Abstract
Adults of three amphidromous gobiid fishes, Tridentiger brevispinis, Rhinogobius similis, and Gymnogobius petschiliensis, are euryhaline and generally found in both freshwater (FW) and brackish water (BW) areas. The determining factors for their choice of habitat with different salinity have never been explored. In this study, a salinity-choice experiment was conducted using the above species captured in the FW region of the Isazu River, northern Kyoto Prefecture. For comparison, the fluvial goby Rhinogobius flumineus and BW-acclimated G. petschiliensis were also tested. We found that the three euryhaline species, including BW-acclimated G. petschiliensis, preferred BW to FW, whereas R. flumineus preferred FW. These results suggest that salinity preference did not determine habitat in these euryhaline gobiids, which were found in FW. Surveys were also conducted focusing on competitors and predators in their potential habitats. Thus, net sampling captured many other gobiid species, and an environmental DNA method detected Japanese temperate bass, a voracious predator, in the estuarine areas, suggesting that biotic factors are major determinants in the distribution of euryhaline species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, fish cannot move easily between hypotonic and hypertonic environments. Stenohaline species, including freshwater (FW) and marine fishes, are not able to control the osmotic pressure of their body fluids and suffer fatal damage soon after they enter an environment with inadequate salinity (Parry 1966). In contrast, euryhaline fish modify their physiological processes as they acclimatize to a new environment with different salinity; for example, they control the level and activity of Na+/K+-ATPase or change the distribution of aquaporin in gill chloride cells (Tipsmark et al. 2002; Jung et al. 2012). In addition, utilization of different metabolic substrates supplies the energy during salinity acclimation, thus, an energy supply has priority over the preservation of metabolic substrates on such occasions (Hwang et al. 2011). This indicates that significant environmental changes (e.g., salinity change) cause new energy requirements in fish, which need energy to maintain physiological functions. Therefore, even euryhaline fish cannot avoid an energetic cost when they enter a habitat with different osmotic characteristics.
Euryhaline species are found in various taxa (Schultz and McCormick 2013). In their study on sea catfishes (Ariidae), Betancur et al. (2012) suggested that euryhaline or FW species tend to occur in FW areas inhabited by few related species. Moreover, intraspecific polymorphism in utilizing habitats with different salinity is a part of the life history strategy of Japanese eel Anguilla japonica (Tsukamoto et al. 1998), Japanese temperate bass (Fuji et al. 2014) and amphidromous goby Awaous stamineus (Hogan et al. 2014). Thus, the benefit that arises when euryhaline fishes access habitats with different salinity can outweigh the cost of osmoregulation. For example, the use of habitats with different salinity enables euryhaline fish to access more food resources (Fuji et al. 2014), avoid high competitive pressure (Alcaraz et al. 2008), and mitigate water temperature variation (Iwatsuki et al. 1993), all of which suggest that benefits of euryhalinity are not trivial in terms of niche width.
Gobiid fishes are highly diverse, particularly in regard to their habitat (Seno 2004). Their distribution area includes ocean, estuary, stream, lake, and marsh ecosystems; diadromous species also exist in this taxon. In addition, the ability of adults to utilize habitats with different salinities is thought to have evolved independently in several euryhaline gobiid species, because such a trait is uncommon yet appears in different genera (Akihito et al. 2013). This renders these species suitable models to reveal relationships between the cost and benefit of habitat choice along a salinity gradient, because ecological factors rather than phylogenetic constraints are considered to have caused such a polyphyletic trait. It is not known what determines habitat choice in such euryhaline species, and answering this question may provide valuable information about their strategy of habitat utilization.
