Abstract
The longitudinal and cross-sectional differences in habitat use between Gymnogobius oppeiens and Gymnogobius urotaenia (sister species) were investigated from June to July 2011 in the Shubuto River System, southwestern Hokkaido, Japan. Generalized linear model revealed that watercourse distance from the sea had a significant influence on the abundances of both G. opperiens and G. urotaenia but in different ways. That is, G. opperiens had a distributional peak at the middle reaches, but the abundance of G. urotaenia gradually decreased with increasing distance from the sea. In addition, the lateral distribution patterns of G. opperiens and G. urotaenia, and all the local environmental variables were significantly different between the fringe and the mid-channel habitats. Both G. opperiens and G. urotaenia were most abundant along the margins of the river. However, the former species was frequently collected from the mid-channel, whereas the latter species was never collected in the habitat. These results coincide with previous observations asserting habitat segregation of the two goby species. The differential habitat use between the two species may be related to the differences in their population sizes and morphologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of valid fish species is estimated to be 27,977 (Nelson 2006). Among them, only one family accounts for approximately 5–10 % of the taxon; i.e., the family Gobiidae consists of approximately 1,500 species (Van Tassell et al. 2011) and more than a hundred of undescribed species (e.g., Senou et al. 2004; Akihito et al. 2013). This family can be found in a variety of marine and freshwater environments and its morphological diversity may in part reflect its wide-ranging habitat use (e.g., Senou et al. 2004; Patzner et al. 2011). Although external morphology is a key predictor of habitat use (e.g., Wood and Bain 1995; Helfman et al. 2009), some closely related species of Gobiidae, such as Luciogobius spp. and Rhinogobius spp., exhibit differential habitat preference despite their “apparent” morphological similarity (e.g., Senou et al. 2004; Akihito et al. 2013; Yamasaki et al. 2015).
The genus Gymnogobius, which belongs to the family Gobiidae, was taxonomically revised by Stevenson (2002). This genus currently includes 13 species found in shallow marine, estuarine, and fresh waters throughout Japan, the Russian Far East, the Kuril Islands, the Korean Peninsula, and the Yellow Sea. Stevenson (2002) described a new species Gymnogobius opperiens, which used to be known as the “middle-reach type” of Chaenogobius annularis described by Nakanishi (1978a, b). The other two types of C. annularis, “freshwater type” and “brackishwater type,” were referred to as Gymnogobius urotaenia and Gymnogobius petschiliensis, respectively (Stevenson 2002). Specifically, C. annularis was previously regarded as one species in Japan, but the species is currently divided into three valid species, G. opperiens, G. urotaenia, and G. petschiliensis. They all share a marine amphidromous life cycle (see McDowall 1988), although they are discernible based on slight difference in external morphology with reproductive isolation (Aizawa et al. 1994; Suk et al. 1996).
Their distribution extensively overlaps in Japanese river systems (Akihito et al. 2013). In such rivers, the two or three species frequently coexist in the low and middle reaches, but their longitudinal and/or cross-sectional distribution patterns have been suggested to be slightly different (Nakanishi 1978b; Ishino et al. 1983). Gymnogobius petschiliensis strongly depends on brackish water (lower reaches influenced by flood tides). Conversely, G. opperiens mainly inhabit lotic environments (riffle) of freshwater bodies, while G. urotaenia prefer lentic environments (pool and lake). Therefore, these related species have been assumed to segregate their habitats (Ishino 1987). Although the above-mentioned studies provided fundamental knowledge of their habitat uses, no rigorous evaluation has been performed to support their assertion.
This study aimed to evaluate the difference in habitat use between G. opperiens and G. urotaenia, a premise of habitat segregation, by extensive field surveys covering the entire system of the Shubuto River in Hokkaido, Japan.
Methods
Study sites. The investigations were conducted in the Shubuto River System, which is located at the northern part of Oshima Peninsula in southwestern Hokkaido, Japan (42°40′N, 140°18′E). The mean annual temperature and mean annual precipitation are 7.4 °C and 1461.8 mm, respectively (averaged for 1981–2010; Japan Meteorological Agency 2012). The water catchment area encompasses 367 km2 of forested and mountainous terrain, and the length of the main stem is approximately 40 km.
