morphology in Acacia s.l. seems uniform, there are variations in floral ontogeny traits. Analyses of floral development showed different types of calyx initiation (Ramírez-Domenech and Tucker 1990), distinct mechanisms of calyx and tubular corolla formation, as well as different pathways of androecium development (Buttrose et al. 1981; Derstine and Tucker 1991; Gómez-Acevedo 2021; Gómez-Acevedo et al. 2007; Pedersoli et al. 2023; Prenner 2011; Rico-Alvarado and Gómez-Acevedo 2022). Previous analyses proposed the patterns of androecium initiation, congenital and postgenital union of the perianth and androcecium, the origin of the staminal tube and petal stomata as potential traits for use in the delimitation of genera of Acacia s.l. (Gómez-Acevedo et al. 2007). However, no other study has analyzed these or other ontogenetic traits to characterize the taxa of the Acacia s.l. group. Therefore, a comparative analysis using these and other characteristics of floral ontogeny and anatomy could provide valuable information for the characterization of these groups.
This work aimed to analyze the morphology and ontogenetic sequence of inflorescences and flowers of four neotropical species of Senegalia belonging to the section Monacanthea p.p. (Ringelberg et al. 2022) to determine whether the union and initiation of calyx, the corolla union type, the presence of the staminal tube, and the distribution of stomata and trichomes can be important in the characterization of this section. Inflorescences and flower ontogenies were described, giving their potential to provide informative characters considering that they are little explored in mimosoids; the type of petal union was mainly studied in S. grandistipula and S. polyphylla. All results were compared with previous studies, especially those conducted in Acacia s.l.
Materials and methods
Study species
Four species were selected to cover the diversity of habit and external morphology of inflorescences and flowers Senegalia polyphylla (DC.) Britton, S. grandistipula (Benth.) Seigler & Ebinger, S. riparia (Kunth) Britton & Rose ex Britton & Killip and S. tubulifera (Benth.) Seigler & Ebinger (Table 1). The plant material of the four species was collected in different locations in Brazil, and the specimens were deposited in the HUFU and RB herbaria (at the University of Uberlândia and The Rio de Janeiro Botanical Garden).
Floral organography and development
Inflorescence and floral buds were collected and fixed in FAA 70% (formaldehyde-acetic acid-ethanol; Johansen 1940) and stored in 70% ethanol. Ten flowers of each species were dissected and described for organography using the Leica MZ 75 stereomicroscope. We counted the number of flowers in ten head-like inflorescence per species to determine the floral abundance per head-like inflorescence (refer to Fig. 1 to observe the head-like inflorescence). To define the structure of inflorescence, we used specimens from herbaria (Supplementary Data).
Inflorescence morphology of Senegalia. a Schematic diagram illustrating the inflorescence morphology of the four studied Senegalia species. b Fascicle of head-like inflorescences in S. tubulifera with a single bract on the peduncle. c Flower detail of S. tubulifera. d Fascicle of head-like inflorescences in S. riparia displaying a single bract on the peduncle. e Flower detail of S. riparia. f Fascicle of head-like inflorescences in S. grandistipula supporting two bracts on the peduncle. g Flower detail of S. grandistipula. h Fascicle of head-like inflorescences in S. polyphylla, exhibiting a peduncle without bracts. i Flower detail of S. polyphylla. The black arrows indicate the position of the first-order bracts. The white arrows indicate the position of the second-order bracts. Drawing: Marcus J. A. Falcão
For surface analyses under a scanning electron microscope (SEM), the materials were dissected, dehydrated in an ethanol series (Tucker 1993), critical point dried in a Bal Tec CPD 030 (AG, Liechtenstein–JBRJ-RJ) dryer, mounted on metal supports with carbon-coated adhesive tape and then covered with gold in an Emitech K550X (Ashford, UK—JBRJ-RJ). Dissection, measurement, dehydration, and metallization were performed at JBRJ, Rio de Janeiro, Brazil. Observations and images were obtained at CENABIO-UFRJ using a Zeiss EVO 10, and at the Centro Brasileiro de Pesquisas Fisicas-CBPF using a JEOL-JSM-6490LV scanning electron microscope at 15, 20, or 30 kV, all located in Rio de Janeiro, Brazil. The electron micrographs were processed using Adobe Photoshop CS5.
Anatomical study
For anatomical analyses (light microscopy), floral buds in pre-anthesis were gradually dehydrated in an ethanol series, embedded in historesin (Gerrits et al. 1991), and sectioned transversely (4–6 μm thick) using a rotary microtome (Leica RM 2245, Wetzlar, Germany). The sections were stained with 0.05% toluidine blue in phosphate buffer (pH = 6.8) (O’Brien et al. 1964) and mounted on water in temporary slides (Gerlach 1969). The anatomical sections were observed and photographed under a light microscope (Olympus BX50) coupled to a digital camera (Olympus DP73) with the scale bars under the same optical conditions. All steps were performed in the Rio de Janeiro Botanical Garden (JBRJ) and the Plant Micromorphology Laboratory at the Federal University of Rio Janeiro, Rio de Janeiro, Brazil.
Illustrations and terminology
The images were processed using Adobe Photoshop CS5. The nomenclature for the position and order of initiation of floral organs follows Tucker (1987) and Teixeira et al. (2014). The terminology for inflorescences follows Endress (2010) and Weberling (1992). The term “subunits of inflorescences” refers to the maximum branching level of the inflorescences, i.e. the racemes and spikes that support the flowers (Grimes 1999; Troll 1965; Weberling 1989). The term congenital union refers to when several organs of the same whorl develop as a ring wall, which may form a tubular structure in time. In contrast, postgenital union refers to when the free parts join during floral development after they have emerged (Endress 1994). The terminology for the type and extent of corolla connation follows Pedersoli et al. (2023); connation-coherence refers to when petals are connate in the basal portion and coherent in the mid and apical portion and complete coherence when petals are interlaced with papillae along their entire length. The initial stage of development refers to the set of stages from which it is possible to observe the floral symmetry, the order of initiation of the appendages, the order of initiation between whorls, the number and type of whorls, the number of organs per whorl and the possible omission of some organs. The intermediate stage begins after the initiation of the organs and mainly concerns the elongation of the organs. The late stage begins when the floral organs show cell specialization (Tucker 1997). Differentiation between bracts and bracteoles follows Tucker (1987) and Endress (1994).
Results
Organography of inflorescences
Synflorescences in the four species are composed of terminal and lateral panicles, with 30.39 ± 2.18 cm long in S. grandistipula, 40.38 ± 4.24 cm in S. polyphylla, 32.04 ± 1.69 cm in S. riparia and 18.79 ± 3.25 cm in S. tubulifera. The axes display alternately and helically inserted head-like subunits arranged in fascicles (Fig. 1a). Each fascicle is subtended by three bracts, one abaxial, and two laterals (first-order bracts). Multicellular trichomes grow on the peduncle of S. grandistipula (Figs. 1f and 2l), while in the other species, the peduncle has no trichomes. The peduncles of the head-like subunits bear two bracts in S. grandistipula (Fig. 1f), one in S. tubulifera (Fig. 1b) and in S. riparia (Fig. 1d), and none in S. polyphylla (Fig. 1h) (second-order bracts). The head-like subunits are spiciform in S. grandistipula (Fig. 1f) and capitate in S. polyphylla (Fig. 1h), S. riparia (Fig. 1d), and S. tubulifera (Fig. 1b). The number of flowers per head-like subunit of each inflorescence is 60–64 in S. grandistipula, 18–21 in S. polyphylla, 18–20 in S. riparia, and 20–23 in S. tubulifera. A single bract subtends each flower in each of the four species.