Three gobiid species, Tridentiger brevispinis, Rhinogobius similis, and Gymnogobius petschiliensis, live almost exclusively in the FW area of the Isazu River, in the northern Kyoto Prefecture (Oto, unpublished data). Their potential distribution, however, extends from the middle or downstream basin to the estuarine areas (Ishino et al. 2005; Akihito et al. 2013). In general, when many gobiid species coexist, their habitat pattern shifts as a result of interactions including competition. Microhabitat niche shift occurs in the middle basin where more than one species of Rhinogobius exists. Mizuno et al. (1979) revealed that when Rhinogobius nagoyae dominates in flat riffle, three other species (Rhinogobius fluviatilis, Rhinogobius sp. “cobalt,” and Rhinogobius brunneus) utilize other parts of the stream, whereas in the absence of Rhinogobius nagoyae, the other three species enter the flat riffles. Other studies showed that when Rhinogobius nagoyae and Rhinogobius fluviatilis coexist, the former species tends to shift its habitat to that with a faster current and coarser substrate as compared to allopatric conditions (Sone et al. 2001, 2006). The Isazu River empties into Maizuru Bay, which is characterized by a high level of species richness of gobiids (Matsui et al. 2014). Many gobiid species live in both the estuary and the FW habitats of this river and various and intense interactions are assumed to arise among these species.
Habitat utilization of gobiid fish is also affected by the presence of predators (Schofield 2003). Japanese temperate bass Lateolabrax japonicus is a typical top predator (e.g., Nip et al. 2003; Kawamura 2005; Nakane et al. 2010). It is distributed widely in Japanese coastal waters (Hatooka 2013). It has high osmoregulatory ability and is known to enter rivers (Hirai et al. 1999; Miyata et al. 2016; Yamanaka and Minamoto 2016). Both adults and juveniles of this species preferentially prey upon gobies in inner bay and estuarine areas, as has been reported by many authors. For example, in Matsushima Bay and Sendai Bay, northeastern Japan, the diet of larval and juvenile temperate bass (standard length 18–209 mm) included fish at the rate of 6.2–1.7% of all prey individuals, mainly comprising gobies (Hatanaka and Sekino 1962). In and around Ise Bay and Mikawa Bay, central Japan, the diet of temperate bass (body length 80–600 mm) included fish individuals at the rate of 50% or more, the majority of which were gobies (Funakoshi 1993). Therefore, Japanese temperate bass should have a major effect on gobiid species in the Isazu River, where this species dominates as a predator.
The goal of this study was to reveal potential factors that determine habitat selection in terms of salinity in euryhaline gobiids T. brevispinis, R. similis, and G. petschiliensis. We first evaluated the physiological preference to salinity by a choice experiment. If salinity preference was consistent with habitat salinity, the preference was considered a major causal factor. If salinity preference did not correspond to habitat salinity, then another factor was assumed to be a determinant. To rule out the possibility that the fish will choose brackish water (BW) to compensate for mineral requirements, experimental trials using fluvial goby, Rhinogobius flumineus, and G. petschiliensis acclimated in BW were conducted.
In addition, distributions of competitors and predators were examined as factors that may affect habitat utilization of the target euryhaline gobiids. The distribution of related gobiid species was investigated from the estuary to the middle basin by capturing fish with hand nets. The distribution of the predator Japanese temperate bass was investigated by detecting environmental DNA (eDNA). eDNA comprises fragmented DNA released into surrounding water by aquatic organisms through their mucus, feces, urine, and remains, and it is used to detect the presence of specific species (Ficetola et al. 2008). This method has been applied to detect endangered or invading species in FW systems (e.g., Takahara et al. 2013) but not in an ethological context.
Materials and methods
Target species
Tridentiger brevispinis , Rhinogobius similis, and Gymnogobius petschiliensis were used for the salinity-choice experiment. They are all amphidromous species from the subfamily Gobionellinae. The adults of their congeneric species reside in FW areas, whereas the above three species inhabit FW but also tidal areas (Akihito et al. 2013). In addition, the FW goby Rhinogobius flumineus, which is sympatric with the above three species, was used for comparison.
Fish sampling
The sampling was conducted from 25 September to 25 November 2015 on the Isazu River (approximately 20 km in length), Maizuru City, Kyoto Prefecture (Fig. 1). Sampling station (St.) 1–3 were located in tidal and BW areas. Salinity was measured by using a salinometer (TCX-999i; Toko-Kagaku, Tokyo); the salinity at St. 1–3 ranged from 1.0 to 19.9.