The riverine environments remain relatively intact except for the large loss of floodplains (Miyazaki et al. 2011; Kuromatsunai Town 2012). No dams or weirs prevent the migration and dispersal of fishes in the main stem, although some small weirs (height <5 m) are present in the upstream reaches of tributaries. Water quality is suitable for most freshwater organisms throughout the river system; dissolved oxygen >95 % in degrees of saturation, biochemical oxygen demand is 0.2–1.7 mg/L, and ammonia concentration <0.05 mg/L (Ministry of Land, Infrastructure, Transport and Tourism of Japan 2007; Terui et al. 2011, 2014; Kuromatsunai Town 2014).
The two species of Gymnogobius (G. opperiens and G. urotaenia) have been recorded in this river system (Miyazaki et al. 2011, 2013a, b). However, G. petschiliensis, which highly depends on brackish waters, is not recorded in the river. Adults of the former two amphidromous species spawn in the river, and the larvae drift down to the sea immediately after being hatched (Nakanishi 1978b; Goto 1991). Developed juveniles return to the river in August (Miyazaki and Terui 2015), following an approximately 1–2-month period of marine life stage (Nakanishi 1978b).
Field protocols. Field surveys were conducted in the summer (23 June–28 July) of 2011 when 0+ larvae and juveniles of G. opperiens and G. urotaenia did not migrate from the sea to the river; i.e., 0+ larvae and juveniles of these species were absent during this period (Miyazaki and Terui 2015). Fish sampling was conducted at 46 sampling sites, among which 19 and 27 sites were located in the main stem and in its 17 tributaries, respectively. The tributary sites did not have weirs under their channels except for upper two sites at Neppu River (its height: approximately 2 m). At each site, we established three 40 m2 belt lines (20 m in length, 2 m in width), one at the mid-channel and another on each side (river fringe), for a total of 120 m2 sampling area. In cases in which the river width did not exceed 6 m, we established one 120 m2 belt line at mid-channel (60 m in length, 2 m in width; 14 out of 46 sites). Fishes were captured from the lower to higher borders of the belt lines by three investigators using an electric shocker (LR-20B Backpack Electrofisher, Smith-Root Inc., Vancouver) and five hand nets (2 mm mesh) with same fishing effort for each belt line.
We identified all the collected species of Gymnogobius following Stevenson (2002) and Senou et al. (2004). After identification, digital images of all sampled Gymnogobius spp. were captured alongside a ruler using a digital camera (μTough-8000, Olympus Corporation, Tokyo, Japan). These were subsequently analyzed using ImageJ (National Institutes of Health, Bethesda, Maryland, USA) to roughly calculate standard length (SL) in millimeters. We released all captured fish back into the sites where they were captured, except for some specimens that were deposited in museums (Miyazaki et al. 2013a).
Habitat attributes. Local scale variables—Physical attributes (water depth, current velocity, and substrate coarseness) were measured concurrently with the fish abundance surveys, using an individual quadrat (0.25 m2) as a unit of measurement. We placed 4 (>6 m in the river width) or 12 (<6 m in the river width) quadrats in each belt line, measured the water depth with a meter stick and current velocity with a flow meter at 60 % depth (VE20, VET-200-10PII; KENNEK, Tokyo), and quantified the substratum composition. We visually estimated the coverage of the substrate in each quadrat as follows: particles <2 mm = silt + sand, 2–64 mm = gravel, 64–256 mm = cobble, and >256 mm = boulder. Percent cover of silt–sand was used in the subsequent analyses because it was found to be important for lentic fish but not for lotic species (Sullivan and Watzin 2009).
Reach scale variables—Assuming that the sea is the largest source of migrating larvae and/or juveniles for G. opperiens and G. urotaenia, we used watercourse distance from the sea to the site as the simplest measure of spatial factor (McDowall and Taylor 2000). We calculated watercourse distances as the shortest distance from the mouth of the river to the reach following the connecting waterways. The catchment area was also calculated to account for variation in habitat capacity, as it has been proved to be a good proxy for gross primary production and discharge (Finlay 2011; Altermatt 2013). Watercourse distance and catchment area were estimated using ArcGIS 10.1 with 1:25,000 topographic and digitized elevation maps.