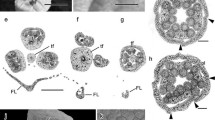
Organogenesis of inflorescences in Senegalia. a-d, S. polyphylla. f, g, h, l, S. grandistipula. e, i, S. riparia. J, K, S. tubulifera. a, e, f Lateral view of inflorescences primordia of S. polyphylla, S. riparia, and S. grandistipula. b Detail of the inflorescence primordium apex of S. polyphylla showing the inception of three bracts. c, g, i, j Indeterminate growth of inflorescence subunits in S. polyphylla, S. grandistipula, S. riparia, and S. tubulifera. The asterisk shows the apex of the inflorescence subunit. d, h, k Rounded or elliptical floral meristems before organ initiation in S. polyphylla, S. grandistipula, and S. tubulifera. (l) Detail of a group of primordia of inflorescence subunits forming a fascicle in S. grandistipula showing first and second-order bracts and multicellular trichomes on the peduncle (arrow). b = first order bract, b1 = second order bract, f = floral meristem, ped = peduncle, si = inflorescence subunit. Scale bars: a, d, e, g, h, k, = 100 μm; b, i = 20 μm; c, j = 50 μm; l = 200 μm
Organography of flowers
The floral symmetry is radial. The flowers are pedicellate in S. grandistipula (Fig. 1g) and sessile in S. polyphylla (Fig. 1i), S. riparia (Fig. 1e), and S. tubulifera (Fig. 1c). The calyx and corolla are gamosepalous and gamopetalous with free lobes in all four species. The base of the filaments is united, forming a staminal tube. The anthers are versatile with longitudinal dehiscence. The ovary is stipitate, and the style is curved, exceeding the height of the stamens. The gynoecium length (from the base stipe to the stigma) is about 8 mm long in S. grandistipula, 6.5 mm in S. polyphylla, 7 mm in S. riparia, and 6 mm in S. tubulifera. The merism and length of floral organs, and the distribution and type of indument on the floral organs vary among species (Table 2).
Organogenesis of inflorescences
Primordia of head-like inflorescence subunits emerge in an acropetal and helical order from the inflorescence apex along with the three first-order bracts that subtend them in S. polyphylla (Fig. 2a, b), S. riparia (Fig. 2e), and S. grandistipula (Fig. 2f). The new head-shaped inflorescence primordia emerge and cluster between the first order bracts forming fascicles. These inflorescences emerge asynchronously within each fascicle, but synchronously with other head-shaped inflorescences in other parts of the synflorescence. (Fig. 2a, e, f). In S. grandistipula, the second-order bracts protect each inflorescence head-like subunit in the early developmental stages (Fig. 2f, l). The formation of floral meristems in each head-like subunit is acropetal (Fig. 2c, g, i, j). The development of floral organs begins after all floral meristems are formed on the inflorescence subunit.
Floral organogenesis
The floral meristem is elliptic or rounded and subtended by a bract (Fig. 2d, h, k), and no bracteoles are initiated. The sepals in S. grandistipula, S. polyphylla, and S. tubulifera arise as individual primordia. In S. grandistipula the order of initiation is helical modified. The first to emerge is the middle adaxial sepal (Fig. 3a), followed by one of the abaxial sepals, the third is the next abaxial lateral sepal (Fig. 3b), the fourth is the adaxial lateral sepal, and the last is the next adaxial lateral sepal (Fig. 3c). Some flower buds showed variations of this initiation pattern (Fig. 3d-f). In S. polyphylla, the order of initiation of the sepals is erratic, with no discernible sequence (Fig. 3g-k). There are deviations from the calyx pentamery. Six sepals can emerge in S. grandistipula, (Fig. 4a), and four in S. polyphylla (Fig. 3l). The petals in S. grandistipula (Fig. 4a, b), S. polyphylla (Fig. 4c, d), and S. tubulifera (Fig. 4f), arise as individualized primordia simultaneously and alternate with the sepals. In S. polyphylla petal primordia initiates after a long plastochrone; at this stage, the enlarged sepals cover the floral meristem (Fig. 4g). The petal primordia emerge when sepals elongate in S. grandistipula (Fig. 4h) and S. tubulifera (Fig. 4i). There are variations in the corolla merism. Six petal primordia can emerge in S. grandistipula (Fig. 4a), and in S. polyphylla, four (Fig. 4c) or even seven petals (Fig. 4e).
Sepal initiation and elongation in Senegalia. a-f, m, S. grandistipula.g-l, n, S. polyphylla. O, S. tubulifera; the bracts were removed, and the abaxial side is positioned at the base in all the images. All images are presented from a polar view. a-c Development of sepals showing a helical modified order of initiation. All sepal primordia arise free. d Floral bud with reversed unidirectional initiation. e Floral bud with the first sepal in a lateral adaxial position. f Floral bud with the median sepal in the abaxial position. g, h Floral bud with the first sepal in an adaxial and lateral position, respectively. i Floral bud showing a reversed unidirectional order of initiation. j Floral bud with two lateral sepals emerged. k Floral buds with three sepals emerged. l Floral bud with four elongated sepals. m, n Flower bud, calyx closed by conspicuous papillose cells arising from the margins of the sepals. o Floral bud, calyx showing cochlear descending aestivation of sepals. b = bract, s = sepal. Scale bars: a, b, e, j, k, h, n = 20 μm; c, d, g, i, f, l = 50 μm; m = 100 μm; o = 200 μm
Petal initiation and elongation in Senegalia. a, b, h, J, S. grandistipula. c-e, g, k, S. polyphylla. L, S. riparia. f, i, S. tubulifera; the bracts and sepals were completely removed, and the abaxial side is always at the base. The images a-i, k and l are presented from a polar view, the image j is presented from a lateral view. a Floral bud with six sepals and six petals primordia. b, d, f Flower bud with five free petal primordia alternate with the sepals. c Floral bud with four free petal primordia. e Floral bud with seven free petal primordia. g-i Floral buds show differences in plastochrones between the beginning of petals and the development of sepals. j-l Floral bud, elongated free petals with papillae at the tip. p = petals, s = sepals. Scale bars: a, d = 20 μm; b, c, e, f, h, k = 50 μm; g, i, j, l = 100 μm
In S. grandistipula (Fig. 5a, b), S. polyphylla (Fig. 5c), S. riparia (Fig. 5d), and S. tubulifera (Fig. 5h), the androecium and gynoecium inception occur concomitantly and begin after petal elongation. The stamen primordia arise from a ring meristem, initially pentagonal and sectored into five parts by the emergence of stamen primordia, which arise in an antesepalous position and proliferate laterally and centripetally (Fig. 5a, d, f). The carpel primordium is differentiated as a circular dome in the central region of the floral meristem (Fig. 5b, d, f, h).