Tridentiger brevispinis, R. similis, and R. flumineus were sampled in the middle basin at St. 6. The reach type of the river was categorized as Bb according to the method of Kani (1970), where type B is defined as having the sequence of one riffle and one pool pair and type b as having turbulent riffles. G. petschiliensis was sampled in the Manai Pond, which is located about 500 m from the reach (Fig. 1). Although the pond is connected to the main stream via a water channel, its water is mostly supplied by spring welling. Salinity at St. 6 and Manai Pond was 0.1. The fish were captured with a hand net and transported at a density of about ten individuals per 20-L bucket.
Acclimation
Acclimation and the experiments were conducted in the Maizuru Fisheries Research Station, Kyoto University. The fish were acclimated in an outdoor facility with a roof in transparent 100-L tanks at a density of 12 individuals or less per tank for approximately 2 days. They were acclimated in FW, except for the BW-acclimated group of G. petschiliensis, which was acclimated in water with salinity of 20.4 (equivalent to the salinity used in experimental BW). Bricks were placed in the tanks as shelters, and the tanks were aerated. The fish were given bloodworm (chironomid larvae; Sanmi, Hyogo, Japan) at approximately 3.2 g tank−1 once per day starting from 2 days after they were transferred to the tanks. Before the experiments were initiated, it was verified that most individuals of the respective species fed on bloodworm.
The experiment was conducted in a temperature-controlled room with tank water temperatures maintained at 22.5 ± 1.5 °C (mean ± SD). The fish were acclimated to the experimental water overnight, or longer, in the same tanks that were used for the salinity-choice experiment; the light–dark cycle in the room was 12/12 h. BW (salinity 20) was prepared by mixing tap water and filtered seawater pumped from Maizuru Bay. The experimental water was left undisturbed overnight, or longer, before a fish was introduced.
Experimental procedure
The experiment was conducted using six tanks measuring 60 × 30 × 45 cm (length × width × depth), with a 30-cm-high separation dividing each tank into two sections. An opaque blue sheet covered all the sides except the front of each tank, and blackout curtains were set around the experimental areas each holding two tanks. A video camera (HDR-CX480; Sony, Tokyo) was set in each experimental area and operated through a window in the curtain.
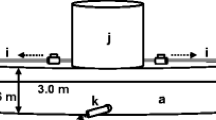
The experiment was composed of the preliminary and test stage. A whole tank was filled with FW in the preliminary stage of the FW-acclimated treatments, and BW was introduced into the left section of the tank in the test stage (Fig. 2). In the BW-acclimated treatment, BW instead of FW was introduced into the whole tank in the preliminary stage. During the test stage, FW and BW were separated by their difference in specific gravity. Two 200-L stock tanks were filled with FW or BW and connected to the experimental tank via tubes. Before the test stage of FW-acclimated treatments, FW was drained via an outlet from the left section and BW was introduced using the same outlet. FW was added from the inlet in the right section of the tank, filling up the tank above the level of the separation wall so that the fish could move between the two sections. The same protocol was used to prepare the tank for the BW-acclimated group, except that BW was drained from the right section and was replaced with FW. The fish in the section that was drained after the preliminary stage was transferred to the other section using a small hand net. The tank walls were rinsed with the same type of water, FW or BW, before introducing the experimental water. After the test stage, the salinity in the FW section was 1.9 ± 1.8, and that in BW was 19.7 ± 0.9. Each stage lasted 1 h and was recorded by a video camera. Between each stage, fish were left in the tanks for 1 h to relieve their stress. Standard length, body weight and sex of individual fish were recorded after each experiment (Table 1).
Experimental tanks used for the salinity-preference experiment. In the preliminary stage of the experiment, the tanks were filled with freshwater (FW) or brackish water (BW) for FW-acclimated groups or BW-acclimated groups, respectively (a). In the test stage of the experiment, the left section of the tank was filled with BW (b)
Statistical analysis
From 1-h video recordings, 361 still frames were extracted at 10-s intervals. Then, the number of frames in which the fish was in the lower-left section where BW was introduced during the test stage was extracted. The position of the fish’s eyes defined its location. The average number of frames showing the fish in the lower-left section was compared between the preliminary and the test stage by paired t-test, and the result determined whether a salinity preference existed; if the number of frames in the test stage was significantly higher or lower than in the preliminary stage, the fish were considered to have BW or FW preference, respectively. In addition, the BW preference index (BPI) representing the degree of salinity preference was calculated as follows:
where n p and n t are the number of frames when the fish is in the lower-left section in the preliminary and test stage, respectively. The BPI was also compared between male and female individuals for all species, except for T. brevispinis in which sex differentiation is unclear. Inter-specific difference of BPI was tested by Bonferroni pairwise t-test. R statistical software version 3.2.0 (R Core Team 2015) was used for the analyses.