Statistical analysis. We examined the differences in the longitudinal and cross-sectional distribution patterns for G. opperiens and G. urotaenia in the Shubuto River System. We used a generalized linear model (GLM) to reveal the factors influencing the longitudinal distribution patterns of the targeted species (i.e., reach scale). Response variables were the number of individuals of either G. opperiens or G. urotaenia at each site and were assumed to follow a negative binomial error distribution. The independent variables were water watercourse distance from the sea and catchment area. We also included a quadratic term of “watercourse distance from the sea” in the models to address the non-linearity of longitudinal distribution patterns, because G. opperiens is considered the “middle-reach type” of C. annularis (see Nakanishi 1978a, b), as mentioned above. No strong collinearity was found among the explanatory variables (Pearson’s correlation coefficients = −0.44).
To compare the within-reach cross-sectional distribution patterns of the two species and the environments (i.e., local scale), we performed likelihood ratio tests with chi-square approximation between the null and the alternative models. We constructed the null and the alternative models with a generalized mixed model (GLMM; random effect = individual sampling site), the response variables of which were either the number of fish individuals or the local environments in each belt line. The alternative model includes the position of the survey belt lines (river fringe or mid-channel) as an explanatory variable. Note that the samples for this analysis were confined to the sites with three belt lines (i.e., ≥6 m river width). The error structures were assumed to follow a negative binomial distribution for the number of individuals and a Gaussian distribution for local environmental factors. All the statistical analyses were conducted with R v. 3.0.1 (R Development Core Team 2013).
Results
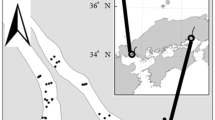
We collected 2,162 individuals of Gymnogobius opperiens and 110 individuals of G. urotaenia in the Shubuto River System from June to July 2011 (Fig. 1). All sites with the genus Gymnogobius were dominated by G. opperiens except for two sites (Fig. 1).
Distribution pattern of Gymnogobius opperiens and G. urotaenia based on the collected data of the river sites. Grey in circles denotes the dominance of G. opperiens, and black in circles denotes the dominance of G. urotaenia. Dots in the river are the study sites where Gymnogobius species were not collected
The ranges of SL are 2.7–9.7 mm (average ± SE: 5.2 ± 0.0) for G. opperiens and 4.2–12.3 mm (average: 9.1 ± 0.2) for G. urotaenia, respectively [Electronic Supplementary Material (ESM) Fig. S1]. So that, G. urotaenia has grown larger than G. opperiens in the river system.
The results of the GLM revealed a significant effect of watercourse distance from the sea on the two species (Table 1), but it influenced on the abundances of the two species differently (Fig. 2). Gymnogobius opperiens was most abundant in the middle-reach (95 % CI of the liner and quadratic terms did not include zero), while G. urotaenia was found frequently in the lower reach (only the liner term was significant).
Relationship between watercourse distance from the sea and the numbers of collected individuals per study site of Gymnogobius opperiens (open circles) and G. urotaenia (solid circles) in the Shubuto River System in June and July 2011. Dashed and solid lines show the value predicted using the regression models of G. opperiens and G. urotaenia, respectively
The cross-sectional distribution patterns of G. opperiens, G. urotaenia, and all the local environmental variables were significantly different between the fringe and the mid-channel habitats. Both G. opperiens and G. urotaenia were most abundant along the margins of the river (both P < 0.001; Fig. 3). However, the former species was frequently collected from the mid-channel [21 of 32 sites, 9.9 ± 3.0 individuals (average ± SE)], but the latter species was never collected in that habitat (Fig. 3). In fact, the occurrence ratios in the mid-channel were significantly different from each other (Fisher’s exact test: P < 0.001). The fringe habitats were characterized by fine-grain substrata, shallower water depth, and slower current velocity, whereas the mid-channel was characterized by coarse-grain substrata, deeper water depth, and faster flow velocity (P < 0.001; Fig. 4).