Androecium and gynoecium initiation and elongation in Senegalia. a-c, e, k, S. grandistipula. f, g, j, l, S. polyphylla. d, m, S. riparia. h, i, n, S. tubulifera. The bracts, sepals, and petals were completely removed in a-j. The abaxial side is at the base in a-j. The images a-j are presented from a polar view, and the images k-N are presented from a lateral view. a, d, f, h Floral bud showing an alternipetalous stamens initiation. The inception of the remaining stamens is in lateral and centripetal mode. Arrows show the direction of stamens initiation. The carpel inception is appreciated as a bulge at the center of the floral meristem. b Inception of the remaining stamen primordia. The carpel is completely delimited in the central region of the floral meristem. c, g, i Somewhat later stage shows the stamen elongation beginning for the antesepalous regions and the carpel cleft formation (arrow). e, j Lateral view of the inflorescence. The arrows indicate the carpel cleft orientation in each flower. k-n Longitudinal section of the floral bud shows the carpel cleft fusing (arrow). The asterisk shows filaments with different lengths. The anthers have the microsporangia completely differentiated. A = androecium, b = bract, c = Carpel, p = petals, rm = ring meristem, s = sepals. Scale bars: a, c, d = 20 μm; b, e, h, i, k-n = 100 μm; f, g, j = 50 μm
Middle and late stages of floral development
In the middle stage of development, the sepals enlarge and unite postgenitally, with lobes joined by interlacing epidermal papillae, forming a valvate aestivation in S. grandistipula (Fig. 3m) and S. polyphylla (Fig. 3n). In S. tubulifera, the sepal aestivation is imbricated with the adaxial sepal outermost (cochlear descending) (Fig. 3o). In the later stages of development, the calyx is opened by the elongation of the corolla, and the basal part remains united, forming a tubular calyx with free lobes in all Senegalia species (Fig. 6a, d, g, j). In S. grandistipula and S. riparia, the calyx of preanthetic flowers reaches approximately two-thirds of the corolla length (Fig. 6a, g); half the length of the corolla in S. polyphylla (Fig. 6d) and one-third of the corolla length in S. tubulifera (Fig. 6j). In S. grandistipula (Fig. 6a), non-glandular scattered trichomes are formed and cover the abaxial sepal surface concentrating mainly on the tips of the lobes of the sepals (Fig. 6c). In S. polyphylla (Fig. 6d), the abaxial sepal surface of preanthetic flowers is covered by stomata, non-glandular trichomes, and glandular trichomes (Fig. 6f, i). In S. riparia (Fig. 6g), the sepal abaxial surface is glabrous (Fig. 6l), and in S. tubulifera (Fig. 6j), the sepal abaxial surface is covered by non-glandular trichomes (Fig. 6n).
Late stage of development of sepals and petals in Senegalia. a-c, S. grandistipula. d-f, h, i, S. polyphylla. g, k, l, S. riparia. J, M, N, S. tubulifera. a, d, g, j Preanthetic flower, lateral view, calyx unite at the base with open aestivation. Protection of the androecium and gynoecium organs is by the valvately closed petals. b Magnification of the corolla showing the glabrous surface. c Magnification of the sepal surface showing non-glandular trichomes. e Inner surface of petal tip, frontal view, showing scattered stomata. f, i Magnification of the sepal surface showing stomata, non-glandular, and glandular trichomes. h Magnification of the corolla surface showing a dense layer of non-glandular trichomes. k, l Magnification of the calyx and corolla shows the glabrous surface. m Magnification of the calyx showing non-glandular trichomes on the sepal surface. n Magnification of the corolla showing non-glandular trichomes on the petal surface. Scale bars: a = 1 mm; b, h = 100 μm; c, f, k-n = 50 μm; d, j = 500 μm; e = 30 μm; g = 200 μm; i = 10 μm
In the middle stage of development, the petals enlarge and unite postgenitally, with lobes joined by interlacing epidermal papillae, forming a valvate aestivation in all Senegalia species (Figs. 4j-l and 6j). In the later stages of development, petals exceed the sepals in length, involving the androecium and gynoecium inside the floral bud in all Senegalia species (Fig. 6a, d, g, j). The abaxial surface of the petals is glabrous in S. grandistipula (Fig. 6a, b) and S. riparia (Fig. 6g, k), densely covered by non-glandular trichomes in S. polyphylla (Fig. 6d, h), and covered by scattered non-glandular trichomes in S. tubulifera (Fig. 6j, m). Stomata can occur at the tip of the inner surface of the petals in S. polyphylla (Fig. 6e). In S. grandistipula (Fig. 7a) the corolla union is postgenital, the apical portion is joined by papillate cells (Fig. 7b, c), while the middle (Fig. 7d, e) and basal parts of the petals are joined (Fig. 7f), forming a single tissue (connate-coherent type). In S. polyphylla (Fig. 7g) the postgenital union of the corolla is the type of full coherence, where the apical (Fig. 7h, i), median (Fig. 7j, kß), and basal portions are united by epidermal papillae (Fig. 7l, m).
Corolla union in S. grandistipula and S. polyphylla. a-f, Senegalia grandistipula. g-m, Senegalia polyphylla. a, g Floral bud, lateral view, representing the height of the anatomical sections shown. b Apical portion is united by marginal epidermal papillae (arrows). c Magnification of (b) shows the floral bud margins being closed by papillae (arrow). d The middle portion of the flower showing the corolla union forming a continuous tissue. The corolla regions with tissue fused are thinner (arrows). e Magnification of (d) shows a corolla region alternate to the sepals where the tissue is completely fused. f Basal portion of the flower with a fused corolla forms a single tissue. Scale bars: a, g = 1 mm; b-d, f, h-l = 50 μm; e, m = 200 μm
In all Senegalia species, the stamens elongate asynchronously, concomitant with their inception, and the differentiation of the anthers and filaments is synchronous (Fig. 5k-m); the stamens remain folded in preanthetic flowers (Fig. 8b, g, l, q); finally a bithecate dorsifixed anther with longitudinal dehiscence develops (Fig. 8c, d, h, i, m, n, r, s); and the base of the filaments becomes united forming a staminal tube (Fig. 8w-y, aa). In S. grandistipula, S. riparia, and S. tubulifera, the staminal tube has scattered stomata in the inner upper part (Fig. 8v, z, ab).
Late stage of development of androecium and gynoecium in Senegalia. a-e, v, w, S. grandistipula. f-j, x, S. polyphylla. k-o, y, z, S. riparia. p-t, aa, ab, S. tubulifera. a, f, k, p Gynoecium of preanthetic flower. The style is conspicuously folded, and the stipe is noticeable. b, g, l, q Androecium of preanthetic flower. The stamens remain conspicuously folded. e Inner surface of petal tip, frontal view, showing scattered stomata. c, d, h, i, m, n, r, s Dorsifixed and bithecate anthers of anthetic flowers showing longitudinal dehiscence. e, j, o, t Cup-shaped stigma of an anthetic flower. (u) Detail of the ovary surface showing scattered non-glandular trichomes and stomata (arrows). v, z, ab Detail of the upper portion of the staminal tube of an anthetic flower showing stomata. (w, x, y, aa) Longitudinal section of an anthetic flower with the flower base magnified showing the staminal tube. A = androecium, f = filament, G = ovary stipe, O = ovary, p = petals, st = staminal tube, sy = style. s = sepals. Scale bars: a, b, f, g, p = 500 μm; c = 100 μm; d, i, j, m, n, z, ab = 20 μm; e, h, o, r-v = 50 μm; k, l, q, w, x, y, aa = 200 μm
In S. grandistipula (Fig. 5c) and S. tubulifera (Fig. 5i), the carpel cleft forms after the stamen primordia have differentiated and begin to elongate. In contrast, in S. polyphylla (Fig. 5g) this differentiates before the ring meristem has fully differentiated into stamen primordia. In S. grandistipula, the orientation of the carpel cleft of each flower in the head-like inflorescence is variable (Fig. 5e), whereas in S. polyphylla is always oriented towards the adaxial side (Fig. 5j). In all Senegalia species, the carpel cleft closes (Fig. 5k-m), the style grows remaining folded in the apical part of the ovary in preanthetic flowers (Fig. 8a, f, k, p), and the stigma is cup-shaped (Fig. 8e, j, o, t). In S. grandistipula (Fig. 8a) and S. polyphylla (Fig. 8f) the ovary of preanthetic flowers is densely covered by non-glandular trichomes; in S. riparia is glabrous (Fig. 8k), and in S. tubulifera is covered by scattered non-glandular trichomes (Fig. 8p) and stomata (Fig. 8u).