Survey of competitors in estuarine and downstream basin
The distribution of competitor gobiid species was surveyed by hand net at St. 1, 3, 4, 6, and Manai Pond on 25 and 27 September 2015. The salinity at St. 4, 6, and Manai Pond was 0.1. Captured species and their approximate densities were recorded. The density was assigned a score according to a three-grade evaluation: minus, representing none; plus, representing approximately 1–10 individuals per 1 h; and plus plus, representing >10 individuals h−1, respectively.
Survey of predators
One liter of river water was sampled from St. 1–10 on 18 December 2015 to detect species-specific DNA of temperate bass. Considering the tidal range in the estuarine basin, water sampling was conducted at both low and high tides at St. 1–4. Three replicates of water specimens were collected for each sampling occasion.
DNA extraction and quantitative determination were conducted at the Maizuru Fisheries Research Station. Bottled water specimens were transported on ice and filtered on a 47-mm-diameter microfiber paper (nominal pre-size 0.7 µm; GF/F grade; Whatman International, Little Chalfont, UK) within 6 h of collection. DNA was extracted by the Salivette method according to the protocol described by Yamamoto et al. (2016). Species-specific DNA of L. japonicus was quantified following the Taq Man probe method (Yamamoto et al. 2016), using a real-time polymerase chain reaction (PCR) (LightCycler 96; Roche Diagnostics, Mannheim, Germany), and the primers reported by Yamanaka and Minamoto (2016). The PCR thermal cycle protocol was as follows: 2 min at 50 °C, followed by 10 min at 95 °C, 45 cycles of 15 s at 95 °C, and 1 min at 60 °C. A triplicate negative control was adopted by using distilled water. Four levels of quantitative standards (species-specific DNA at 3.0 × 104, 3.0 × 103, 3.0 × 102, 3.0 × 101 copies) were used to draw a calibration curve. The R 2-value of the calibration curve was 0.98 or more.
Results
Salinity-choice experiment
The FW-acclimated T. brevispinis, R. similis, and G. petschiliensis and the BW-acclimated G. petschiliensis showed a significantly higher preference for BW than for FW, whereas R. flumineus had significantly higher preference for FW than for BW (Table 2). There was no significant difference in the salinity preference between the sexes in all species (t-test; R. similis, P = 0.233; G. petschiliensis, P = 0.088; R. flumineus, P = 0.562). The BPI of R. flumineus was significantly different from that in other groups (Bonferroni pairwise t-test, P < 0.01), but there was no difference in the BPI between other pairs of the groups (Bonferroni pairwise t-test, P > 0.8; Fig. 3).
Salinity preference of the fish of four experimental groups represented by BW preference index (BPI), which was calculated as follows: [(the number of frames when a fish was in the lower left part of the tank in the test stage)–(the number of frames when a fish was in the lower left part of the tank in the preliminary stage)]/(the total number of frames = 361). For values significantly higher than zero, the group was considered to prefer BW (salinity of 20). Black circles represent means (n = 13) and bars SEs. Different letters indicate significant difference among fishes or treatments (Bonferroni pairwise t-test). Gp Gymnogobius petschiliensis, Rf Rhinogobius flumineus, Rs Rhinogobius similis, Tb Tridentiger brevispinis; for other abbreviations, see Fig. 2
Distribution of competitors and predators
Tridentiger bifasciatus, a congeneric species of T. brevispinis, was captured at a high density in the waters of St. 1 and 3. Gobiids of the genera Acanthogobius and Luciogobius were also recorded at the same stations. Although juveniles of T. brevispinis were captured, the adults were not found (Table 3).