Cross-sectional distribution patterns of (a) Gymnogobius opperiens and (b) G. urotaenia in the river sites. Significant differences were observed by likelihood ratio tests based on the opposite and null models, respectively (both P < 0.001). The number of line transects of the river fringe is 64, and that of the mid-channel is 32
Comparison of (a) sand + silt proportion of bottom substrate, (b) water depth, and (c) current velocity between the fringe and the middle of the river. The box boundaries represent the 25th and 75th percentiles, the horizontal line is the median, and the whiskers extend to the most extreme data point that is no more than 1.5 times the interquartile range from the box. Data points outside of the whiskers are represented by open circles. All local environmental factors were found to have significant differences by the likelihood ratio tests based on the opposite and null models, respectively (all P < 0.001)
Discussion
Our study first identified the longitudinal and cross-sectional differences in habitat use between Gymnogobius opperiens and G. urotaenia with statistical supports, and it confirmed the previous observations by Nakanishi (1978b), Ishino et al. (1983), and Ishino (1987).
We revealed the difference of longitudinal distributions between G. opperiens and G. urotaenia. The peak of G. urotaenia abundance was in the lower reach of the Shubuto River System, and that of G. opperiens was in the middle reach of the river system (Fig. 2). This finding corresponds with the fact that G. opperiens used to be called the “middle-reach type” of Chaenogobius annularis in Nakanishi (1978a). These results support the hypothesis that the two species segregate their habitats on the reach scale.
The longitudinal difference in habitat use may reflect historical distribution of lentic habitats. Downstream areas of the Shubuto River System encompassed an extensive floodplain until 1950s (Miyazaki et al. 2012), likely a major habitat for the lentic goby G. urotaenia in the past (see results of fine-scale habitat preference: Figs. 3, 4). Such a legacy of the historical landscape could persist in the species and may result in their settling into the “previously” suitable localities (i.e., downstream reaches). Furthermore, the rapid loss of floodplain habitats seems to have caused a population decline of G. urotaenia (see Miyazaki et al. 2012, 2013b), likely leading to a stochastic failure of migration from the sea. Another possible reason is that G. urotaenia possessed inferior swimming ability or shorter freshwater life stage. However, this is an unlikely explanation because, in comparison with G. opperiens, G. urotaenia was larger in body size (see ESM Fig. S1) and has slightly delayed maturity, which implies a longer duration of the freshwater life stage (Ishino 1987, 1989). However, we do not have direct evidence supporting the above-mentioned inference, so further experimental studies are required to reveal the mechanism(s) behind the longitudinal habitat selection.
On the local scale, the two species exhibited some differences in cross-sectional habitat use. There are two possible explanations for the observed pattern. First, interspecific competition played a role in differentiating habitat use. Exploitive and/or interference competition can have an influence on their habitat selection since the two species share primary food resources (Ishino 1989). Second, the lower body depth (Stevenson 2002) and slightly depressed head shape (Y. Miyazaki, personal observation) of G. opperiens could provide an advantage to selecting lotic habitats, such as utilizing the void structure interspaces among cobbles in a riffle bed. These processes are not mutually exclusive and may act in concert in natural conditions.
Although darters and sculpins, which are ecologically similar benthic fishes with gobies but are not taxonomically related, usually show habitat segregations (e.g., Kessler and Thorp 1993; Stauffer et al. 1996; van Sink Gray and Stauffer 1999; White and Harvey 1999; Henry and Grossman 2008), these studies have focused on local environmental factors such as current velocity, water depth, and bottom substrata (Kessler et al. 1995; Welsh and Perry 1998; Compton and Taylor 2013). Our study shows that the watercourse distance from the sea is an important variable for the abundances of G. opperiens and G. urotaenia, and it likely reflects their marine amphidromous life cycles (Miyazaki et al. 2011; Miyazaki and Terui 2015). Therefore, our study emphasizes the importance of considering spatial factors to better understand habitat segregation among related species.