Discussion
Inflorescences
All species of Senegalia we studied have inflorescences in terminal and lateral panicles that support subunits in head-like inflorescences, i.e., racemes with very short internodes. In mimosoids, such inflorescences are common; likewise, it is usual for the number of flowers per head-like inflorescences to vary among species (Derstine and Tucker 1991; Ramírez-Domenech and Tucker 1989; Stone et al. 1999; Tucker 1988). The asynchrony in the emergence of head-like inflorescences may be because in Leguminosae, perennial species, such as theSenegalia species studied by us, have axillary buds that often remain dormant until the next vegetative or flowering period. This would give rise to new head-like inflorescences from the same architecture as the previous flowering shoots (Weberling 1989).
In each inflorescence subunit, the organ initiation in each floral bud occurs only after the last flower primordium of that inflorescence subunit is formed. This character is typical of mimosoids, where each flower bud pauses its development after initiation until all flowers are initiated on the inflorescence subunit, so flower development on each raceme is synchronous (Tucker 2003a). Considering the synchrony in anthesis of each flower of each inflorescence subunit, they would be acting together to attract pollinators, and for this reason, they are considered the unit of pollination in mimosoids (Arroyo 1981; Harder et al. 2004).
Initiation and position of the perianth organs
In flowers of the mimosoid clade, it is possible to find the median sepal in the adaxial position (Tucker 2003a). Although this condition is common in this group, it can also be found in other Caesalpinioideae outside the mimosoid clade, such as Gleditsia (Tucker 1991), Ceratonia, and Erythrophleum (Tucker 1992a, b). This condition may be related to radial symmetry, as a bilateral symmetry in Leguminosae is not known with the median sepal in the adaxial position (Endress 1999; Sinjushin 2021). However, the merism’s instability can cause the median sepal’s position to present deviations, as observed in S. grandistipula (Fig. 4a), Inga grandis T.D. Penn., and I. hispida Schott ex Benth. (Paulino et al. 2017), or instability, as observed in Inga congesta T.D. Penn., Mimosa caesalpiniifolia Benth. and M. bimucronata (DC.) Kuntze. (Gonçalves et al. 2023). Likewise, some flowers of S. grandistipula have the middle sepal in the abaxial position (Fig. 3f), a feature already found in other mimosoids such as Inga grandis T.D. Penn., Pentaclethra macroloba (Willd.) Kuntze., Anadenanthera microsperma Teijsm &Binn, Parkia multijuga Benth., Stryphnodendron adstringens (Mart.) Coville. and Neptunia pubescens Benth. which also show meristic variation (Barros et al. 2017a, b; Paulino et al. 2017; Pedersoli and Teixeira 2016; Ramírez-Domenech 1989; Tucker 1988).
The erratic pattern of calyx initiation found in S. polyphylla has also been reported in the mimosoid Vachellia pennatula (= Acacia pennatula) (Schltdl. & Cham.) Seigler & Ebinger and Vachellia cornigera (= Acacia cornigera) (L.) Seigler & Ebinger (Gómez-Acevedo et al. 2007; Gómez-Acevedo 2021). Valvar estivation of the calyx is common in mimosoid (Tucker 1987); however, imbricated aestivations were reported in Pentaclethra macroloba (Willd.) Kuntze (Barros et al. 2017a, b), Adenanthera microsperma Teijsm. & Binn., Calliandra angustifolia Spruce ex Benth. (Prenner 2004a; Ramírez-Domenech and Tucker 1990), and in S. tubulifera, reported in this study. The different types of sepal initiation may show different developmental pathways by which radial symmetry arose in the mimosoid clade (Ramírez-Domenech and Tucker 1990).
The simultaneous petal initiation in S. grandistipula, S. polyphylla, S. tubulifera, and valvar estivation of the corolla in S. grandistipula, S. polyphylla, and S. riparia are common and stable characters that define the mimosoid clade (Ramírez-Domenech and Tucker 1990; Tucker 2003a). This pattern contrasts with the imbricate corolla estivation of the other groups of Leguminosae (Falcão et al. 2020; Kochanovski et al. 2018; Mansano et al. 2002; Prenner and Klitgaard 2008; Tucker 1996). The protective function in flowers is commonly associated with sepals and the floral attraction to petals; however, in some groups of plants, the petals can assume the protective function (Endress 2011). The protective whorls are relatively thicker, covered by tector and glandular trichomes, and are usually green. Also, the valvar estivation has been related to whorls with a protective function (Endress 2004, 2011). In many mimosoids, the flowers have colorful stamens that are the conspicuous floral whorl responsible for the floral attraction and, as in the case of the species of Senegalia studied, the floral protective function is assumed by the petals (Koenen et al. 2020).
Lability of perianth merism
Fluctuations in perianth merism are rare in core eudicots, where the pentamerous and tetramerous pattern predominates (Endress 2011; Ronse De Craene 2022). This is confirmed in mimosoid, where pentamerous and tetramerous flowers are more common (Tucker 2003a). However, merism changes within core eudicots have been reported in natural populations, being more common in families such as Styracaceae, Polemoniaceae, and Gentianaceae (Ronse De Craene 2016). Perianth merism lability in Leguminosae is comparatively an uncommon character; however, there are records for several clades, some examples are in the genus Acacia (Prenner 2011), Apuleia (Falcão et al. 2020), Calliandra (Prenner 2004a), Ceratonia (Tucker 1992b), Dialium (Marcus José de Azevedo Falcão Junior personal communication; Tucker 1998), Inga (Paulino et al. 2017), Lecointea (Mansano et al. 2002), Mimosa (Gonçalves et al. 2023), Mendoravia (Zimmerman et al. 2017), Parkia (Renan Siqueira Moraes personal communication), Stryphnodendron (Pedersoli and Teixeira 2016), Swartzia (Paulino et al. 2013) and Vachellia (Gómez-Acevedo et al. 2007).
This study adds two species having pentamerous flowers with a meristic variation of the merism within the same inflorescence: S. grandistipula, which can initiate six sepals and petals primordia, and S. polyphylla with four sepals and petals primordia. More specifically, changes in the perianth merism between five and six or seven sepals have already been reported for other mimosoids such as Calliandra angustifolia Spruce ex Benth. (Prenner 2004a) Inga congesta T.D. Penn., Inga feuillei DC., Inga grandis T.D. Penn., Inga hispida Schott ex Benth. (Paulino et al. 2017), Parkia platycephala Benth. Renan Siqueira Moraes personal communication), Stryphnodendron adstringens (Mart.) Coville (Pedersoli and Teixeira 2016), Mimosa bimucronata (DC.) Kuntze., Mimosa candollei R. Grether (Gonçalves et al. 2023), Neptunia pubescens Benth. (Tucker 1988), and Vachellia pennatula (Schltdl. & Cham.) Seigler & Ebinger (Gómez-Acevedo et al. 2007). On the other hand, perianth merism variation from five to four or fewer members, as observed in S. polyphylla, has been less reported, so far reported only in C. angustifolia (Prenner 2004a), Inga bella M. Sousa (Paulino et al. 2017), M. candollei and M. caesalpiniifolia (Gonçalves et al. 2023)d pubescens (Tucker 1988).