On average, 481 copies of eDNA of temperate bass were detected in the water from St. 1 at low tide during daytime and 59.1 copies were detected at St. 2 during high tide at night. No eDNA of this predator species was detected at other stations (Fig. 4).
Discussion
The salinity-choice experiment revealed that the three euryhaline gobies, T. brevispinis, R. similis, and G. petschiliensis, preferred BW to FW. A relationship between salinity and fitness has been reported in various euryhaline fishes. For example, Micropogonias furnieri, a euryhaline species of croaker, exhibits better growth in BW than in FW, suggesting that physiological processes in this species require saline environments (Mont’Alverne et al. 2016). Trachinotus marginatus, a euryhaline species of pompano, is also known to show the best growth at a salinity of 6 (Anni et al. 2016). For the three euryhaline gobies tested in the present study, BW may have provided a physiologically better environment. Nevertheless, it cannot be excluded that the euryhaline gobies might have chosen FW if the experiment had been extended over a longer period.
Salinity preference of the three euryhaline gobies was not consistent with that of their habitat. This suggests that a requirement for physiological salinity is not a priority in their habitat selection. It is likely that the basic habitat of the three euryhaline gobies is estuarine and that the tolerance to FW is a derived trait; thus external factors might have shifted their habitat utilization and forced them to enter the FW area.
The present results suggest that one of these external factors is interspecific competition. Many T. bifasciatus and Acanthogobius species were captured in the Isazu River estuary, and the presence of these related species may increase competition for food and spatial resources. Many studies suggest that the presence of competitors, particularly competition with related species, causes habitat shift in gobiid species (Sone et al. 2001, 2006; Schofield 2003). Other studies show that Gymnogobius opperiens and Gymnogobius urotaenia, sister species of G. petschiliensis, tend to use different habitat along and across the river flow, respectively (Miyazaki and Terui 2016). In the case of T. brevispinis and T. obscurus, there is reproductive interference between them because the fitness of their hybrid is low (Mukai et al. 2000). It is also known that both of these species show parapatric distribution; T. brevispinis lives in the upper stream when the two species live in the same river (Mukai 2001). In contrast, salinity can influence the intensity of competition between euryhaline species. Alcaraz et al. (2008) revealed that when two related euryhaline species, mosquitofish and cyprinodont, coexist, the feeding probability of the latter increases with increasing salinity level. Furthermore, Herichthys cyanoguttatus, a euryhaline cichlid, enhances its aggression level in BW compared to FW, suggesting elevated competition in estuaries (Lorenz et al. 2015). Taken together, these data indicate that the three euryhaline gobies studied here were likely chased out by these competitors, or they shifted their salinity habitat to avoid the competition. Although other Rhinogobius species coexisted with the three euryhaline species at St. 6, their density was low and each species occupied a separate habitat: R. flumineus and R. nagoyae lived in areas of swift current, whereas T. brevispinis and R. similis inhabited flat riffles and pools (Oto, unpublished data). Thus, structural complexity of the stream might mitigate interspecific competition and enable many species to coexist.
Predation is one of the most important factors that can affect animal behavior (e.g., Lima and Dill 1990; Brown and Kotler 2004). The eDNA of the typical predator temperate bass was detected at two estuarine stations in the Isazu River in December 2015. Moreover, many adults of this species were captured by lure fishing, or their presence was visually confirmed in the estuary of this river between June and September 2015 (Oto, unpublished data). Thus, both the eDNA data and the observations indicate that the Japanese temperate bass may be considered a resident of the estuary, or at least a frequent visitor. The northern distributional limit of Japanese temperate bass is southern Hokkaido, where its density is relatively low (Yokogawa 2002; Hibino et al. 2007). It is noteworthy that G. petschiliensis mainly lives in the FW area in most rivers of Honshu and Shikoku, Japan, whereas its main habitat is BW in southern Hokkaido (Goto et al. 1978; Nakanishi 1978; Hatama and Ohhashi 2009; Tsuji 2015). This supports the hypothesis that predation pressure shapes the habitat choice of this goby in terms of salinity.