References
Aizawa T, Hatsumi M, Wakahama K (1994) Systematic study on the Chaenogobius species (family Gobiidae) by analysis of allozyme polymorphisms. Zool Sci 11:455–465
Akihito, Sakamoto K, Ikeda Y, Aizawa M (2013) Gobioidei. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, third edition. Tokai University Press, Hadano, pp 1347–1608, 2109–2211
Altermatt F (2013) Diversity in riverine metacommunities: a network perspective. Aquat Ecol 47:365–377
Compton M, Taylor C (2013) Spatial scale effects on habitat associations of the Ashy Darter, Etheostoma cinereum, an imperiled fish in the southeast United States. Ecol Freshwat Fish 22:178–191
Finlay JC (2011) Stream size and human influences on ecosystem production in river networks. Ecosphere 2:art87
Goto A (1991) Fish. In: Maeda Ippoen Foundation (ed) Guide book of natural environment in Hokkaido. Maeda Ippoen Foundation, Akan, pp 271–304
Helfman GS, Collette BB, Facey DE, Bowen BW (2009) The diversity of fishes, second edition: biology, evolution, and ecology. Wiley-Blackwell, Oxford
Henry BE, Grossman GD (2008) Microhabitat use by blackbanded (Percina nigrofasciata), turquoise (Etheostoma inscriptum), and tessellated (E. olmstedi) darters during drought in a Georgia piedmont stream. Environ Biol Fish 83:171–182
Ishino K (1987) Species complex of Chaenogobius annularis: adaptation to habitat and differentiation. In: Mizuno N, Goto A (eds) Japanese freshwater fishes: their distribution, variation and speciation. Tokai University Press, Tokyo, pp 189–197
Ishino K (1989) Chaenogobius urotaenia. In: Kawanabe H, Mizuno N (eds) Freshwater fishes of Japan. Yama-Kei Publishers, Tokyo, pp 618–620
Ishino K, Goto A, Hamada K (1983) Studies on the freshwater fish in Hokkaido, Japan-III. Distribution of three types of a goby, Chaenogobius annularis. Bull Fac Fish Hokkaido Univ 34:192–207
Japan Meteorological Agency (2012) Monthly data in an average year (1981–2010) at the Kuromatsunai Meteorological Station. http://www.data.jma.go.jp/obd/stats/etrn/view/nml_amd_ym.php?prec_no=16&block_no=0061&year=&month=&day=&view=p1. Accessed 19 August 2015
Kessler RK, Casper AF, Weddle GK (1995) Temporal variation in microhabitat use and spatial relations in the benthic fish community of a stream. Am Midl Nat 134:361–370
Kessler RK, Thorp JH (1993) Microhabitat segregation of the threatened spotted darter (Etheostoma maculatum) and closely related orangefin darter (E. bellum). Can J Fish Aquat Sci 50:1084–1091
Kuromatsunai Town (2012) Kuromatsunai-town Biodiversity Strategies and Action Plans. Environmental Policy Division of Kuromatsunai Town, Kuromatsunai
Kuromatsunai Town (2014) Annual survey of water quality in the Shubuto River System. Environmental Policy Division of Kuromatsunai Town, Kuromatsunai
McDowall RM (1988) Diadromy in fishes: migrations between freshwater and marine environments. Croom Helm, London
McDowall RM, Taylor MJ (2000) Environmental indicators of habitat quality in a migratory freshwater fish fauna. Environ Manage 25:357–374
Ministry of Land, Infrastructure, Transport and Tourism of Japan (2007) National survey on natural environment in river and watershore. http://mizukoku.nilim.go.jp/ksnkankyo/. Accessed 19 August 2015
Miyazaki Y, Terui A (2015) Temporal dynamics of fluvial fish community caused by marine amphidromous species in the Shubuto River, southwestern Hokkaido, Japan. Ichthyol Res DOI:10.1007/s10228-015-0474-7
Miyazaki Y, Terui A, Kubo S, Hatai N, Takahashi K, Saitoh H, Washitani I (2011) Ecological evaluation of the conservation of fish fauna in the Shubuto River system, southwestern Hokkaido. Jpn J Conserv Ecol 16:213–219
Miyazaki Y, Terui A, Senou H, Washitani I (2013a) Illustrated checklist of fishes from the Shubuto River System, southwestern Hokkaido, Japan. Check List 9:63–72
Miyazaki Y, Terui A, Yoshioka A, Kaifu K, Washitani I (2013b) Fish species composition of temporary small lentic habitats in the floodplains of the Shubuto River System: factors affecting species richness and suggestions for conservation and restoration. Jpn J Conserv Ecol 18:55–68
Miyazaki Y, Yoshioka A, Washitani I (2012) Attempt to reconstruct the past fish fauna of the Shubuto River System using museum specimens and interviews. Jpn J Conserv Ecol 17:235–244