The propensity for meristic fluctuations in the mimosoid clade can be explained by the mechanical forces within flowers (Ronse De Craene 2016, 2018); however, floral evolution is also strongly influenced by pollination systems (Hodges and Arnold 1995; Xiang et al. 2023). Most groups with variations in the perianth merism have actinomorphic flowers (Gonçalves et al. 2023; Mansano et al. 2002; Paulino et al. 2017; Ronse De Craene 2016; Tucker 1999), which exhibit, in general, more generalist pollination systems relative to zygomorphic flowers (Buckhari et al. 2017; Fenster et al. 2004; Sinjushin and Karasyova 2017). Zygomorphy allows precision in the placement of pollen on the pollinator; therefore, variations in perianth merism in these flowers may alter the bilateral symmetry, making them non-functional or less attractive to pollinators (Citerne et al. 2010; Lázaro and Totland 2014). Meristic variation in flowers of highly congested inflorescences, such as in Senegalia and other mimosoids, would not affect attractiveness because they do not act as a pollination unit by themselves but the inflorescence as a whole (Harder et al. 2004).
Union of perianth organs in mimosoid
In this study, a postgenital union of the calyx and corolla in S. grandistipula, S. polyphylla, and S. tubulifera was evidenced, in which each primordium of sepals and petals initiates separately from the others, elongates, and subsequently unites with the adjacent members. Postgenital calyx unions have been mentioned in other mimosoids such as Acacia saligna (Labill.) H.L. Wendl. (Gómez-Acevedo et al. 2007), A. microsperma, Wallaceodendron celebicum Koord. (Ramírez-Domenech and Tucker 1990), Parkia multijuga Benth. and S. adstringens (Pedersoli and Teixeira 2016). Congenital calyx unions have been reported in Acacia celastrifolia Benth. (Prenner 2011), Acaciella angustissima (Mill.) Kuntze (Rico-Alvarado and Gómez-Acevedo 2022), Mimosa albida Humb. & Bonpl. ex Willd., Mimosa pigra L., Mimosa strigillosa Torr. & A. Gray (Ramírez-Domenech and Tucker 1990), S. berlandieri, V. pennatula (Gómez-Acevedo et al. 2007)d cornigera (Gómez-Acevedo 2021).
Sympetaly is a remarkable character in the mimosoid clade that differentiates them from other Leguminosae, where the corollas are mostly free (Lewis et al. 2005; Tucker 2003a). The corolla union in the mimosoid clade occurs mainly postgenitally (Pedersoli et al. 2023), with exceptions reported in A. saligna, V. cornigera, and V. pennatula (Gómez-Acevedo et al. 2007; Gómez-Acevedo 2021). Postgenital union of the corolla may occur via papillose cells or wall-to-wall junctions, which may involve the apical, middle, and/or basal parts (Pedersoli et al. 2023). In S. grandistipula, the corolla shows a connation-coherence type union, where connation occurs in the middle and basal part and coherence in the apical part by the action of papillose cells. In S. polyphylla, total coherence occurs, where the apical, middle, and basal parts are joined by papillose cells. Conjunction-coherence type unions have been reported in other mimosoid species: Abarema cochliacarpos (Gomes) Barneby & J.W. Grimes, Inga laurina (Sw.) Willd., Inga vera Kunth, P. multijuga, Pithecellobium dulce (Roxb.) Benth. and Samanea saman (Jacq.) Merr. (Pedersoli and Teixeira 2016; Pedersoli et al. 2023). Corollas with full coherence in the mimosoids: A. celastrifolia, Adenanthera pavonina L., Entada acaciifolia Benth., Mimosa artemisiana Heringer & Paula, P. macroloba, Piptadenia gonoacantha (Willd.) Kuntze, Stryphnodendron adstringens, S.rotundifolium var. rotundifolium Mart. and Tetrapleura tetraptera (Schumach. & Thonn.) Taub (Pedersoli and Teixeira 2016; Pedersoli et al. 2023). Within the ingoid clade, there is a tendency toward connate corollas, except for A. celastrifolia (Pedersoli et al. 2023; Prenner 2011), and the one reported in this study, S. polyphylla.
Androecium features
Polyandry can be produced by the action of ring meristems, which allow a prolongation of stamen production after carpel initiation, without depending on the apical meristem of the flower (Endress 2006; Kong and Becker 2021). The evolution of polyandry in eudicots is unclear; however, the diversity of ring meristems suggests recurrent emergence in evolution, probably produced by similar pollination environments (Kong and Becker 2021; Luckow et al. 2003; Wessinger and Hileman 2020). Similar developmental mechanisms control the development of the free multistaminate androcecium characteristic of Acacia s.l.; however, the underlying molecular mechanisms that generate and regulate ring meristems are unknown and need to be studied (Kong and Becker 2021; Luckow et al. 2003).
The order of emergence of individual stamen from the ring primordium in a centripetal sequence, as described here in S. grandistipula, S. polyphylla, and S. riparia, is common in the mimosoid clade (Ramírez-Domenech 1989), with exceptions reported in A. baileyana, which showed combined centrifugal and centripetal initiation (Derstine and Tucker 1991) and A. angustissima with a synchronous initiation of the stamen primordia, this latter is a novel developmental pattern in the mimosoid clade (Rico-Alvarado and Gómez-Acevedo 2022). In Leguminosae, polyandry is present in other groups such as in Detarioideae (Colophospermum, Maniltoa, Polystemonanthus) and Papilionoideae (Alexa, Swartzia, Bocoa, Cordyla) (Da Silva 2023; Sinjushin 2021). In Swartzioid (Papilionoideae), a ring meristem with centrifugal and centripetal initiation has been reported (Paulino et al. 2013; Tucker 2003b). Nevertheless, to date, there are no precise data on the developmental processes of the multi-staminate androcecium in all legume groups.
At the beginning, the ring meristems in S. grandistipula, S. polyphylla, and S. riparia appear sectored in five parts around the flower. The formation of these sectors is due to the delayed initiation of the stamen primordia that occurs on the sides of the pentagonal-shaped floral meristem. This delay is probably attributed to the pressure exerted by the petals on the ring meristem during the initial stages of development. Consequently, this pressure induces a temporal mismatch in the meristematic activity of the androecium, leading to the early proliferation of the stamen primordia located between the petal margins (Bull-Hereñu et al. 2022). Then, as the flower bud develops, the sectors are no longer evident. This is because the pressure exerted by the petals ceases to exist because the petals have elongated (Ronse de Craene 2018). This type of initiation is evident in studies of some mimosoid species with multistaminate androecia, such as A. baileyana (Derstine and Tucker 1991), A. saligna, S. berlandieri, V. cornigera, and V. pennatula (Gómez-Acevedo et al. 2007; Gómez-Acevedo 2021).
The formation of the staminal tube occurs through the union of the stamen bases as in P. multijuga (Pedersoli and Teixeira 2016) or by the growth of the receptacle below the bases of the stamens as in Calliandra houstoniana (Mill.) Standl. (Tucker 2003a). In the Senegalia species studied, the staminal tube was formed by the growth of the base of the filaments. The presence of the staminal tube is directly associated with pollination mechanisms because these can limit the range of floral visitors that can access the nectar that is normally produced and accumulated in the space between the tube and the pistil (Polhill 1981; Rodríguez-Riaño et al. 1999; Tucker 1987).
Gynoecium features
In S. grandistipula, S. polyphylla, and S. riparia, carpel initiation occurred before the initiation of all stamen primordia, a common condition in Leguminosae (Tucker 1987). The adaxial orientation of the carpel cleft is also usual in Leguminosae (Sinjushin 2021; Tucker 1987), with some exceptions reported in Caesalpinioideae: Ceratonia siliqua L. (Tucker 1992b), Gleditsia J. Clayton (Tucker 1991), A. baileyana (Derstine and Tucker 1991), A. saligna, S. berlandieri, V. pennatula (Gómez-Acevedo et al. 2007) and cornigera (Gómez-Acevedo 2021). Likewise, slight lateral carpel cleft deviations of the adaxial side have been previously described in some genera of Papilionoideae (Prenner 2004b). In this study, S. grandistipula also presented unusual orientations of the carpel cleft (abaxial and lateral). These erratic initiation patterns are associated with radially symmetric taxa (Sinjushin 2021; Tucker 1999).