Piscine predators are rare in the middle and upper basin of the Isazu River. According to monthly underwater visual surveys conducted from June 2012 to January 2013 and from March to November 2015, only one individual of Anguilla japonica and one individual of Oncorhynchus keta were recorded as predatory species around St. 6 (Kumagai, unpublished data; Oto, unpublished data). Therefore, predation risk is most likely to be higher in BW than in FW areas. Combining the present results with this circumstantial information indicates that the presence of Japanese temperate bass may well have forced the three euryhaline gobies to avoid estuarine areas.
Another factor, seasonal migration, can also determine habitat utilization of the three euryhaline gobies. Some of the euryhaline wanderers born in FW areas are known to migrate to FW areas in the low temperature season. For instance, Tribolodon species migrate to streams in winter, which is called “wintering migration,” due to physiological restrictions (Sakai 1995; Nakamura et al. 2016). The three euryhaline gobies are also born in FW areas, but their densities did not change significantly during the sampling conducted from September to November 2015 despite the substantial temperature reduction then, suggesting that there was no clear wintering migration. Spawning migration is not a likely causal factor either because of the lack of eggs and the presence of immature reproductive organs in the captured fish. Food resources are known to vary greatly along a salinity gradient and may affect habitat utilization (Islam et al. 2006; Fuji et al. 2014); however, this was not investigated in the present study.
Inconsistency between the fishes’ preferred salinity and that of the actual habitat provides an insight into the driving force of the process of acquiring euryhalinity. Future studies should focus on quantifying the costs and benefits of utilizing environments with a different salinity. Our next goal is to reveal whether, and if so how much, salinity restricts the physiological performance of euryhaline species by comparing the response of body weight and nutritional status between fish kept in FW and those kept in BW. Otolith microstructure analysis is another promising tool which can be used to answer these questions by combining environmental factors such as temperature, water quality, and topography of the estuary.
References
Akihito Sakamoto K, Ikeda Y, Aizawa M (2013) Gobiidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, 3rd edn. Tokai University Press, Hadano, pp 1361–1541 (in Japanese)
Alcaraz C, Bisazza A, García-Berthou E (2008) Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 155:205–213
Anni ISA, Bianchini A, Barcarolli IF, Junior ASV, Robaldo RB, Tesser MB, Sampaio LA (2016) Salinity influence on growth, osmoregulation and energy turnover in juvenile pompano Trachinotus marginatus Cuvier 1832. Aquaculture 455:63–72
Betancur-R R, Ortí G, Stein AM, Marceniuk AP, Alexander Pyron R (2012) Apparent signal of competition limiting diversification after ecological transitions from marine to freshwater habitats. Ecol Lett 15:822–830
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Ficetola GF, Miaud C, Pompanon F, Taberlet P (2008) Species detection using environmental DNA from water samples. Biol Lett 4:423–425
Fuji T, Kasai A, Ueno M, Yamashita Y (2014) Growth and migration patterns of juvenile temperate seabass Lateolabrax japonicus in the Yura River estuary, Japan—combination of stable isotope ratio and otolith microstructure analyses. Environ Biol Fish 97:1221–1232
Funakoshi S (1993) Characteristics of the food habits of the main fishes in and around Ise Bay and Mikawa Bay. Summer–autumn phase. Bull Aichi Fish Res Inst 1:1–18 (in Japanese with English abstract)
Goto A, Nakanishi T, Utoh H, Hamada K (1978) A preliminary study of the freshwater fish fauna of rivers in Southern Hokkaido. Bull Fac Fish Hokkaido Univ 29:118–130 (in Japanese with English abstract)