Nakanishi T (1978a) Comparison of color pattern and meristic characters among the three types of Chaenogobius annularis Gill. Bull Fac Fish Hokkaido Univ 29:223–232
Nakanishi T (1978b) Comparison of ecological and geographical distributions among the three types of Chaenogobius annularis Gill. Bull Fac Fish Hokkaido Univ 29:233–242
Nelson JS (2006) Fishes of the world, fourth edition. John Wiley & Sons, New Jersey
Patzner RA, Van Tassell JL, Kovačiĉ M, Kapoor BG (eds) (2011) The biology of Gobies. CRC Press Taylor and Francis Group & Science Publishers, Enfield
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 19 August 2015
Senou H, Suzuki T, Shibukawa K, Yano K (2004) A photographic guide to the gobioid fishes of Japan. Heibonsha, Tokyo
Stauffer JR Jr, Boltz JM, Kellogg KA, van Sink ES (1996) Microhabitat partitioning in a diverse assemblage of darters in the Allegheny River system. Environ Biol Fish 46:37–44
Stevenson DE (2002) Systematics and distribution of fishes of the Asian goby genera Chaenogobius and Gymnogobius (Osteichthyes: Perciformes: Gobiidae), with the description of a new species. Species Divers 7:251–312
Suk HY, Kim JB, Min MS, Yang SY (1996) Genetic differentiation and reproductive isolation among three types of the floating goby (Chaenogobius annularis) in Korea. Korean J Zool 39:147–158
Sullivan SMP, Watzin MC (2009) Stream-floodplain connectivity and fish assemblage diversity in the Champlain Valley, Vermont, U.S.A. J Fish Biol 74:1394–1418
Terui A, Miyazaki Y, Matsuzaki SS, Washitani I (2011) Population status and factors affecting local density of endangered Japanese freshwater pearl mussel, Margaritifera laevis, in the Shubuto river basin, Hokkaido. Jpn J Conserv Ecol 16:149–157
Terui A, Miyazaki Y, Yoshioka A, Kaifu K, Matsuzaki SS, Washitani I (2014) Asymmetric dispersal structures a riverine metapopulation of the freshwater pearl mussel Margaritifera laevis. Ecol Evol 4:3004–3014
van Sink Gray E, Stauffer JR Jr (1999) Comparative microhabitat use of ecologically similar benthic fishes. Environ Biol Fish 56:443–453
Van Tassell JL, Tornabene L, Taylor MS (2011) A history of Gobioid morphological systematics. In: Patzner RA, Van Tassell JL, Kovačić M, Kapoor BG (eds) The biology of gobies. Science Publishers and CRC Press, Enfield and Boca Raton, pp 3–22
Welsh SA, Perry SA, (1998) Influence of spatial scale on estimates of substrate use by benthic darters. N Am J Fish Manage 18:954–959
White JL, Harvey BC (1999) Habitat separation of prickly sculpin, Cottus asper, and coastrange sculpin, Cottus aleuticus, in the mainstem Smith River, northwestern California. Copeia 1999:371–375
Wood BM, Bain MB (1995) Morphology and microhabitat use in stream fish. Can J Fish Aquat Sci 52:1487–1498
Yamasaki YY, Nishida M, Suzuki T, Mukai T, Watanabe K (2015) Phylogeny, hybridization, and life history evolution of Rhinogobius gobies in Japan, inferred from multiple nuclear gene sequence. Mol Phylogenet Evol 90:20–33
Acknowledgments
We thank M. Wakami, H. Suzuki, K. Takahashi, N. Hatai, K. Kataoka, H. Saito, K. Kaifu, S. Kubo and I. Washitani for generous help in research in the Shubuto River System. This study was partly supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to I. Washitani, No. 22310143 and 26292181), the Global Center of Excellence for Asian conservation ecology as a basis of human–nature mutualism, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and “The Creation projects for Green Innovations originated from the Universities; Green Network of Excellence (GRENE) in the field of Environmental Information” funded through the Ministry of Education, Culture, Sports, Science and Technology. This study was also partly funded by Research Fellowship for Young Scientist (to Y. Miyazaki, No. 25·11038) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Miyazaki and A. Terui contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Miyazaki, Y., Terui, A. Difference in habitat use between the two related goby species of Gymnogobius opperiens and Gymnogobius urotaenia: a case study in the Shubuto River System, Hokkaido, Japan. Ichthyol Res 63, 317–323 (2016). https://doi.org/10.1007/s10228-015-0501-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-015-0501-8