Cell specializations
The presence, timing of formation, and distribution pattern of trichomes on the surfaces of sepals, petals, and ovaries were different among the Senegalia species analyzed here. These late-expressed characteristics in floral development, such as the size and shape of organs, distribution, and type of indumentum, tend to differentiate species of the same genus (Tucker 1992a, 1997). Intertwined papillate epidermal cells in the apical parts of petals and sepals in the middle and late stages of development are responsible for flower bud closure, a common corolla closure mechanism in mimosoids (Pedersoli et al. 2023). This has been proposed as a potential ontogenetic synapomorphy for the mimosoid clade (Gonçalves et al. 2023).
In Leguminosae, glandular trichomes on the surface of the sepals have been reported mainly in Caesalpinioideae and Papilionoideae (Marazzi et al. 2019). Likewise, secretory trichomes are located on the inflorescence axis, base of the floral receptacle, margins, and surfaces of bracts, bracteoles, sepals, and petals (Barros et al. 2017a, b). Floral trichomes and stomata can emit volatile compounds to repel herbivores or attract pollinators (Callow et al. 2000; Effmert et al. 2005). In S. polyphylla, glandular trichomes and stomata were found on the outer surface of the sepals, as well as stomata on the inner surface of the petal apex. Considering that petals in Leguminosae are the main sites of fragrance emission (Marinho et al. 2014) and that the sepals mainly have a protective function (Endress 1994; Ghazoul 2001), trichomes and stomata on the sepals could be more related to protection from herbivory, while the presence of stomata on the petals related to the release of fragrances to attract potential pollinators. However, it is necessary to analyze in detail the compounds produced by these trichomes and stomata to understand better their function in these flowers.
Stomata were found on the surface of the ovary in S. tubulifera. This feature has been reported in other angiosperms (Endress and Igersheim 1999) and legumes within Caesalpinioideae, mainly in pluricarpellar species of the mimosoid clade: Acacia celastrifolia, Archidendron glabrum (K. Schum.) K. Schum. & Lauterb., A. lucyi F. Muell., Inga bella, I. congesta, I. gereauana (Pipoly & Vásquez) T.D. Penn., I. grandis, I. hispida (Paulino et al. 2014; Prenner 2011). The presence of stomata on the surface of the carpel has been related to a photosynthetic function (Galen et al. 1993); however, they may also be associated with the release of volatiles (Effmert et al. 2005).
Nectar secretion in Acacia s.l. flowers is not common, and polyads are the main floral resource for floral visitors (mainly bees) (Ancibor 1969; Stone et al. 2003). Some species of Acacia s.l. with reports of nectar production are: Acacia zanzibarica (S. Moore) Taub., A. tortilis (Forssk.) Hayne, Senegalia brevispica (Harms) Seigler & Ebinger, S. mellifera (Vahl) Seigler & Ebinger, S. senegal (L.) Britton, S. berlandieri and Acaciella angustissima (Gómez-Acevedo et al. 2007; Rico-Alvarado and Gómez-Acevedo 2022; Stone et al. 2003;). In this study, S. grandistipula, S. riparia, and S. tubulifera showed stomata in the apical part of the staminal tube, which could indicate possible nectar secretion. Still, studies are needed to corroborate nectar production.
Ontogenetic traits and phylogenetic relationships in Acacia s.l.
Ontogenetic characters leading to flower formation in the segregate genera of Acacia s.l. show a high variation (see Table 3). The order of appearance of sepals, and the process of calyx and corolla union are the most variable characters. Calyx initiation patterns change even within the same inflorescence, which is rarely reported in other legume groups, i.e. in Astragalus (Derstine and Tucker 1991; Naghiloo et al. 2012). Some floral developmental characteristics that are specific for genera of Acacia s.l. could be the postgenital union of corolla in Senegalia, simultaneous initiation and congenital union of the calyx in Acacia, and an erratic initiation with congenital union of sepals and petals in Vachellia. The presence of a staminal tube was reported in Acaciella angustissima and Senegalia, and simultaneous initiation of stamen primordia only in Acaciella. Due to the large number of species in many of these clades, along with the small number of species studied, it remains difficult to associate developmental trends to specific genera, and further ontogenetic studies are needed.
Conclusions
The postgenital union of the corolla and the staminal tube may be typical characteristics of the Senegalia sect. Monacanthea p.p., however, is necessary to analyze if these characteristics also occur in the African and Asian sections Senegalia and Monocanthea s.s. Potential distinctive characteristics suggested to differentiate groups within Acacia s.l., such as the inception patterns of androecium and the presence of stomata in the petals, were not shown to be characteristic of all Senegalia species. Similarly, the mechanisms of postgenital union of the corolla vary among Senegalia species, indicating that this is not an informative character for the morphological characterization of the section., The results demonstrate the great diversity of ontogenetic pathways in the construction of the multistaminate flower in Senegalia sect. Monacanthea p.p. and the mimosoid clade. Further studies of floral anatomy and ontogeny in species of the different genera of Acacia s.l., as well as in the mimosoid clade, will help to establish patterns more precisely.