Hatama T, Ohhashi Y (2009) Distributions of fishes and decapod crustaceans in the inland water in Yamaguchi Prefecture (I). Bull Yamaguchi Pref Fish Res Ctr 7:19–61 (in Japanese with English abstract)
Hatanaka M, Sekino K (1962) Ecological studies on the Japanese sea bass, Lateolabrax japonicus. I. Feeding habit. Bull Jpn Soc Sci Fish 28:851–856 (in Japanese with English abstract)
Hatooka K (2013) Lateolabracidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, 3rd edn. Tokai University Press, Hadano, p 748
Hibino M, Ohta T, Isoda T, Nakayama K, Tanaka M (2007) Distribution of Japanese temperate bass, Lateolabrax japonicus, eggs and pelagic larvae in Ariake Bay. Ichthyol Res 54:367–373
Hirai N, Tagawa M, Kaneko T, Seikai T, Tanaka M (1999) Distributional changes in branchial chloride cells during freshwater adaptation in Japanese sea bass Lateolabrax japonicus. Zool Sci 16:43–49
Hogan JD, Blum MJ, Gilliam JF, Bickford N, McIntyre PB (2014) Consequences of alternative dispersal strategies in a putatively amphidromous fish. Ecology 95:2397–2408
Hwang P-P, Lee T-H, Lin L-Y (2011) Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol 301:28–47
Ishino K, Tsuji K, Iwata A, Suzuki T, Mizuno N, Koshikawa T, Kishi Y, Sawara Y, Takahashi S (2005) Gobiidae. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan, 3rd edn. Yama-to-keikoku-sha, Tokyo, pp 585–623 (in Japanese)
Islam MS, Hibino M, Tanaka M (2006) Distribution and diets of larval and juvenile fishes: influence of salinity gradient and turbidity maximum in a temperate estuary in upper Ariake Bay, Japan. Estuar Coast Shelf Sci 68:62–74
Iwatsuki Y, Tashiro K, Hamasaki T (1993) Distribution and fluctuations in occurrence of the Japanese centropomid fish, Lates japonicus. Jpn J Ichthyol 40:327–332
Jung D, Sato JD, Shaw JR, Stanton BA (2012) Expression of aquaporin 3 in gills of the Atlantic killifish (Fundulus heteroclitus): effects of seawater acclimation. Mol Integr Physiol 161:320–326
Kani T (1970) Ecology of torrent-inhabiting insects. Works of Kani Tokichi. Shisakusha, Tokyo, pp 3–91 (in Japanese)
Kawamura K (2005) Lateolabrax japonicus. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan, 3rd edn. Yama-to-keikoku-sha, Tokyo, p 485
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lorenz OT, Riccobono SA, Smith P (2015) Effects of salinity on the survival and aggression of the invasive Rio Grande cichlid (Herichthys cyanoguttatus). Mar Freshwater Behav Physiol 49:1–8
Matsui S, Inui R, Kai Y (2014) Annotated checklist of gobioid fishes (Perciformes, Gobioidei) from Wakasa Bay, Sea of Japan. Bull Osaka Mus Nat Hist 68:1–25
Miyata N, Mori T, Kagehira M, Miyazaki N, Suzuki M, Sato K (2016) Micro CTD data logger reveals short-term excursions of Japanese sea bass from seawater to freshwater. Aquat Biol 25:97–106
Miyazaki Y, Terui A (2016) Difference in habitat use between the two related goby species of Gymnogobius opperiens and Gymnogobius urotaenia: a case study in the Shubuto River System, Hokkaido, Japan. Ichthyol Res 63:317–323
Mizuno N, Uehara S, Maki M (1979) Studies on a freshwater fish, Rhinogobius brunneus (Gobiidae). IV. Habitat segregation among sympatric populations of 4 colour types. Jpn J Ecol 29:137–147 (in Japanese with English abstract)
Mont’Alverne R, Jardine TD, Pereyra PER, Oliveira MCLM, Medeiros RS, Sampaio LA, Tesser MB, Garcia AM (2016) Elemental turnover rates and isotopic discrimination in a euryhaline fish reared under different salinities: implications for movement studies. J Exp Mar Biol Ecol 480:36–44
Mukai T (2001) Hybridization and introgression in the speciation process of fishes. Jpn J Ichthyol 48:1–18 (in Japanese with English abstract)
Mukai T, Sato T, Morisawa M (2000) Natural hybridization and gene flow between two Tridentiger gobies in Lake Hinuma (Ibaraki Prefecture, Japan). Ichthyol Res 47:175–181
Nakamura M, Masuda R, Tsukamoto K, Otake T (2016) Narrowed temperature adaptability in non-natal osmotic environments of two euryhaline wanderers, dace and black porgy: implications for seasonal habitat changes. Fish Sci 82:261–268
Nakane Y, Suda Y, Sano M (2010) Food habits of fishes on an exposed sandy beach at Fukiagehama, South–West Kyushu Island, Japan. Helgol Mar Res 65:123–131
Nakanishi T (1978) Comparison of ecological and geographical distributions among the three types of Chaenogobius annularis Gill. Bull Fac Fish Hokkaido Univ 29:233–242 (in Japanese with English abstract)
Nip THM, Ho W-Y, Wong CK (2003) Feeding ecology of larval and juvenile black seabream (Acanthopagrus schlegeli) and Japanese seaperch (Lateolabrax japonicus) in Tolo Harbour, Hong Kong. Environ Biol Fishes 66:197–209
Parry G (1966) Osmotic adaptation in fishes. Biol Rev 41:392–440
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Sakai H (1995) Life-histories and genetic divergence in three species of Triborodon (Cypriniodae). Mem Fac Fish Hokkaido Univ 42:1–98
Schofield PJ (2003) Habitat selection of two gobies (Microgobius gulosus, Gobiosoma robustum): influence of structural complexity, competitive interactions and presence of a predator. J Exp Mar Biol Ecol 288:125–137
Schultz ET, McCormick SD (2013) Euryhalinity in an evolutionary context. In: McCormick SD, Farrell AP, Brauner CJ (eds) Euryhaline fishes. Academic Press, New York, pp 477–533
Seno H (2004) Review article. In: Seno H (ed) A photographic guide to the gobioid fishes of Japan. Heibon-sha, Tokyo, pp 12–15 (in Japanese)
Sone S, Inoue M, Yanagisawa Y (2001) Habitat use and diet of two stream gobies of the genus Rhinogobius in south-western Shikoku, Japan. Ecol Res 16:205–219
Sone S, Inoue M, Yanagisawa Y (2006) Competition between two congeneric stream gobies for habitat in southwestern Shikoku, Japan. Ichthyol Res 53:19–23
Takahara T, Minamoto T, Doi H (2013) Using environmental DNA to estimate the distribution of an invasive fish species in ponds. PLoS One 8:e56584
Tipsmark CK, Madsen SS, Seidelin M, Christensen AS, Cutler CP, Cramb G (2002) Dynamics of Na+, K+, 2Cl− cotransporter and Na+, K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool 293:106–118
Tsuji K (2015) Fish fauna of the Iwamatsu River system in Ehime Prefecture, Japan. Bull Tokushima Pref Mus 25:1–24
Tsukamoto K, Nakai I, Tesch WV (1998) Do all freshwater eels migrate? Nature 396:635–636
Yamamoto S, Minami K, Fukaya K, Takahashi K, Sawada H et al (2016) Environmental DNA as a ‘snapshot’ of fish distribution: a case study of Japanese jack mackerel in Maizuru Bay Sea of Japan. PLoS One 11:e0149786
Yamanaka H, Minamoto T (2016) The use of environmental DNA of fishes as an efficient method of determining habitat connectivity. Ecol Indic 62:147–153
Yokogawa K (2002) Genus Lateolabrax distributed in the East Asian coastal water. In: Tanaka M, Kinoshita I (eds) Temperate bass and biodiversity—new perspective for fisheries biology. Koseisha-kouseikaku, Tokyo, pp 114–126 (in Japanese)
Acknowledgements
We are grateful to K. Sakemi for helping with fish sampling and A. Tanimoto for supporting the water collection. Y. Kumagai (Kyoto University) provided unpublished data of the fish visual survey on the Isazu River, and T. Fuji (Japan Fisheries Research and Education Agency) informed us about the ecology of Japanese sea bass. Comments from H. Nakagawa (Kyoto University) and two anonymous reviewers on the manuscript and advice from members of the laboratory during the research were also greatly appreciated. This study was supported by the CREST program of the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
About this article
Cite this article
Oto, Y., Nakamura, M., Murakami, H. et al. Inconsistency between salinity preference and habitat salinity in euryhaline gobiid fishes in the Isazu River, northern Kyoto Prefecture. J Ethol 35, 203–211 (2017). https://doi.org/10.1007/s10164-017-0510-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-017-0510-3