References
Ancibor E (1969) Los Nectarios florales en leguminosas. Darwiniana 15:128–142
Arroyo MK (1981) Breeding systems and pollination biology in Leguminosae. In: Polhill RM, Raven PH (eds) Advances in legume systematics, part 2. Royal Botanic Gardens, Kew, pp 723–769
Barros TC, Marinho CR, Pedersoli GD, Paulino JV, Teixeira SP (2017a) Beyond pollination: diversity of secretory structures during flower development in different legume lineages. Acta Bot Brasilica 31:358–373
Barros TC, Pedersoli GD, Paulino JV, Teixeira SP (2017b) In the interface of caesalpinioids and mimosoids: comparative floral development elucidates shared characters in Dimorphandra Mollis and Pentaclethra macroloba (Leguminosae). Am J Bot 104:218–232
Buckhari G, Zhang J, Stevens PF, Zhang W (2017) Evolution of the process underlying floral zygomorphy development in pentapetalous angiosperms. Am J Bot 104:1846–1856
Bull-Hereñu K, dos Santos P, Toni JFG, El Ottra JHL, Thaowetsuwan P, Jeiter J, De Ronse LP, Iwamoto A (2022) Mechanical forces in floral development. Plants 11:661
Buttrose MS, Grant WJR, Sedgley M (1981) Floral development in Acacia pycnantha Benth. In Hook. Aust J Bot 29:385–395
Callow JA, Hallahan DL, Gray JC (2000) Plant trichomes, vol 31. Academic, San Diego
Citerne H, Jabbour F, Nadot S, Damerval C (2010) The evolution of floral symmetry. Adv Bot Res 54:85–137
Da Silva GS, Torke BM, Mansano VDF (2023) Alexa Duckeana (Leguminosae-Papilionoideae): a new species from the Brazilian Amazon. Phytotaxa 629:255–265
Derstine KS, Tucker SC (1991) Organ initiation and development of inflorescences and flowers of Acacia baileyana. Am J Bot 78:816–832
Effmert U, Große J, Röse US, Ehrig F, Kägi R, Piechulla B (2005) Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae). Am J Bot 92:2–12
Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge
Endress PK (1999) Symmetry in flowers: diversity and evolution. Int J Plant Sci 160(S6):S3–S23
Endress PK (2004) Structure and relationships of basal relictual angiosperms. Austral Syst Bot 17:343–366
Endress PK (2006) Angiosperm floral evolution: morphological developmental framework. Adv Bot Res 44:1–61
Endress PK (2010) Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. J Syst Evol 48:225–239
Endress PK (2011) Evolutionary diversification of the flowers in angiosperms. Am J Bot 98:370–396
Endress PK, Igersheim A (1999) Gynoecium diversity and systematics of the basal eudicots. Bot J Linn Soc 130:305–393
Falcão MJ, Paulino JV, Kochanovski FJ, Figueiredo RC, Basso-Alves JP, Mansano VF (2020) Development of inflorescences and flowers in Fabaceae subfamily Dialioideae: an evolutionary overview and complete ontogenetic series for Apuleia and Martiodendron. Bot J Linn Soc 193:19–46
Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD (2004) Pollination syndromes and floral specialization. Ann Rev Ecol Evol Syst 35:375–403
Galen C, Dawson TE, Stanton ML (1993) Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95:187–193
Gerlach G (1969) Botanische Mikrotechnik. George Thieme Verlag, Stuttgart
Gerrits PO, Eppinger B, Goor HV, Horobin RW (1991) A versatile, low-toxicity glycol methacrylate embedding medium for use in biological research, and for recovered biomaterials prostheses. Cells Mater 1:189–198
Ghazoul J (2001) Can floral repellents pre-empt potential ant-plant conflicts? Ecol Lett 4:295–299
Gómez-Acevedo SL (2021) Floral development of the myrmecophytic Acacia cornigera (Leguminosae). Bot Sci 99:588–598
Gómez-Acevedo SL, Magallon S, Rico-Arce L (2007) Floral development in three species of Acacia (Leguminosae, Mimosoideae). Aust J Bot 55:30–41
Gonçalves BCF, Mansano VDF, Moraes RS, Paulino JV (2023) Comparative floral development in Mimosa (Fabaceae: Caesalpinioideae) brings new insights into merism lability in the mimosoid clade. J Plant Res 1–26
Grimes J (1999) Inflorescence morphology, heterochrony, and phytogeny in the mimosoid tribes ingeae and acacieae (Leguminosae: Mimosoideae). Bot Rev 65:317–347
Harder LD, Jordan CY, Gross WE, Routley MB (2004) Beyond floricentrism: the pollination function of inflorescences. Plant Species Biol 19:137–148
Hodges SA, Arnold ML (1995) Spurring plant diversification: are floral nectar spurs a key innovation. Proc Royal Soc Lond 262:343–348
Johansen DA (1940) Plant microtechnique. McGraw-Hill, London
Kochanovski FJ, Paulino JV, Teixeira SP, Tozzi AMGDA, Mansano VF (2018) Floral development of Hymenaea verrucosa: an ontogenetic approach to the unusual flower of Fabaceae subfamily detarioideae. Bot J Linn Soc 187:46–58
Koenen EJ, Kidner C, de Souza ÉR, Simon MF, Iganci JR, Nicholls JA, Gillian KB, de Queiroz LP, Luckow M, Lewis GP, Pennington RT, Hughes CE (2020) Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. Am J Bot 107:1710–1735
Kong D, Becker A (2021) Then there were plenty-ring meristems giving rise to many stamen whorls. Plants 10:1140
Lázaro A, Totland Ø (2014) The influence of floral symmetry, dependence on pollinators and pollination generalization on flower size variation. Ann Bot 114:157–165
Lewis GP (2005) Tribe Acacieae. In: Lewis G, Schrire B, Mackinder B, Lock M (eds) Legumes of the world. Royal Botanic Gardens, London, pp 187–191
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world. Royal Botanic Gardens, Kew
Luckow M, Miller JT, Murphy DJ, Livshultz T (2003) A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. In: Klitgaard BB, Bruneau A (eds) Advances in legume systematics, part, 10. Royal Botanic Garden, London, pp 197–220
Mansano VF, Tucker SC, Tozzi AMGA (2002) Floral ontogeny of Lecointea, Zollernia, Exostyles, and Harleyodendron (Leguminosae: Papilionoideae: Swartzieae Sl). Am J Bot 89:1553–1569
Marazzi B, Gonzalez AM, Delgado-Salinas A, Luckow MA, Ringelberg JJ, Hughes CE (2019) Extrafloral nectaries in Leguminosae: phylogenetic distribution, morphological diversity and evolution. Aust Syst Bot 32:409–458
Marinho CR, Souza CD, Barros TC, Teixeira SP (2014) Scent glands in legume flowers. Plant Biol 16:215–226
Maslin BR, Stirton CH (1997) Generic and infra-generic classification in Acacia (Leguminosae: Mimosoideae): a list of critical species on which to build a comparative data set. Bull Int Group Stud Mimosoideae 20:22–44
Maslin BR, Miller JT, Seigler DS (2003) Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust Syst Bot 16:1–18
Miller JT, Seigler DS (2012) Evolutionary and taxonomic relationships of Acacia s.l. (Leguminosae: Mimosoideae). Aust Syst Bot 25:217–224
Naghiloo S, Dadpour MR, Movafeghi A (2012) Floral ontogeny in Astragalus compactus (Leguminosae: Papilionoideae: Galegeae): variable occurrence of bracteoles and variable patterns of sepal initiation. Planta 235:793–805
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Paulino JV, Mansano VF, Teixeira SP (2013) Elucidating the unusual floral features of Swartzia dipetala (Fabaceae). Bot J Linn Soc 173:303–320
Paulino JV, Prenner G, Mansano VF, Teixeira SP (2014) Comparative development of rare cases of a polycarpellate gynoecium in an otherwise monocarpellate family, Leguminosae. Am J Bot 101:572–586
Paulino JV, Mansano VF, Prenner G, Teixeira SP (2017) High developmental lability in the perianth of Inga (Fabales, Fabaceae): a neotropical woody rosid with gamopetalous corolla. Bot J Linn Soc 183:146–161
Pedersoli GD, Teixeira SP (2016) Floral development of Parkia multijuga and Stryphnodendron adstringens, two andromonoecious mimosoid trees (Leguminosae). Int J Plant Sci 177:60–75
Pedersoli GD, Mansano VF, Barros TC, Paulino JV, Teixeira SP (2023) Sympetaly in the Mimosoid Clade (Leguminosae, Caesalpinioideae): an asterid characteristic in a Rosid Group. Perspect Plant Ecol Evol Syst 60:125747
Polhill RM, Raven PH (1981) Advances in legume systematics, vol 1. Royal Botanic Gardens, Kew
Prenner G (2004a) Floral ontogeny in Calliandra Angustifolia (Leguminosae: Mimosoideae: Ingeae) and its systematic implications. Int J Plant Sci 165:417–426
Prenner G (2004b) The asymmetric androecium in Papilionoideae (Leguminosae): definition, occurrence, and possible systematic value. Int J Plant Sci 165:499–510
Prenner G (2011) Floral ontogeny of Acacia Celastrifolia: an enigmatic mimosoid legume with pronounced polyandry and multiple carpels. Flowers Tree Life 1:256–278
Prenner G, Klitgaard BB (2008) Towards unlocking the deep nodes of Leguminosae: floral development and morphology of the enigmatic Duparquetia orchidacea (Leguminosae, Caesalpinioideae). Am J Bot 95:1349–1365
Ramírez-Domenech JI (1989) Floral ontogeny of mimosoid legumes. Dissertation, Louisiana State University and Agricultural & Mechanical College
Ramírez-Domenech JI, Tucker SC (1989) Phylogenetic implications of inflorescence and floral ontogeny of Mimosa strigillosa. Am J Bot 76:1583–1593
Ramírez-Domenech JI, Tucker SC (1990) Comparative ontogeny of the perianth in mimosoid legumes. Am J Bot 77:624–635
Rico-Alvarado D, Gómez-Acevedo S (2022) Desarrollo floral de Acaciella Angustissima (Leguminosae: Caesalpinioideae: Acacieae). Bot Sci 100:412–422
Ringelberg JJ, Koenen EJM, Iganci JR, de Queiroz LP, Murphy DJ, Gaudeul M, Bruneau A, Luckow M, Lewis GP, Hughes CE (2022) Phylogenomic analysis of 997 nuclear genes reveals the need for extensive generic re-delimitation in Caesalpinioideae (Leguminosae). In: Hughes CE, de Queiroz LP, Lewis GP (eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations. PhytoKeys 205:3–58. https://doi.org/10.3897/phytokeys.205.85866
Rodríguez-Riaño T, Ortega-Olivencia A, Devesa JA (1999) Types of androecium in the Fabaceae of SW Europe. Ann Bot 83:109–116
Ronse De Craene LP (2016) Meristic changes in flowering plants: how flowers play with numbers. Flora 221:22–37
Ronse de Craene LP (2018) Understanding the role of floral development in the evolution of angiosperm flowers: clarifications from a historical and physico-dynamic perspective. J Plant Res 131:367–393
Ronse de Craene LP (2022) Floral diagrams: an aid to understanding flower morphology and evolution. Cambridge University Press, Cambridge
Seigler DS, Ebinger JE, Miller JT (2006) Mariosousa, a new segregate genus from Acacia s.l. (Fabaceae, Mimosoideae) from Central and North America. Novon J Bot Nomenclature 16:413–420
Seigler DS, Ebinger JE, Riggins CW, Terra V, Miller JT (2017) Parasenegalia and Pseudosenegalia (Fabaceae): new genera of the Mimosoideae. Novon J Bot Nomenclature 25:180–205
Sinjushin AA (2021) Evolutionary history of the leguminous flower. Biol Bull Rev 11:400–413
Sinjushin AA, Karasyova TA (2017) Stability of the floral structure in Leguminosae with flag versus non-flag blossom. Wulfenia 24:1–10
Stone GN, Willmer PG, Rowe JA, Nyundo B, Abdallah R (1999) The pollination ecology of Mkomazi Acacia species. In: Coe MJ, McWilliam N, Stone GN, Packer M (eds) Mkomazi, the ecology, biodiversity and conservation of a Tanzanian Savanna. Royal Geogr Soc, London, pp 337–360
Stone GN, Raine NE, Prescott M, Willmer PG (2003) Pollination ecology of Acacia (Fabaceae, Mimosoideae). Aust Syst Bot 16:103–118
Teixeira SP, Marinho CR, Paulino JV (2014) A Flor: aspectos morfofuncionais. In: Rech AR, Agostini K, Oliveira PE, Machado IC (eds) Biologia Da Polinização. Editora Projeto Cultural, Rio de Janeiro, pp 45–69
Terra V, Garcia FC, de Queiroz L, van der Bank M, Miller JT (2017) Phylogenetic relationships in Senegalia (Leguminosae-Mimosoideae) emphasizing the south American lineages. Syst Bot 42:458–464
Terra V, Ringelberg JJ, Maslin B, Koenen EJ, Ebinger J, Seigler D, Hughes CE (2022) Dilemmas in generic delimitation of Senegalia and allies (Caesalpinioideae, mimosoid clade): how to reconcile phylogenomic evidence with morphology and taxonomy? PhytoKeys 205:261–278
Troll W (1965) Botanischer teil. In: Kommission für biologische forschung, Bericht. Akademie der Wissenschaften und Literatur Mainz, Jahrbuch. 1964, pp 93–109
Tucker SC (1987) Floral initiation and development in legumes. In: Stirton CH (ed) Advances in legume systematics, part 3. Royal Botanic Gardens, Kew, pp 183–239
Tucker SC (1988) Heteromorphic flower development in Neptunia pubescens, a mimosoid legume. Am J Bot 75:205–224
Tucker SC (1991) Helical floral organogenesis in Gleditsia, a primitive Caesalpinioideae legume. Am J Bot 78:1130–1149
Tucker SC (1992a) The role of floral development in studies of legume evolution. Can J Bot 70:692–700
Tucker SC (1992b) The developmental basis for sexual expression in Ceratonia siliqua (Leguminosae: Caesalpinioideae: Cassieae). Am J Bot 79:318–327
Tucker SC (1993) Floral ontogeny in Sophoreae (Leguminosae: Papilionoideae). I. Myroxylon (Myroxylon group) and Castanospermum (Angylocalyx group). Am J Bot 80:65–75
Tucker SC (1996) Trends in evolution of floral ontogeny in Cassia sensu stricto, Senna, and Chamaecrista (Leguminosae: Caesalpinioideae: Cassieae: Cassiineae): a study in convergence. Am J Bot 83:687–711
Tucker SC (1997) Floral evolution, development, and convergence: the hierarchical-significance hypothesis. Int J Plant Sci 158(6 suppl):143–161
Tucker SC (1998) Floral ontogeny in legume genera Petalostylis, Labichea, and Dialium (Caesalpinioideae: Cassieae), a series in floral reduction. Am J Bot 85:184–208
Tucker SC (1999) Evolutionary lability of symmetry in early floral development. Int J Plant Sci 160(6 suppl):25–39
Tucker SC (2003a) Floral development in legumes. Plant Physiol 131:911–926
Tucker SC (2003b) Floral ontogeny in Swartzia (Leguminosae: Papilionoideae: Swartzieae): distribution and role of the ring meristem. Am J Bot 90:1271–1292
Weberling F (1989) Structure and evolutionary tendencies of inflorescences in the Leguminosae. Adv Legume Biol 29:35–58
Weberling F (1992) Morphology of flowers and inflorescences. CUP Archive, New York
Wessinger CA, Hileman LC (2020) Parallelism in flower evolution and development. Annu Rev Ecol Evol Syst 51:387–408
Xiang GJ, Lázaro A, Dai XK, Xia J, Yang CF (2023) Pollinator proboscis length plays a key role in floral integration of honeysuckle flowers (Lonicera spp). Plants 12:1629
Zimmerman E, Herendeen PS, Lewis GP, Bruneau A (2017) Floral evolution and phylogeny of the Dialioideae, a diverse subfamily of tropical legumes. Am J Bot 104:1019–1041
Acknowledgements
The authors thank Elaine Zózimo de Souza (Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Brazil), Raquel Pires (Centro Brasileiro de Pesquisas Físicas, Rio de Janeiro, Brazil) and Jean Pierre (CENABIO - Centro Nacional de Biologia Estrutural e Bioimagem, Rio de Janeiro, Brazil) for technical support during electron microscopy work; Marcus José de Azevedo Falcão for technical and theoretical support during the all phases. This research was supported by CAPES with the scholarship for the first author.
Author information
Authors and Affiliations
Contributions
A.J.A.R., V.S.T. and J.V.P. analysed the anatomical and MEV images. V.S.T. and V.F.M. contributed to the study conception and design. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvarado-Reyes, A.J., Paulino, J.V., Terra, V. et al. Floral ontogeny reveals potential synapomorphies for Senegalia sect. Monacanthea p.p. (Leguminosae). J Plant Res 137, 907–925 (2024). https://doi.org/10.1007/s10265-024-01554-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-024-01554-z